Real-World Effectiveness of Boosting Against Omicron Hospitalization in Older Adults, Stratified by Frailty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Period and Design
2.2. Frailty
2.3. COVID-19 Outcomes
2.4. Covariates
2.5. Statistical Analysis
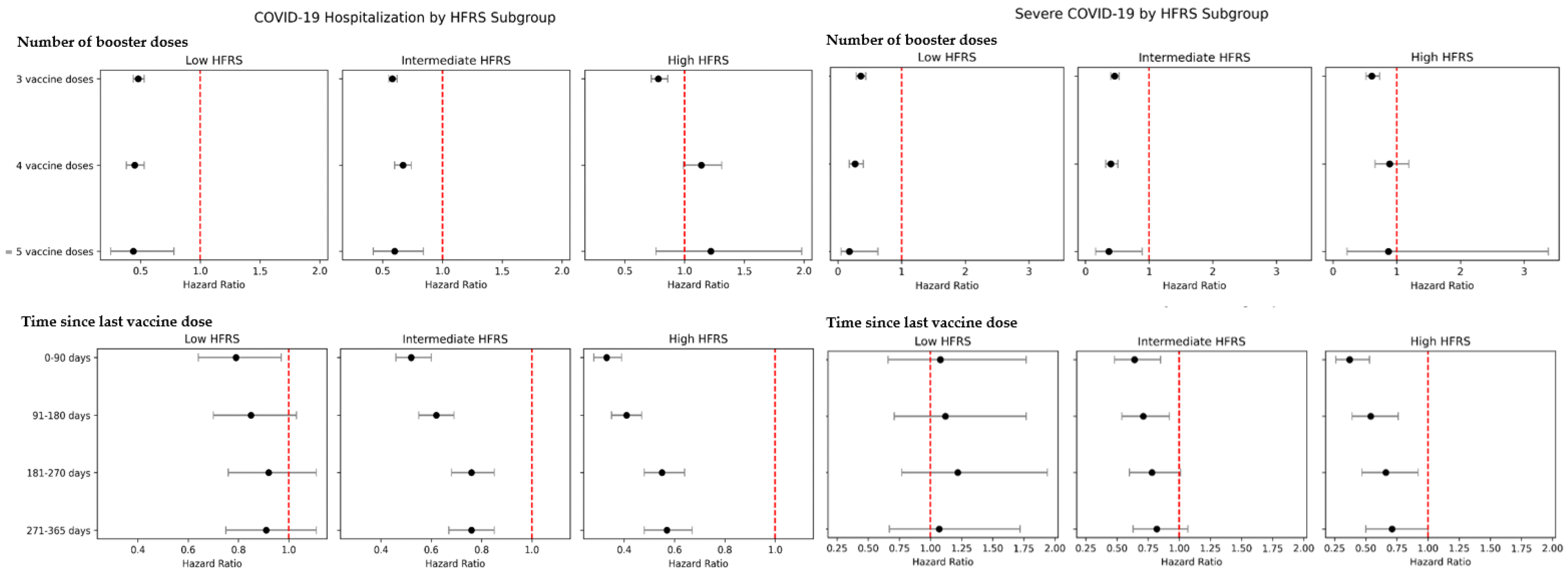
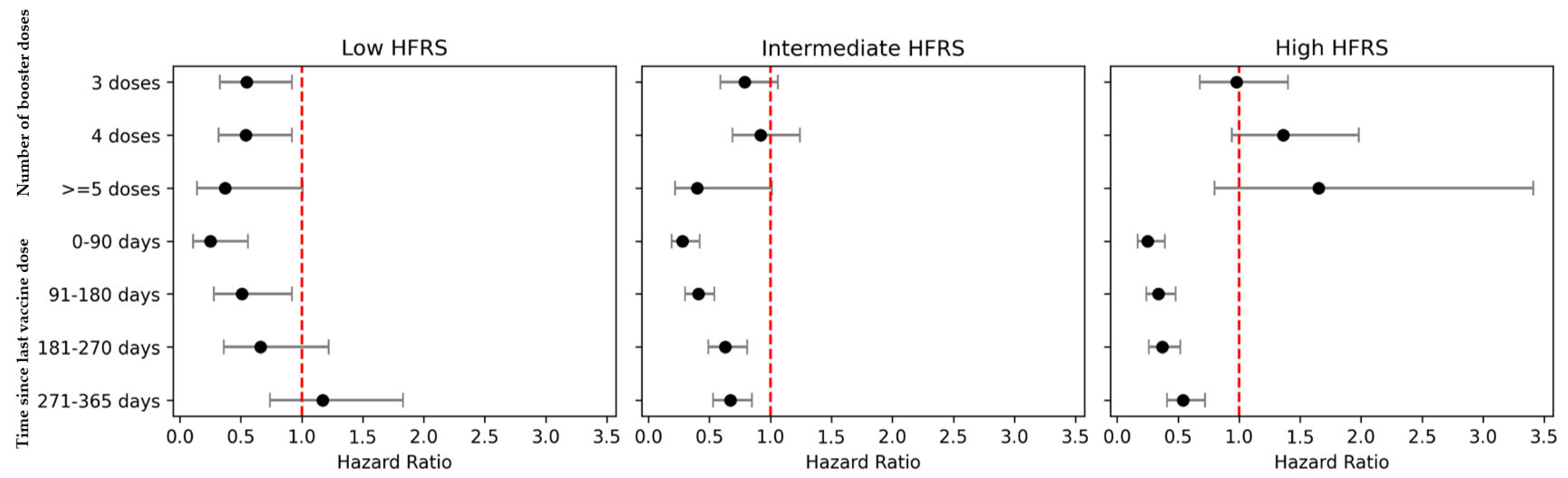
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VE | Vaccine effectiveness |
| HFRS | Hospital Frailty Risk Score |
| ICD-10 | International-Classification-of-Diseases, Tenth-Revision |
| CFS | Clinical Frailty Scale |
References
- Tana, C. Editorial: Frailty in older patients during the COVID-19 era. Front. Med. 2024, 10, 1348468. [Google Scholar] [CrossRef]
- Makovski, T.T.; Ghattas, J.; Monnier-Besnard, S.; Cavillot, L.; Ambrožová, M.; Vašinová, B.; Feteira-Santos, R.; Bezzegh, P.; Bollmann, F.P.; Cottam, J.; et al. Multimorbidity and frailty are associated with poorer SARS-CoV-2-related outcomes: Systematic review of population-based studies. Aging Clin. Exp. Res. 2024, 36, 40. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Zhang, S.; Kong, Y.; Shen, Z.; Zhang, J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: A systematic review and analysis. J. Med. Virol. 2022, 94, 5790–5801. [Google Scholar] [CrossRef]
- van Raaij, B.F.M.; Noordam, R.; Smits, R.A.L.; van der Klei, V.M.G.T.H.; Jansen, S.W.M.; van der Linden, C.M.J.; Polinder-Bos, H.A.; Minnema, J.; Tap, L.; van der Bol, J.M.; et al. Preparing for future pandemics: Frailty associates with mortality in hospitalized older people during the entire COVID-19 pandemic, a Dutch multicentre cohort study. Eur. Geriatr. Med. 2024. ahead of print. [Google Scholar] [CrossRef]
- Kojima, N.; Adams, K.; Self, W.H.; Gaglani, M.; McNeal, T.; Ghamande, S.; Steingrub, J.S.; Shapiro, N.I.; Duggal, A.; Busse, L.W.; et al. Investigating Respiratory Viruses in the Acutely Ill (IVY) Network. Changing Severity and Epidemiology of Adults Hospitalized With Coronavirus Disease 2019 (COVID-19) in the United States After Introduction of COVID-19 Vaccines, March 2021–August 2022. Clin. Infect. Dis. 2023, 77, 547–557. [Google Scholar] [CrossRef]
- Kakugawa, T.; Mimura, Y.; Mimura-Kimura, Y.; Doi, K.; Ohteru, Y.; Kakugawa, H.; Oishi, K.; Kakugawa, M.; Hirano, T.; Matsunaga, K. Kinetics of pro- and anti-inflammatory spike-specific cellular immune responses in long-term care facility residents after COVID-19 mRNA primary and booster vaccination: A prospective longitudinal study in Japan. Immun. Ageing. 2024, 21, 41. [Google Scholar] [CrossRef]
- Smith, C.L.; Didion, E.; Aung, H.; Tamilselvan, B.; Bej, T.; Oyebanji, O.; Shive, C.L.; Wilson, B.M.; Cameron, M.; Cameron, C.; et al. Longitudinal analysis of nursing home residents’ T cell responses after SARS-CoV-2 mRNA vaccinations shows influence of biological sex and SARS-CoV-2 infection history. J. Infect. Dis. 2024, 230, 635–644. [Google Scholar] [CrossRef]
- Semelka, C.T.; DeWitt, M.E.; Callahan, K.E.; Herrington, D.M.; Alexander-Miller, M.A.; Yukich, J.O.; Munawar, I.; McCurdy, L.H.; Gibbs, M.A.; Weintraub, W.S.; et al. Frailty and COVID-19 mRNA Vaccine Antibody Response in the COVID-19 Community Research Partnership. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1366–1370. [Google Scholar] [CrossRef]
- Meeraus, W.; Joy, M.; Ouwens, M.; Taylor, K.S.; Venkatesan, S.; Dennis, J.; Tran, T.N.; Dashtban, A.; Fan, X.; Williams, R.; et al. AZD1222 effectiveness against severe COVID-19 in individuals with comorbidity or frailty: The RAVEN cohort study. J. Infect. 2024, 88, 106129. [Google Scholar] [CrossRef]
- Tang, F.; Hammel, I.S.; Andrew, M.K.; Ruiz, J.G. COVID-19 mRNA vaccine effectiveness against hospitalisation and death in veterans according to frailty status during the SARS-CoV-2 delta (B.1.617.2) variant surge in the USA: A retrospective cohort study. Lancet Healthy Longev. 2022, 3, e589–e598. [Google Scholar] [CrossRef]
- Tang, F.; Hammel, I.S.; Andrew, M.K.; Ruiz, J.G. Frailty Reduces Vaccine Effectiveness Against SARS-CoV-2 Infection: A Test-Negative Case Control Study Using National VA Data. J. Nutr. Health Aging 2023, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Carazo, S.; Skowronski, D.M.; Brisson, M.; Sauvageau, C.; Brousseau, N.; Fafard, J.; Gilca, R.; Talbot, D.; Ouakki, M.; Febriani, Y.; et al. Effectiveness of previous infection-induced and vaccine-induced protection against hospitalisation due to omicron BA subvariants in older adults: A test-negative, case-control study in Quebec, Canada. Lancet Healthy Longev. 2023, 4, e409–e420. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Hayes, K.N.; Zullo, A.R.; Mor, V.; Chachlani, P.; Deng, Y.; McCarthy, E.P.; Djibo, D.A.; McMahill-Walraven, C.N.; Gravenstein, S. Comparative Risks of Potential Adverse Events Following COVID-19 mRNA Vaccination Among Older US Adults. JAMA Netw. Open. 2023, 6, e2326852. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Pang, D.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Vaccine effectiveness against Delta, Omicron BA.1, and BA.2 in a highly vaccinated Asian setting: A test-negative design study. Clin. Microbiol. Infect. 2023, 29, 101–106. [Google Scholar] [CrossRef]
- Wee, L.E.; Pang, D.; Chiew, C.; Tan, J.; Lee, V.; Ong, B.; Lye, D.C.; Tan, K.B. Long-term Real-world Protection Afforded by Third mRNA Doses Against Symptomatic Severe Acute Respiratory Syndrome Coronavirus 2 Infections, Coronavirus Disease 19-related Emergency Attendances and Hospitalizations Amongst Older Singaporeans During an Omicron XBB Wave. Clin. Infect. Dis. 2023, 77, 1111–1119. [Google Scholar] [CrossRef]
- Chong, C.; Wee, L.E.; Jin, X.; Zhang, M.; Malek, M.I.A.; Ong, B.; Lye, D.; Chiew, C.J.; Tan, K.B. Risks of SARS-CoV-2 JN.1 infection and COVID-19 associated emergency-department (ED) visits/hospitalizations following updated boosters and prior infection: A population-based cohort study. Clin. Infect. Dis. 2024, 79, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Street, A.; Maynou, L.; Gilbert, T.; Stone, T.; Mason, S.; Conroy, S. The use of linked routine data to optimise calculation of the Hospital Frailty Risk Score on the basis of previous hospital admissions: A retrospective observational cohort study. Lancet Healthy Longev. 2021, 2, e154–e162. [Google Scholar] [CrossRef]
- Rosario, B.H.; Quah, J.L.; Chang, T.Y.; Barrera, V.C.; Lim, A.; Sim, L.E.; Conroy, S.; Dhaliwal, T.K. Validation of the Hospital Frailty Risk Score in older adults hospitalized with community-acquired pneumonia. Geriatr. Gerontol. Int. 2024, 24 (Suppl. 1), 135–141. [Google Scholar] [CrossRef]
- Sim, L.; Chang, T.Y.; Htin, K.K.; Lim, A.; Selvaratnam, T.; Conroy, S.; Goh, K.S.; Rosario, B.H. Modified Hospital Frailty Risk Score (mHFRS) as a tool to identify and predict outcomes for hospitalised older adults at risk of frailty. J. Frailty Sarcopenia Falls 2024. ahead of print. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in older adults. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Y.; Wong, B.; Lim, R.; Lee, C.L.; Tan, J.; Tan, K.B.; Wee, L.E. Factors associated with delayed diagnosis of symptomatic adult COVID-19 cases presenting to primary care: A population-wide study during transition from Delta to Omicron BA.1 in Singapore. Lancet Reg. Health West. Pac. 2023, 41, 100919. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Yap, A.J.W.; Dickens, B.; Tan, S.; Ong, B.; Lye, D.C.; Tan, K.B. Access to COVID-19 vaccination by socio-economic status in older Singaporean adults: A population-based cohort study. Public Health 2024, 233, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hernández-Díaz, S.; Solomon, D.H.; Jackson, J.W.; Gagne, J.J.; Glynn, R.J.; Franklin, J.M. Matching Weights to Simultaneously Compare Three Treatment Groups: Comparison to Three-way Matching. Epidemiology 2017, 28, 387–395. [Google Scholar] [CrossRef]
- Gao, J.; Lun, P.; Ding, Y.Y.; George, P.P. COVID-19 Vaccination for Frail Older Adults in Singapore—Rapid Evidence Summary and Delphi Consensus Statements. J. Frailty Aging 2022, 11, 236–241. [Google Scholar] [CrossRef]
- Niyomnaitham, S.; Chokephaibulkit, K.; Pheerapanyawaranun, C.; Toh, Z.Q.; Licciardi, P.V.; Satayasanskul, A.; Jansarikit, L.; Assantachai, P. Immunogenicity of BNT162b2 as a first booster after a ChAdOx1 primary series in a Thai geriatric population living with frailty. J. Nutr. Health Aging 2024, 28, 100315. [Google Scholar] [CrossRef]
- Kuijpers, Y.; Kaczorowska, J.; Picavet, H.S.J.; de Zeeuw-Brouwer, M.L.; Kuijer, M.; Slits, I.; Gijsbers, E.; Rutkens, R.; de Rond, L.; Verschuren, W.M.M.; et al. Health characteristics associated with persistence of SARS-CoV-2 antibody responses after repeated vaccinations in older persons over time: The Doetinchem cohort study. Immun. Ageing 2024, 21, 68. [Google Scholar] [CrossRef]
- McAuley, H.J.C.; Evans, R.A.; Bolton, C.E.; Brightling, C.E.; Chalmers, J.D.; Docherty, A.B.; Elneima, O.; Greenhaff, P.L.; Gupta, A.; Harris, V.C.; et al. PHOSP-COVID Study Collaborative Group. Prevalence of physical frailty, including risk factors, up to 1 year after hospitalisation for COVID-19 in the UK: A multicentre, longitudinal cohort study. EClinicalMedicine 2023, 57, 101896. [Google Scholar] [CrossRef]
- Resendes, N.M.; Bradley, J.; Tang, F.; Hammel, I.S.; Ruiz, J.G. The association of non-severe COVID-19 infection and progression to frailty among robust older veterans. J. Nutr. Health Aging 2024, 28, 100296. [Google Scholar] [CrossRef]
- Hammel, I.S.; Tosi, D.M.; Tang, F.; Pott, H.; Ruiz, J.G. Frailty as a risk factor for post-acute sequelae of COVID-19 among US veterans during the Delta and Omicron waves. J. Am. Geriatr. Soc. 2023, 71, 3826–3835. [Google Scholar] [CrossRef]
- Di Fusco, M.; Sun, X.; Moran, M.M.; Coetzer, H.; Zamparo, J.M.; Alvarez, M.B.; Puzniak, L.; Tabak, Y.P.; Cappelleri, J.C. Impact of COVID-19 and effects of booster vaccination with BNT162b2 on six-month long COVID symptoms, quality of life, work productivity and activity impairment during Omicron. J. Patient Rep. Outcomes 2023, 7, 77. [Google Scholar] [CrossRef]
- Huh, K.; Kim, Y.E.; Bae, G.H.; Moon, J.Y.; Kang, J.M.; Lee, J.; Bae, J.W.; Peck, K.R.; Jung, J. Vaccination and the risk of post-acute sequelae after COVID-19 in the Omicron-predominant period. Clin. Microbiol. Infect. 2024, 30, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Iba, A.; Hosozawa, M.; Hori, M.; Muto, Y.; Kihara, T.; Muraki, I.; Masuda, R.; Tamiya, N.; Iso, H. Booster vaccination and post-COVID-19 condition during the Omicron variant-dominant wave: A large population-based study. Clin. Microbiol. Infect. 2025, 31, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Lim, J.T.; Goel, M.; Malek, M.I.A.; Chiew, C.J.; Ong, B.; Lye, D.C.B.; Tan, K.B. Bivalent Boosters and Risk of Postacute Sequelae Following Vaccine-Breakthrough SARS-CoV-2 Omicron Infection: A Cohort Study. Clin. Infect. Dis. 2025, 80, 520–528. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; White, E.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; St Denis, K.; Sheehan, M.L.; et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. EBioMedicine 2022, 80, 104066. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Coyle, P.; Yassine, H.M.; Al Thani, A.A.; Al-Khatib, H.A.; Hasan, M.R.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: A retrospective population-based cohort study. Lancet Infect. Dis. 2023, 23, 816–827. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Zhu, Z.; Yang, J.; Deng, M.; Chen, M.; Lai, C.; Kong, W.; Xiong, S.; Wan, L.; et al. Omicron variants breakthrough infection elicited higher specific memory immunity than third dose booster in healthy vaccinees. Virol. Sin. 2023, 38, 233–243. [Google Scholar] [CrossRef]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
- Tamada, Y.; Takeuchi, K.; Kusama, T.; Maeda, M.; Murata, F.; Osaka, K.; Fukuda, H. Bivalent mRNA vaccine effectiveness against COVID-19 among older adults in Japan: A test-negative study from the VENUS study. BMC Infect. Dis. 2024, 24, 135. [Google Scholar] [CrossRef]
- Liu, B.; Stepien, S.; Sharma, K.; Macartney, K. Effectiveness of bivalent COVID-19 boosters against COVID-19 mortality in people aged 65 years and older, Australia, November 2022 to May 2023. Eurosurveillance 2023, 28, 2300603. [Google Scholar] [CrossRef]
- Gravenstein, S.; DeVone, F.; Oyebanji, O.A.; Abul, Y.; Cao, Y.; Chan, P.A.; Halladay, C.W.; Rudolph, J.L.; Nugent, C.; Bosch, J.; et al. Durability of immunity and clinical protection in nursing home residents following bivalent SARS-CoV-2 vaccination. EBioMedicine 2024, 105, 105180. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.; Rojas-Castro, M.; Lozano, M.; Martínez-Baz, I.; Leroux-Roels, I.; Borg, M.L.; Oroszi, B.; Fitzgerald, M.; Dürrwald, R.; Jancoriene, L.; et al. VEBIS SARI VE network team. Effectiveness of the XBB.1.5 COVID-19 Vaccines Against SARS-CoV-2 Hospitalisation Among Adults Aged ≥ 65 Years During the BA.2.86/JN.1 Predominant Period, VEBIS Hospital Study, Europe, November 2023 to May 2024. Influenza Other Respir. Viruses 2025, 19, e70081. [Google Scholar] [CrossRef]
- Nunes, B.; Humphreys, J.; Nicolay, N.; Braeye, T.; Van Evercooren, I.; Holm Hansen, C.; Moustsen-Helms, I.R.; Sacco, C.; Fabiani, M.; Castilla, J.; et al. VEBIS-EHR working group. Monovalent XBB.1.5 COVID-19 vaccine effectiveness against hospitalisations and deaths during the Omicron BA.2.86/JN.1 period among older adults in seven European countries: A VEBIS-EHR network study. Expert Rev. Vaccines 2024, 23, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Frankland, T.B.; Ackerson, B.K.; Jodar, L.; McLaughlin, J.M. Effectiveness of BNT162b2 XBB Vaccine Against XBB and JN.1 Sublineages. Open Forum Infect. Dis. 2024, 11, ofae370. [Google Scholar] [CrossRef]
- Okoye, C.; Zazzara, M.B.; Ceolin, C.; Fedele, G.; Palmieri, A.; Abbatecola, A.M.; Malara, A.; Trevisan, C.; Timmons, S.; Prato, R.; et al. GeroCovid Vax Working Group. Delirium Incidence and Predictors in SARS-CoV-2 Vaccinated Residents in Long-Term Care Facilities (LTCF): Insights from the GeroCovid Vax Study. J. Am. Med. Dir. Assoc. 2024, 25, 105251. [Google Scholar] [CrossRef]
- Wee, L.E.; Tay, A.T.; Chiew, C.; Young, B.E.; Wong, B.; Lim, R.; Lee, C.L.; Tan, J.; Vasoo, S.; Lye, D.C.; et al. Real-world effectiveness of nirmatrelvir/ritonavir against COVID-19 hospitalizations and severe COVID-19 in community-dwelling older adults Singaporeans during Omicron BA.2, BA.4/5, and XBB transmission. Clin. Microbiol Infect. 2023, 29, 1328–1333. [Google Scholar] [CrossRef]
- Calcaterra, L.; Cesari, M.; Lim, W.S. Long-Term Care Facilities (LTCFs) During the COVID-19 Pandemic-Lessons From the Asian Approach: A Narrative Review. J. Am. Med. Dir. Assoc. 2022, 23, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.; Radhakrishnan, A.; Williams, C.; Ramkissoon, N.; Pham, B.; Cormack, G.V.; Grossman, M.R.; Muller, M.P.; Straus, S.E.; Tricco, A.C. Preventing the transmission of COVID-19 and other coronaviruses in older adults aged 60 years and above living in long-term care: A rapid review. Syst. Rev. 2020, 9, 218. [Google Scholar] [CrossRef]
- Telford, C.T.; Bystrom, C.; Fox, T.; Holland, D.P.; Wiggins-Benn, S.; Mandani, A.; McCloud, M.; Shah, S. COVID-19 Infection Prevention and Control Adherence in Long-Term Care Facilities, Atlanta, Georgia. J. Am. Geriatr. Soc. 2021, 69, 581–586. [Google Scholar] [CrossRef]
- Wong, R.; Grullon, J.R.; Lovier, M.A. COVID-19 risk factors and predictors for handwashing, masking, and social distancing among a national prospective cohort of US older adults. Public Health 2022, 211, 164–170. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Hayashi, M.A.L.; Berendes, D.M.; Sobolik, J.S.; Leon, J.S.; Lopman, B.A. Risk for Fomite-Mediated Transmission of SARS-CoV-2 in Child Daycares, Schools, Nursing Homes, and Offices. Emerg. Infect. Dis. 2021, 27, 1229–1231. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Han, J.; Zhao, E.; Fang, J.; Wang, D.; Cheng, Y.; Shi, Y.; Wang, Z.; Yao, Z.; Lu, P.; et al. The effects of obesity and metabolic abnormalities on severe COVID-19-related outcomes after vaccination: A population-based study. Cell Metab. 2023, 35, 585–600.e5. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, S.; Mueller, B.; Tausendfreund, O.; Rippl, M.; Deissler, L.; Martini, S.; Schmidmaier, R.; Stoecklein, S.; Ingrisch, M.; Blaschke, S.; et al. Impact of sarcopenia and obesity on mortality in older adults with SARS-CoV-2 infection: Automated deep learning body composition analysis in the NAPKON-SUEP cohort. Infection 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.E.; Lim, J.T.; Ho, R.W.L.; Chiew, C.J.; Young, B.; Venkatachalam, I.; Sim, J.X.Y.; Cheong, H.Y.; Ng, T.Y.; Yung, C.F.; et al. Severity of respiratory syncytial virus versus SARS-CoV-2 Omicron and influenza infection amongst hospitalized Singaporean adults: A national cohort study. Lancet Reg. Health West. Pac. 2025, 55, 101494. [Google Scholar] [CrossRef]
- Turcotte, L.A.; Heckman, G.; Rockwood, K.; Vetrano, D.L.; Hébert, P.; McIsaac, D.I.; Rhynold, E.; Mitchell, L.; Mowbray, F.I.; Larsen, R.T.; et al. External validation of the hospital frailty risk score among hospitalised home care clients in Canada: A retrospective cohort study. Age Ageing 2023, 52, afac334. [Google Scholar] [CrossRef]

| Baseline, Before Weighting | Post-Weighting | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Frailty a, N(%) | Intermediate Frailty a, N(%) | High Frailty a, N(%) | SMD, Intermediate vs. Low Frailty | SMD, High vs. Low Frailty | SMD, High vs. Intermediate Frailty | Low a, N(%) | Intermediate Frailty a, N(%) | High Frailty a, N(%) | SMD, Intermediate vs. Low R Frailty | SMD, High vs. Low f Frailty | SMD, High vs. Intermediate Frailty | |
| Age | ||||||||||||
| 60–69 years | 477,130 (61.2%) | 22,855 (28.8%) | 2209 (15.0%) | 0.69 | 1.08 | 0.34 | 114,226 (14.6%) | 13,629 (17.2%) | 2354 (16.0%) | 0.07 | 0.04 | 0.03 |
| 70–79 years | 226,332 (29.0%) | 28,033 (35.4%) | 3941 (26.8%) | 0.14 | 0.05 | 0.19 | 214,366 (27.5%) | 22,470 (28.3%) | 4171 (28.4%) | 0.02 | 0.02 | 0.00 |
| ≥80 years | 76,733 (9.8%) | 28,381 (35.8%) | 8546 (58.2%) | 0.65 | 1.19 | 0.46 | 451,603 (57.9%) | 43,170 (54.5%) | 8171 (55.6%) | 0.07 | 0.05 | 0.02 |
| Ethnicity | ||||||||||||
| Chinese | 629,813 (80.7%) | 62,161 (78.4%) | 11,357 (77.3%) | 0.06 | 0.08 | 0.03 | 611,531 (78.4%) | 61,608 (77.7%) | 11,399 (77.6%) | 0.02 | 0.02 | 0.00 |
| Indian | 53,056 (6.8%) | 6357 (8.0%) | 1384 (9.4%) | 0.05 | 0.10 | 0.05 | 70,410 (9.0%) | 6869 (8.7%) | 1358 (9.2%) | 0.01 | 0.01 | 0.02 |
| Malay | 81,716 (10.5%) | 9836 (12.4%) | 1772 (12.1%) | 0.06 | 0.05 | 0.01 | 89,258 (11.4%) | 9787 (12.3%) | 1749 (11.9%) | 0.03 | 0.01 | 0.01 |
| Others b | 15,610 (2.0%) | 915 (1.2%) | 183 (1.2%) | 0.07 | 0.06 | 0.01 | 8996 (1.2%) | 1006 (1.3%) | 189 (1.3%) | 0.01 | 0.01 | 0.00 |
| Gender | ||||||||||||
| Female | 412,040 (52.8%) | 43,131 (54.4%) | 8595 (58.5%) | 0.03 | 0.11 | 0.08 | 449,928 (57.7%) | 46,116 (58.2%) | 8513 (57.9%) | 0.01 | 0.01 | 0.01 |
| Male | 368,155 (47.2%) | 36,138 (45.6%) | 6101 (41.5%) | 0.03 | 0.11 | 0.08 | 330,267 (42.3%) | 33,153 (41.8%) | 6183 (42.1%) | 0.01 | 0.01 | 0.01 |
| Socioeconomic status (housing type) c | ||||||||||||
| Public, 1–2 room | 52,033 (6.7%) | 8228 (10.4%) | 1884 (12.8%) | 0.13 | 0.21 | 0.08 | 99,075 (12.7%) | 9721 (12.3%) | 1846 (12.6%) | 0.01 | 0.00 | 0.01 |
| Public, 3 room | 145,828 (18.7%) | 18,351 (23.2%) | 3653 (24.9%) | 0.11 | 0.15 | 0.04 | 199,443 (25.6%) | 19,548 (24.7%) | 3626 (24.7%) | 0.02 | 0.02 | 0.00 |
| Public, 4 room | 239,768 (30.7%) | 25,591 (32.3%) | 4472 (30.4%) | 0.03 | 0.01 | 0.04 | 237,066 (30.4%) | 24,378 (30.8%) | 4484 (30.5%) | 0.01 | 0.00 | 0.01 |
| Public, 5-room | 254,797 (32.7%) | 20,656 (26.1%) | 3200 (21.8%) | 0.15 | 0.25 | 0.10 | 173,046 (22.2%) | 17,846 (22.5%) | 3272 (22.3%) | 0.01 | 0.00 | 0.01 |
| Others | 21,262 (2.7%) | 1566 (2.0%) | 643 (4.4%) | 0.05 | 0.09 | 0.14 | 25,818 (3.3%) | 2993 (3.8%) | 601 (4.1%) | 0.03 | 0.04 | 0.02 |
| Private housing | 66,507 (8.5%) | 4877 (6.2%) | 844 (5.7%) | 0.09 | 0.11 | 0.02 | 45,747 (5.9%) | 4782 (6.0%) | 867 (5.9%) | 0.01 | 0.00 | 0.01 |
| Comorbidity burden (Charlson Comorbidity Index, CCMI) d | ||||||||||||
| No comorbidities (CCMI = 0) | 523,248 (67.1%) | 19,035 (24.0%) | 1381 (9.4%) | 0.96 | 1.47 | 0.40 | 74,094 (9.5%) | 7405 (9.3%) | 1472 (10.0%) | 0.01 | 0.02 | 0.02 |
| Mild comorbidity burden (CCMI 1–2) | 190,160 (24.4%) | 26,978 (34.0%) | 4875 (33.2%) | 0.21 | 0.20 | 0.02 | 252,370 (32.3%) | 29,827 (37.6%) | 5171 (35.2%) | 0.11 | 0.06 | 0.05 |
| Moderate comorbidity burden (CCMI 3–4) | 48,709 (6.2%) | 19,275 (24.3%) | 3998 (27.2%) | 0.52 | 0.59 | 0.07 | 222,503 (28.5%) | 21,663 (27.3%) | 4083 (27.8%) | 0.03 | 0.02 | 0.01 |
| Severe comorbidity burden (CCMI ≥5) | 18,078 (2.3%) | 13,981 (17.6%) | 4442 (30.2%) | 0.53 | 0.82 | 0.30 | 231,228 (29.6%) | 20,373 (25.7%) | 3970 (27.0%) | 0.09 | 0.06 | 0.03 |
| Vaccination status pre-Omicron e | ||||||||||||
| Unvaccinated/partially vaccinated | 68,140 (8.7%) | 3706 (4.7%) | 1421 (9.7%) | 0.16 | 0.03 | 0.19 | 61,548 (7.9%) | 8368 (10.6%) | 1358 (9.2%) | 0.09 | 0.05 | 0.04 |
| Fully vaccinated | 126,919 (16.3%) | 24,327 (30.7%) | 6448 (43.9%) | 0.35 | 0.63 | 0.28 | 339,195 (43.5%) | 32,299 (40.7%) | 6157 (41.9%) | 0.06 | 0.03 | 0.02 |
| Boosted | 585,136 (75.0%) | 51,235 (64.6%) | 6826 (46.4%) | 0.23 | 0.61 | 0.37 | 379,601 (48.6%) | 38,598 (48.7%) | 7180 (48.9%) | 0.00 | 0.00 | 0.00 |
| Frailty Risk | Person-Years | Number of COVID-19 Hospitalizations | COVID-19 Hospitalization, aHR (95% CI) a | Number of Severe COVID-19 cases | Severe COVID-19, aHR (95% CI) a |
|---|---|---|---|---|---|
| Omicron wave, first infections (infection-naïve) | |||||
| Frailty, defined using Hospital Frailty Risk Score (HFRS) b | |||||
| Low risk of frailty, HFRS < 5 | 1,106,573 | 12,577 | 1.00 (ref) | 2008 | 1.00 (ref) |
| Intermediate risk of frailty, HFRS 5–15 | 94,698 | 9010 | 2.03 (1.94, 2.13) | 1955 | 2.44 (2.19, 2.72) |
| High risk of frailty, HFRS > 15 | 14,949 | 3665 | 3.38 (3.21, 3.56) | 927 | 4.33 (3.86, 4.85) |
| Frailty, defined using Clinical Frailty Scale (CFS) in subset of population with available data c | |||||
| Low risk of frailty, CFS 1–5 | 475,826 | 10,378 | 1.00 (ref) | 1683 | 1.00 (ref) |
| Intermediate risk of frailty, CFS 6 | 40,720 | 817 | 1.79 (1.66, 1.94) | 176 | 2.07 (1.75, 2.45) |
| High risk of frailty, CFS 7–8 | 6428 | 1107 | 1.89 (1.76, 2.03) | 318 | 2.68 (2.33, 3.09) |
| Omicron wave, reinfections d | |||||
| Frailty, defined using Hospital Frailty Risk Score (HFRS) b | |||||
| Low risk of frailty, HFRS < 5 | 1,466,967 | 928 | 1.00 (ref) | 92 | 1.00 (ref) |
| Intermediate risk of frailty, HFRS 5–15 | 133,322 | 831 | 2.68 (2.26, 3.19) | 114 | 2.86 (1.77, 4.62) |
| High risk of frailty, HFRS > 15 | 23,280 | 414 | 5.29 (4.40, 6.35) | 60 | 5.39 (3.27, 8.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, L.E.; Loy, E.X.H.; Lim, J.T.; Kwok, W.H.; Chiew, C.; Lien, C.; Rosario, B.H.; Leong, I.Y.O.; Merchant, R.A.; Lye, D.C.B.; et al. Real-World Effectiveness of Boosting Against Omicron Hospitalization in Older Adults, Stratified by Frailty. Vaccines 2025, 13, 565. https://doi.org/10.3390/vaccines13060565
Wee LE, Loy EXH, Lim JT, Kwok WH, Chiew C, Lien C, Rosario BH, Leong IYO, Merchant RA, Lye DCB, et al. Real-World Effectiveness of Boosting Against Omicron Hospitalization in Older Adults, Stratified by Frailty. Vaccines. 2025; 13(6):565. https://doi.org/10.3390/vaccines13060565
Chicago/Turabian StyleWee, Liang En, Enoch Xue Heng Loy, Jue Tao Lim, Wei Hao Kwok, Calvin Chiew, Christopher Lien, Barbara Helen Rosario, Ian Yi Onn Leong, Reshma Aziz Merchant, David Chien Boon Lye, and et al. 2025. "Real-World Effectiveness of Boosting Against Omicron Hospitalization in Older Adults, Stratified by Frailty" Vaccines 13, no. 6: 565. https://doi.org/10.3390/vaccines13060565
APA StyleWee, L. E., Loy, E. X. H., Lim, J. T., Kwok, W. H., Chiew, C., Lien, C., Rosario, B. H., Leong, I. Y. O., Merchant, R. A., Lye, D. C. B., & Tan, K. B. (2025). Real-World Effectiveness of Boosting Against Omicron Hospitalization in Older Adults, Stratified by Frailty. Vaccines, 13(6), 565. https://doi.org/10.3390/vaccines13060565





