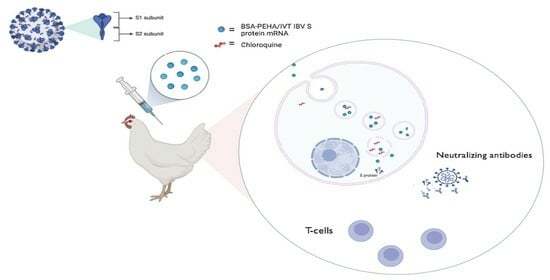
Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cationic BSA NP Synthesis and Characterization
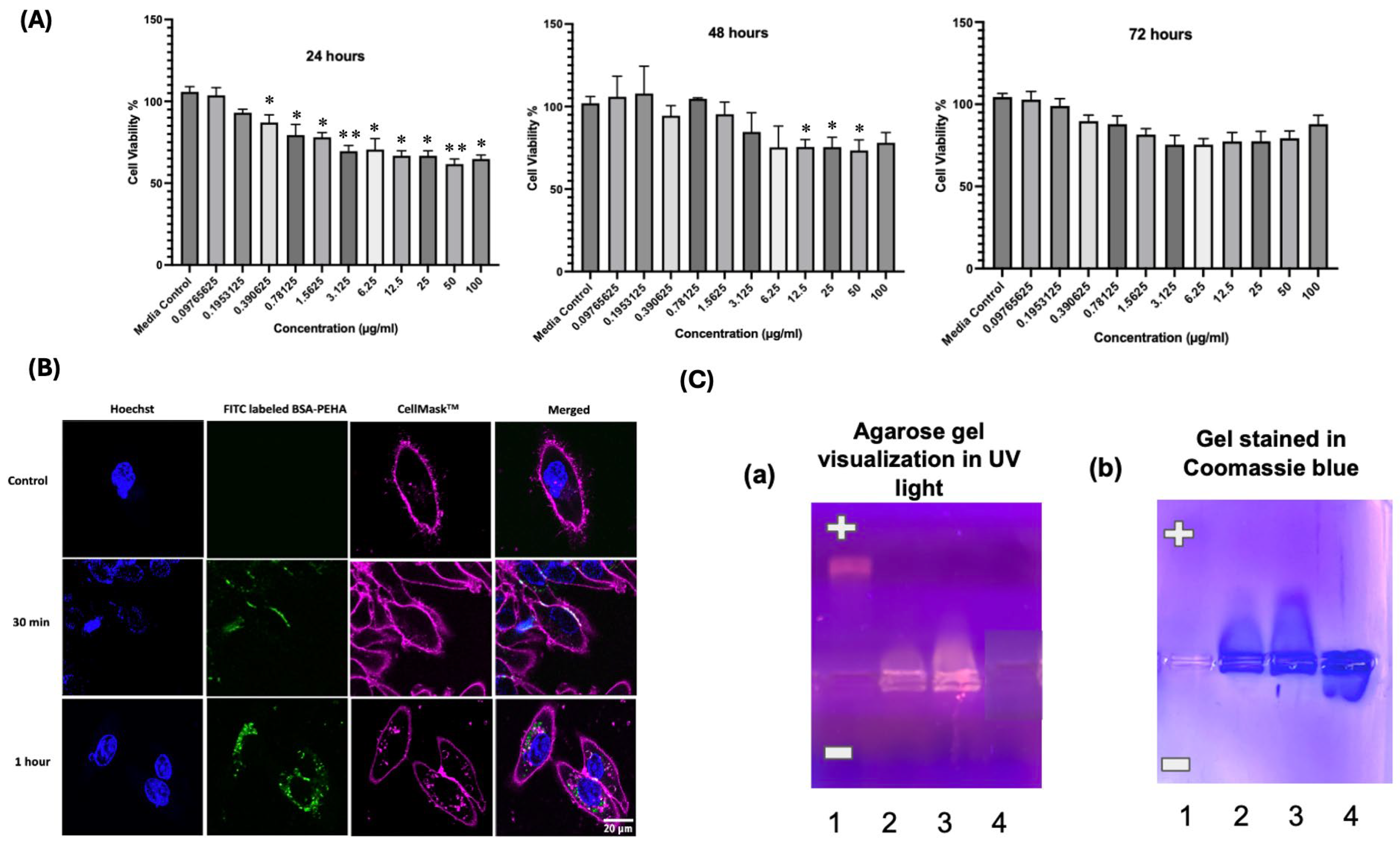
2.2. NP Uptake Assay Using HD11 Cells
2.3. In Vitro Cytotoxicity of BSA-PEHA NPs Determined Using an MTT Assay
In Vitro Cytotoxicity Study Using Flow Cytometry
2.4. Electrophoretic Mobility Shift Assay (EMSA)
2.5. Circular Dichroism
2.6. Transfection of Cells with Lipofectamine 2000 and BSA-PEHA NPs
2.7. Design of IVT S Protein mRNA for IBV and Transfection in HD11 Cells
2.7.1. SDS PAGE
2.7.2. Western Blotting
2.8. Animal Experiment
2.8.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8.2. Lymphocyte Proliferation
2.8.3. Virus Neutralization
2.8.4. Statistical Analysis
3. Results
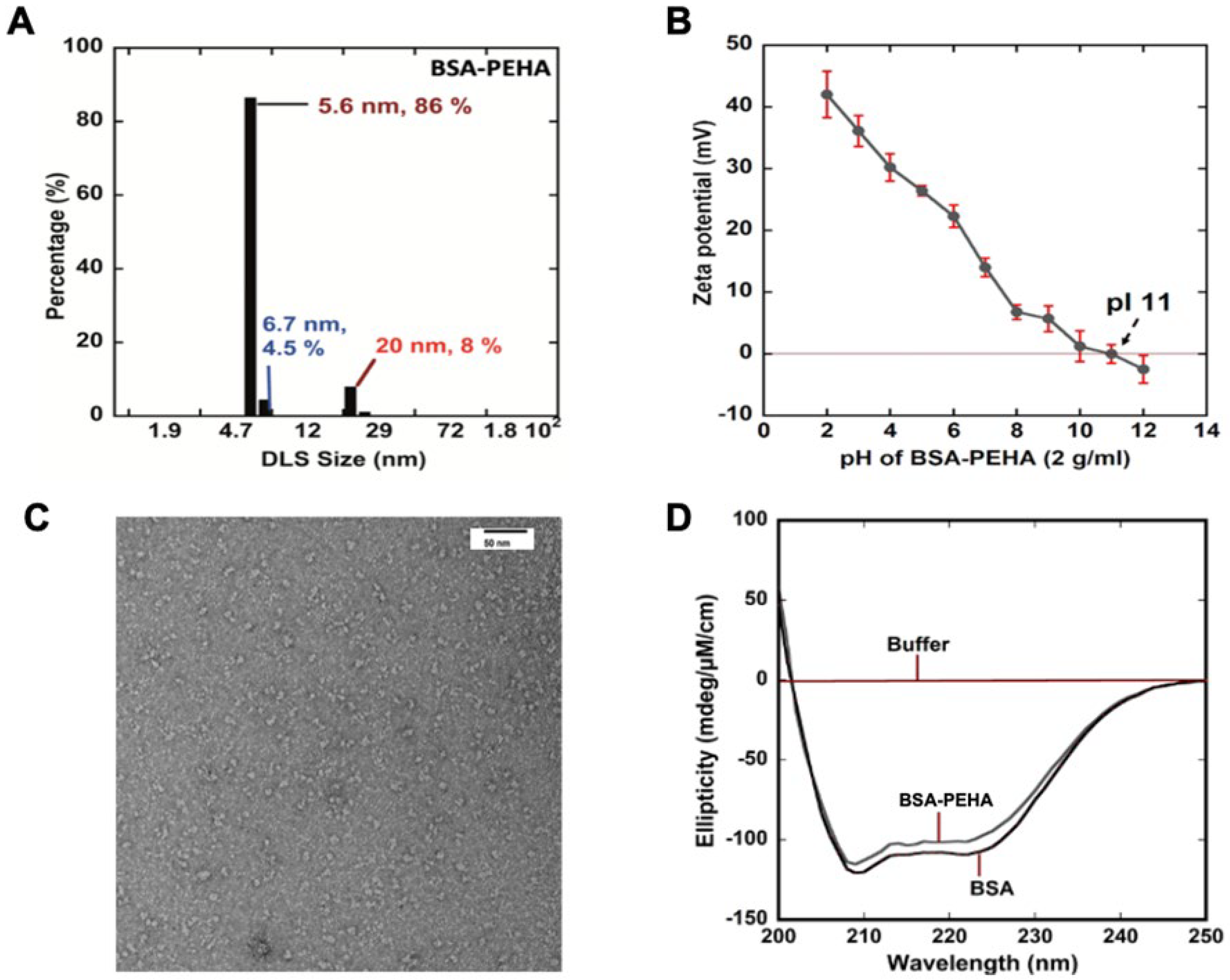
3.1. BSA-PEHA NP Synthesis and Characterization
3.2. Cellular Uptake of BSA-PEHA by Chicken Macrophage-like HD 11 Cells
3.3. In Vitro Cell Cytotoxicity Study of BSA-PEHA NP Using MTT Assay
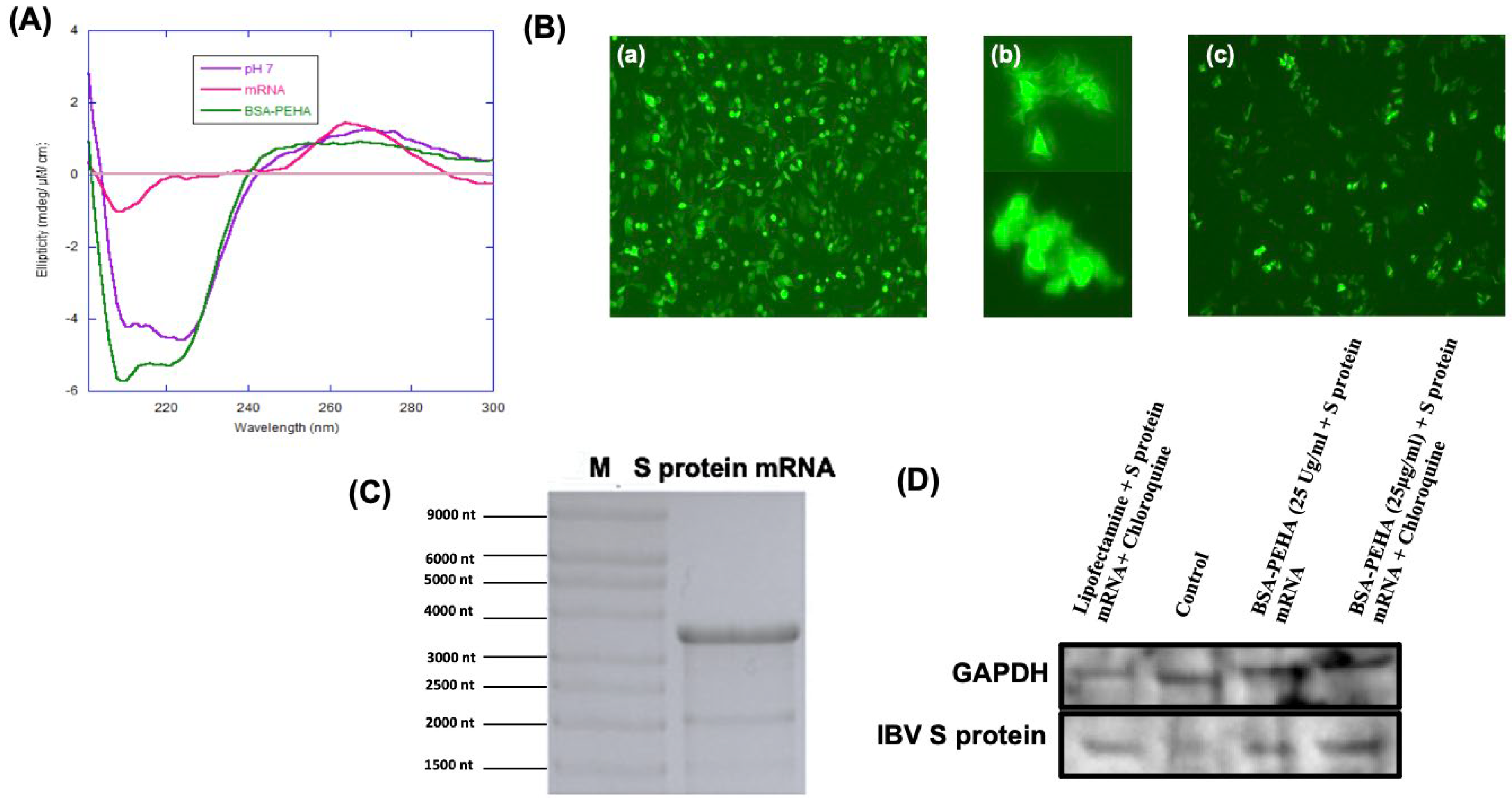
3.4. Binding Study of mRNA with BSA-PEHA NPs Using Electrophoretic Mobility Shift Assay (EMSA) and Evaluation of Structure Change Using CD Spectra
3.5. Expression of eGFP and IVT IBV S Protein Encoding mRNA in HD11 Cells
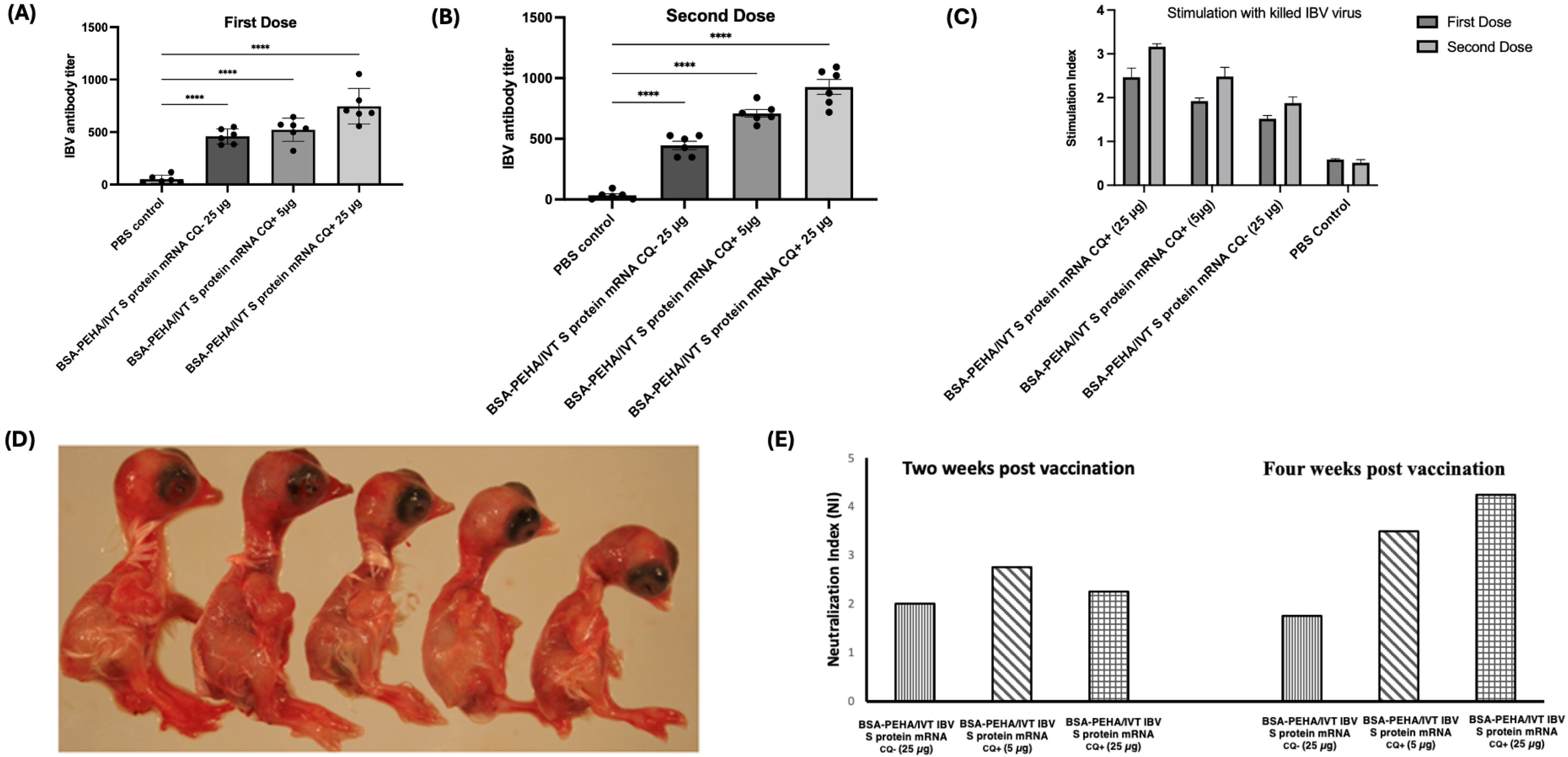
3.5.1. Antibody Response to BSA-PEHA Conjugated with IBV S Protein Encoding mRNA
3.5.2. Stimulation of Cell-Mediated Immune Response in PBMCs from Chickens Vaccinated with the BSA-PEHA/IVT IBV S Protein Encoding mRNA
3.5.3. Evaluation of Efficacy of the BSA-PEHA/IVT IBV S Protein Encoding mRNA Using Serum Neutralization Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavanagh, D. Coronaviruses in Poultry and Other Birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef] [PubMed]
- McKinley, E.T.; Hilt, D.A.; Jackwood, M.W. Avian Coronavirus Infectious Bronchitis Attenuated Live Vaccines Undergo Selection of Subpopulations and Mutations Following Vaccination. Vaccine 2008, 26, 1274–1284. [Google Scholar] [CrossRef]
- Tarpey, I.; Orbell, S.J.; Britton, P.; Casais, R.; Hodgson, T.; Lin, F.; Hogan, E.; Cavanagh, D. Safety and Efficacy of an Infectious Bronchitis Virus Used for Chicken Embryo Vaccination. Vaccine 2006, 24, 6830–6838. [Google Scholar] [CrossRef] [PubMed]
- Ladman, B.S.; Pope, C.R.; Ziegler, A.F.; Swieczkowski, T.; Callahan, C.J.M.; Davison, S.; Gelb, J. Protection of Chickens after Live and Inactivated Virus Vaccination against Challenge with Nephropathogenic Infectious Bronchitis Virus PA/Wolgemuth/98. Avian Dis. 2002, 46, 938–944. [Google Scholar] [CrossRef]
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in avian species—Review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.V. CORONAVIRUSES (CORONAVIRIDAE). In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 291–298. [Google Scholar] [CrossRef]
- Godet, M.; Grosclaude, J.; Delmas, B.; Laude, H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994, 68, 8008–8016. [Google Scholar] [CrossRef]
- Promkuntod, N.; Van Eijndhoven, R.E.W.; De Vrieze, G.; Gröne, A.; Verheije, M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 2014, 448, 26–32. [Google Scholar] [CrossRef]
- Kant, A.; Koch, G.; Van Roozelaar, D.J.; Kusters, J.G.; Poelwijk, F.A.J.; Van Der Zeijst, B.A.M. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J. Gen. Virol. 1992, 73, 591–596. [Google Scholar] [CrossRef]
- Johnson, M.A.; Pooley, C.; Ignjatovic, J.; Tyack, S.G. A Recombinant Fowl Adenovirus Expressing the S1 Gene of Infectious Bronchitis Virus Protects against Challenge with Infectious Bronchitis Virus. Vaccine 2003, 21, 2730–2736. [Google Scholar] [CrossRef]
- Wang, X.; Schnitzlein, W.M.; Tripathy, D.N.; Girshick, T.; Khan, M.I. Construction and Immunogenicity Studies of Recombinant Fowl Poxvirus Containing the S1 Gene of Massachusetts 41 Strain of Infectious Bronchitis Virus. Avian Dis. 2002, 46, 831–838. [Google Scholar] [CrossRef]
- Song, C.S.; Jang, H.K.; Lee, Y.J.; Izumiya, Y.; Mikami, T.; Lee, C.W.; Sung, H.W.; Kim, J.H.; Mo, I.P. Induction of Protective Immunity in Chickens Vaccinated with Infectious Bronchitis Virus S1 Glycoprotein Expressed by a Recombinant Baculovirus. J. Gen. Virol. 1998, 79, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, O.B.; Estevez, C.; Yu, Q.; Suarez, D.L. Passive Antibody Transfer in Chickens to Model Maternal Antibody after Avian Influenza Vaccination. Vet. Immunol. Immunopathol. 2013, 152, 341–347. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.-N.; Wang, X.; Tang, J.-N.; Lu, D.; Zhang, Y.-F.; Guo, Z.-C.; Li, Y.-L.; Gao, R.; Kang, R.-M. The Protective Immune Response against Infectious Bronchitis Virus Induced by Multi-Epitope Based Peptide Vaccines. Biosci. Biotechnol. Biochem. 2009, 73, 1500–1504. [Google Scholar] [CrossRef]
- Ignjatovic, J.; Sapats, S. Identification of Previously Unknown Antigenic Epitopes on the S and N Proteins of Avian Infectious Bronchitis Virus. Arch. Virol. 2005, 150, 1813–1831. [Google Scholar] [CrossRef] [PubMed]
- Kapczynski, D.R.; Hilt, D.A.; Shapiro, D.; Sellers, H.S.; Jackwood, M.W. Protection of Chickens from Infectious Bronchitis by In Ovo and Intramuscular Vaccination with a DNA Vaccine Expressing the S1 Glycoprotein. Avian Dis. 2003, 47, 272–285. [Google Scholar] [CrossRef]
- Tan, B.; Wang, H.; Shang, L.; Yang, T. Coadministration of Chicken GM-CSF with a DNA Vaccine Expressing Infectious Bronchitis Virus (IBV) S1 Glycoprotein Enhances the Specific Immune Response and Protects against IBV Infection. Arch. Virol. 2009, 154, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, H.; Lu, D.; Zhang, Y.; Wang, T.; Kang, R. The Immunoreactivity of a Chimeric Multi-Epitope DNA Vaccine against IBV in Chickens. Biochem. Biophys. Res. Commun. 2008, 377, 221–225. [Google Scholar] [CrossRef]
- Tang, M.; Wang, H.; Zhou, S.; Tian, G. Enhancement of the Immunogenicity of an Infectious Bronchitis Virus DNA Vaccine by a Bicistronic Plasmid Encoding Nucleocapsid Protein and Interleukin-2. J. Virol. Methods 2008, 149, 42–48. [Google Scholar] [CrossRef]
- Babapoor, S.; Almeida, D.D.O.; Fabis, J.J.; Helal, Z.H.; Wang, X.; Girshick, T.; Khan, M.I. Protective Effect of In Ovo Vaccination with IBV-Spike-Recombinant DNA and Chicken Interferon as an Adjuvant. Int. J. Poult. Sci. 2009, 8, 1034–1041. [Google Scholar] [CrossRef]
- Tang, M.-J.; Wang, H.-N.; Zhou, S.; Huang, Y.; Liu, P. Potent immune responses elicited by a bicistronic IBV DNA vaccine expressing S1 and IL-2 gene. Wei Sheng Wu Xue Bao 2007, 47, 1055–1059. [Google Scholar]
- Armesto, M.; Evans, S.; Cavanagh, D.; Abu-Median, A.-B.; Keep, S.; Britton, P. A Recombinant Avian Infectious Bronchitis Virus Expressing a Heterologous Spike Gene Belonging to the 4/91 Serotype. PLoS ONE 2011, 6, e24352. [Google Scholar] [CrossRef] [PubMed]
- Casais, R.; Thiel, V.; Siddell, S.G.; Cavanagh, D.; Britton, P. Reverse Genetics System for the Avian Coronavirus Infectious Bronchitis Virus. J. Virol. 2001, 75, 12359–12369. [Google Scholar] [CrossRef] [PubMed]
- Inayoshi, Y.; Oguro, S.; Tanahashi, E.; Lin, Z.; Kawaguchi, Y.; Kodama, T.; Sasakawa, C. Bacterial Artificial Chromosome-Based Reverse Genetics System for Cloning and Manipulation of the Full-Length Genome of Infectious Bronchitis Virus. Curr. Res. Microb. Sci. 2022, 3, 100155. [Google Scholar] [CrossRef] [PubMed]
- Sakhrie, A.; Ding, J.; Kalluri, A.; Liu, C.; Kumar, C.V.; Khan, M.I. In-Vitro Cytotoxicity Assessment and Cellular Uptake Study of Novel FluoDot Nanoparticles on Human Cervical Carcinoma Cells. Int. J. Nanoparticles Nanotechnol. 2024, 9, 44. [Google Scholar] [CrossRef]
- Sakhrie, A.; Ding, J.; Kalluri, A.; Helal, Z.; Kumar, C.V.; Khan, M.I. Fluodot Nanoparticle—A Promising Novel Delivery System For Veterinary Vaccine. Int. J. Nanoparticle Res. 2020, 3, 14. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; Von Briesen, H.; Schubert, D. Optimization of the Preparation Process for Human Serum Albumin (HSA) Nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer Functionalized Curcumin-Loaded Human Serum Albumin (HSA) Nanoparticles for Targeted Delivery to HER-2 Positive Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-Assembled Amphiphilic Zein-Lactoferrin Micelles for Tumor Targeted Co-Delivery of Rapamycin and Wogonin to Breast Cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Kiani, M.; Farokhi, M.; Kundu, S.C.; Reis, R.L.; Gholami, M.; Bardania, H.; Dinarvand, R.; Geramifar, P.; Beiki, D.; et al. Targeted Delivery System Based on Gemcitabine-Loaded Silk Fibroin Nanoparticles for Lung Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31600–31611. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent Advancement of Gelatin Nanoparticles in Drug and Vaccine Delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Da Silva, N.I.O.; Salvador, E.A.; Rodrigues Franco, I.; De Souza, G.A.P.; De Souza Morais, S.M.; Prado Rocha, R.; Dias Novaes, R.; Paiva Corsetti, P.; Malaquias, L.C.C.; Leomil Coelho, L.F. Bovine Serum Albumin Nanoparticles Induce Histopathological Changes and Inflammatory Cell Recruitment in the Skin of Treated Mice. Biomed. Pharmacother. 2018, 107, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Avci-Adali, M.; Behring, A.; Keller, T.; Krajewski, S.; Schlensak, C.; Wendel, H.P. Optimized Conditions for Successful Transfection of Human Endothelial Cells with in Vitro Synthesized and Modified mRNA for Induction of Protein Expression. J. Biol. Eng. 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Helal, Z.H.; Karch, C.P.; Mishra, N.; Girshick, T.; Garmendia, A.; Burkhard, P.; Khan, M.I. A Self-Adjuvanted Nanoparticle Based Vaccine against Infectious Bronchitis Virus. PLoS ONE 2018, 13, e0203771. [Google Scholar] [CrossRef]
- WOAH. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Thirteenth Edition 2024; AVIAN INFECTIOUS BRONCHITIS; CHAPTER 3.3.2. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.02_AIB.pdf (accessed on 23 July 2021).
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of Nanoparticles to the Clathrin-Mediated Endocytic Pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Shape Effect in Cellular Uptake of PEGylated Nanoparticles: Comparison between Sphere, Rod, Cube and Disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding Biophysicochemical Interactions at the Nano–Bio Interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Zhang, B.; Sai Lung, P.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape Dependent Cytotoxicity of PLGA-PEG Nanoparticles on Human Cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles via the Pathways of Clathrin- and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Donkor, D.A.; Tang, X.S. Tube Length and Cell Type-Dependent Cellular Responses to Ultra-Short Single-Walled Carbon Nanotube. Biomaterials 2014, 35, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wilson, M.R.; MacNee, W.; Stone, V.; Donaldson, K. Size-Dependent Proinflammatory Effects of Ultrafine Polystyrene Particles: A Role for Surface Area and Oxidative Stress in the Enhanced Activity of Ultrafines. Toxicol. Appl. Pharmacol. 2001, 175, 191–199. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-Dependent Toxicity of Metal Oxide Particles—A Comparison between Nano- and Micrometer Size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-Dependent Cytotoxicity of Amorphous Silica Nanoparticles in Human Hepatoma HepG2 Cells. Toxicol. Vitr. 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface Characteristics, Copper Release, and Toxicity of Nano- and Micrometer-Sized Copper and Copper(II) Oxide Particles: A Cross-Disciplinary Study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-Dependent Cytotoxicity of Monodisperse Silica Nanoparticles in Human Endothelial Cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, W.-Y.; Wang, T.-C.; Jia, G.; Wang, M.; Shi, J.-W.; Zhang, F.; Zhao, Y.-L.; Chai, Z.-F. Acute Toxicity of Nano- and Micro-Scale Zinc Powder in Healthy Adult Mice. Toxicol. Lett. 2006, 161, 115–123. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L. Nanoscale and Fine Zinc Oxide Particles: Can in Vitro Assays Accurately Forecast Lung Hazards Following Inhalation Exposures? Environ. Sci. Technol. 2009, 43, 7939–7945. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M.; Hanawa, T. Cytotoxicity Evaluation of Ceramic Particles of Different Sizes and Shapes. J. Biomed. Mater. Res. 2004, 68A, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.M.; Revzin, A. The Use of Gel Electrophoresis to Detect and Study Nucleic Acid—Protein Interactions. Trends Biochem. Sci. 1986, 11, 395–396. [Google Scholar] [CrossRef]
- Sakai, T.T.; Torget, R.; I, J.; Freda, C.E.; Cohen, S.S. The Binding of Polyamines and of Ethidium Bromide to tRNA. Nucl. Acids Res. 1975, 2, 1005–1022. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.E.; Erdmann, V.A. Binding of Spermidine to Transfer Ribonucleic Acid. Biochemistry 1982, 21, 5280–5288. [Google Scholar] [CrossRef]
- Frydman, L.; Rossomando, P.C.; Frydman, V.; Fernandez, C.O.; Frydman, B.; Samejima, K. Interactions between Natural Polyamines and tRNA: An 15N NMR Analysis. Proc. Natl. Acad. Sci. USA 1992, 89, 9186–9190. [Google Scholar] [CrossRef]
- Ruiz-Chica, J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Raman Study of the Effects of Polyamines on DNA: Spermine and Histamine. J. Mol. Struct. 1999, 480–481, 455–458. [Google Scholar] [CrossRef]
- Deng, H. Structural Basis of Polyamine-DNA Recognition: Spermidine and Spermine Interactions with Genomic B-DNAs of Different GC Content Probed by Raman Spectroscopy. Nucleic Acids Res. 2000, 28, 3379–3385. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Chica, A.J.; Medina, M.A.; Sánchez-Jiménez, F.; Ramírez, F.J. Characterization of Polyamine-Induced Aggregates of Oligodeoxyribonucleotides by Raman Spectroscopy. J. Mol. Struct. 2001, 565–566, 141–146. [Google Scholar] [CrossRef]
- Ruiz-Chica, J. Raman Spectroscopy Study of the Interaction between Biogenic Polyamines and an Alternating AT Oligodeoxyribonucleotide. Biochim. Biophys. Acta (BBA)—Gene Struct. Expr. 2003, 1628, 11–21. [Google Scholar] [CrossRef]
- Ouameur, A.A.; Tajmir-Riahi, H.-A. Structural Analysis of DNA Interactions with Biogenic Polyamines and Cobalt(III)Hexamine Studied by Fourier Transform Infrared and Capillary Electrophoresis. J. Biol. Chem. 2004, 279, 42041–42054. [Google Scholar] [CrossRef]
- Xaplanteri, M.A. Localization of Spermine Binding Sites in 23S rRNA by Photoaffinity Labeling: Parsing the Spermine Contribution to Ribosomal 50S Subunit Functions. Nucleic Acids Res. 2005, 33, 2792–2805. [Google Scholar] [CrossRef] [PubMed]
- N’soukpoé-Kossi, C.N.; Ouameur, A.A.; Thomas, T.; Shirahata, A.; Thomas, T.J.; Tajmir-Riahi, H.A. DNA Interaction with Antitumor Polyamine Analogues: A Comparison with Biogenic Polyamines. Biomacromolecules 2008, 9, 2712–2718. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Odoko, M.; Grzeskowiak, K.; Hiyama, Y.; Tsukamoto, K.; Maezaki, N.; Ishida, T.; Tanaka, T.; Okabe, N.; Fukuyama, K.; et al. Polyamines Stabilize Left-Handed Z-DNA: Using X-Ray Crystallographic Analysis, We Have Found a New Type of Polyamine (PA) That Stabilizes Left-Handed Z-DNA. Biochem. Biophys. Res. Commun. 2008, 366, 275–280. [Google Scholar] [CrossRef] [PubMed]
- N’soukpoé-Kossi, C.N.; Ahmed Ouameur, A.; Thomas, T.; Thomas, T.J.; Tajmir-Riahi, H.A. Interaction of tRNA with Antitumor Polyamine Analogues. Biochem. Cell Biol. 2009, 87, 621–630. [Google Scholar] [CrossRef]
- Ouameur, A.A.; Bourassa, P.; Tajmir-Riahi, H.-A. Probing tRNA Interaction with Biogenic Polyamines. RNA 2010, 16, 1968–1979. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Hall, D.; Handel, A. Molecular Evolution and Emergence of Avian Gammacoronaviruses. Infect. Genet. Evol. 2012, 12, 1305–1311. [Google Scholar] [CrossRef]
- Finney, P.M.; Box, P.G.; Holmes, H.C. Studies with a Bivalent Infectious Bronchitis Killed Virus Vaccine. Avian Pathol. 1990, 19, 435–450. [Google Scholar] [CrossRef]
- Gough, R.E.; Allan, W.H.; Nedelciu, D. Immune Response to Monovalent and Bivalent Newcastle Disease and Infectious Bronchitis Inactivated Vaccines. Avian Pathol. 1977, 6, 131–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Vaccine Induces Neutralizing Antibodies and Poly-Specific T Cells in Humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Ouranidis, A.; Vavilis, T.; Mandala, E.; Davidopoulou, C.; Stamoula, E.; Markopoulou, C.K.; Karagianni, A.; Kachrimanis, K. mRNA Therapeutic Modalities Design, Formulation and Manufacturing under Pharma 4.0 Principles. Biomedicines 2021, 10, 50. [Google Scholar] [CrossRef]
- Minnaert, A.-K.; Vanluchene, H.; Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Raemdonck, K.; Sanders, N.N.; Remaut, K. Strategies for Controlling the Innate Immune Activity of Conventional and Self-Amplifying mRNA Therapeutics: Getting the Message Across. Adv. Drug Deliv. Rev. 2021, 176, 113900. [Google Scholar] [CrossRef] [PubMed]
- Presnyak, V.; Alhusaini, N.; Chen, Y.-H.; Martin, S.; Morris, N.; Kline, N.; Olson, S.; Weinberg, D.; Baker, K.E.; Graveley, B.R.; et al. Codon Optimality Is a Major Determinant of mRNA Stability. Cell 2015, 160, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Dalby, B. Advanced Transfection with Lipofectamine 2000 Reagent: Primary Neurons, siRNA, and High-Throughput Applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, L.; Karelsky, S.; Andino, R. Short Interfering RNA Confers Intracellular Antiviral Immunity in Human Cells. Nature 2002, 418, 430–434. [Google Scholar] [CrossRef]
- Yu, J.-Y.; DeRuiter, S.L.; Turner, D.L. RNA Interference by Expression of Short-Interfering RNAs and Hairpin RNAs in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 6047–6052. [Google Scholar] [CrossRef]
- Yamagishi, T.; Sahni, S.; Sharp, D.M.; Arvind, A.; Jansson, P.J.; Richardson, D.R. P-Glycoprotein Mediates Drug Resistance via a Novel Mechanism Involving Lysosomal Sequestration. J. Biol. Chem. 2013, 288, 31761–31771. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal Escape Pathways for Delivery of Biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakhrie, A.; Kalluri, A.; Helal, Z.H.; Kumar, C.V.; Khan, M.I. Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection. Vaccines 2025, 13, 568. https://doi.org/10.3390/vaccines13060568
Sakhrie A, Kalluri A, Helal ZH, Kumar CV, Khan MI. Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection. Vaccines. 2025; 13(6):568. https://doi.org/10.3390/vaccines13060568
Chicago/Turabian StyleSakhrie, Aseno, Ankarao Kalluri, Zeinab H. Helal, Challa V. Kumar, and Mazhar I. Khan. 2025. "Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection" Vaccines 13, no. 6: 568. https://doi.org/10.3390/vaccines13060568
APA StyleSakhrie, A., Kalluri, A., Helal, Z. H., Kumar, C. V., & Khan, M. I. (2025). Nanoparticle-Based mRNA Vaccine Induces Protective Neutralizing Antibodies Against Infectious Bronchitis Virus in In-Vivo Infection. Vaccines, 13(6), 568. https://doi.org/10.3390/vaccines13060568