HPV Infection Prevalence, Vaccination-Related Knowledge, Attitudes, and Barriers Among Women Aged 30–64 in Shenzhen, China: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
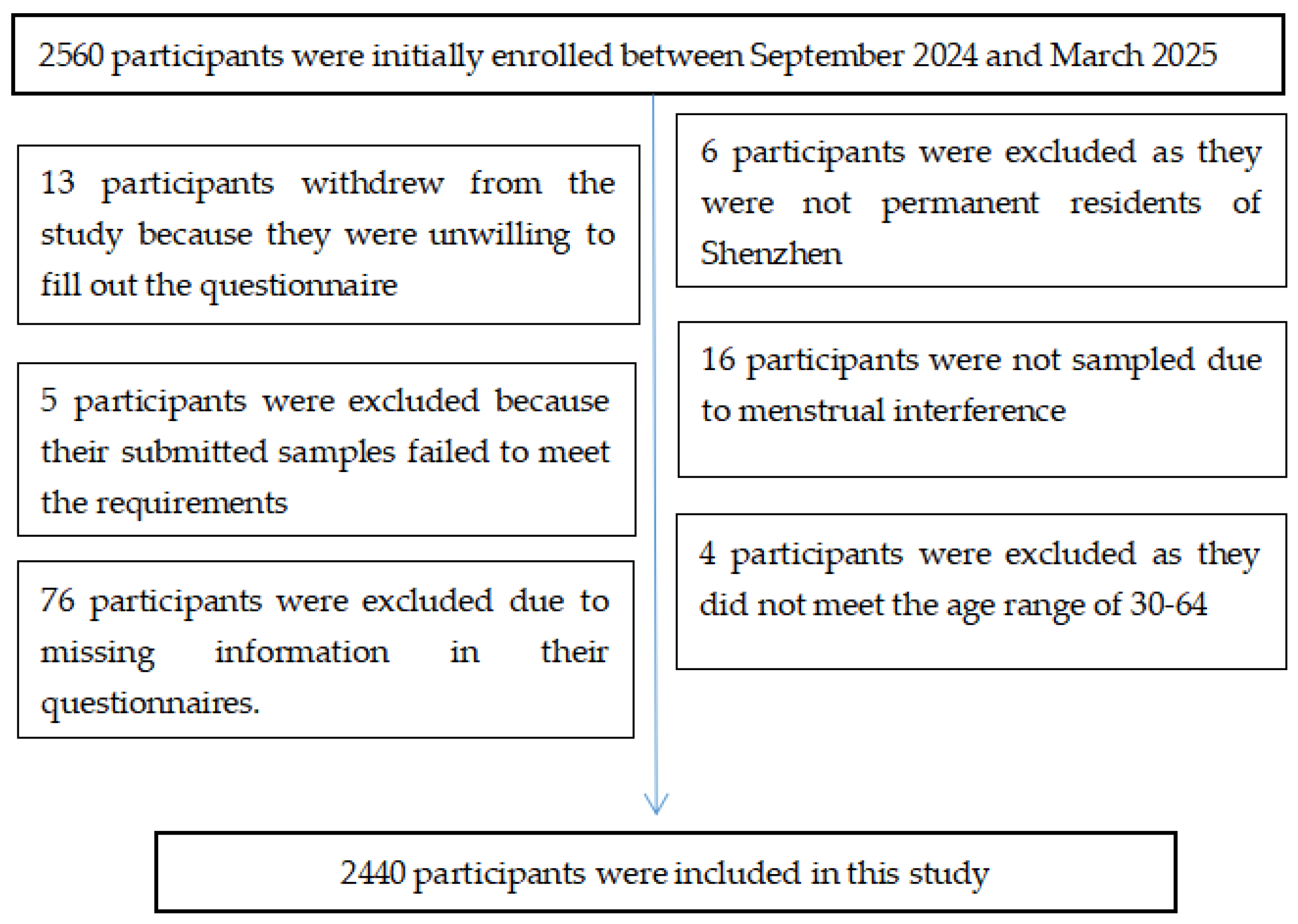
2.2. Criteria for Participants
2.3. Definition of Variables
2.3.1. HPV Knowledge and HPV Vaccination
2.3.2. Practice and Willingness to Receive HPV Vaccination
2.3.3. HPV Genotyping
2.3.4. Histopathological Diagnosis
2.4. Statistical Analysis
3. Results
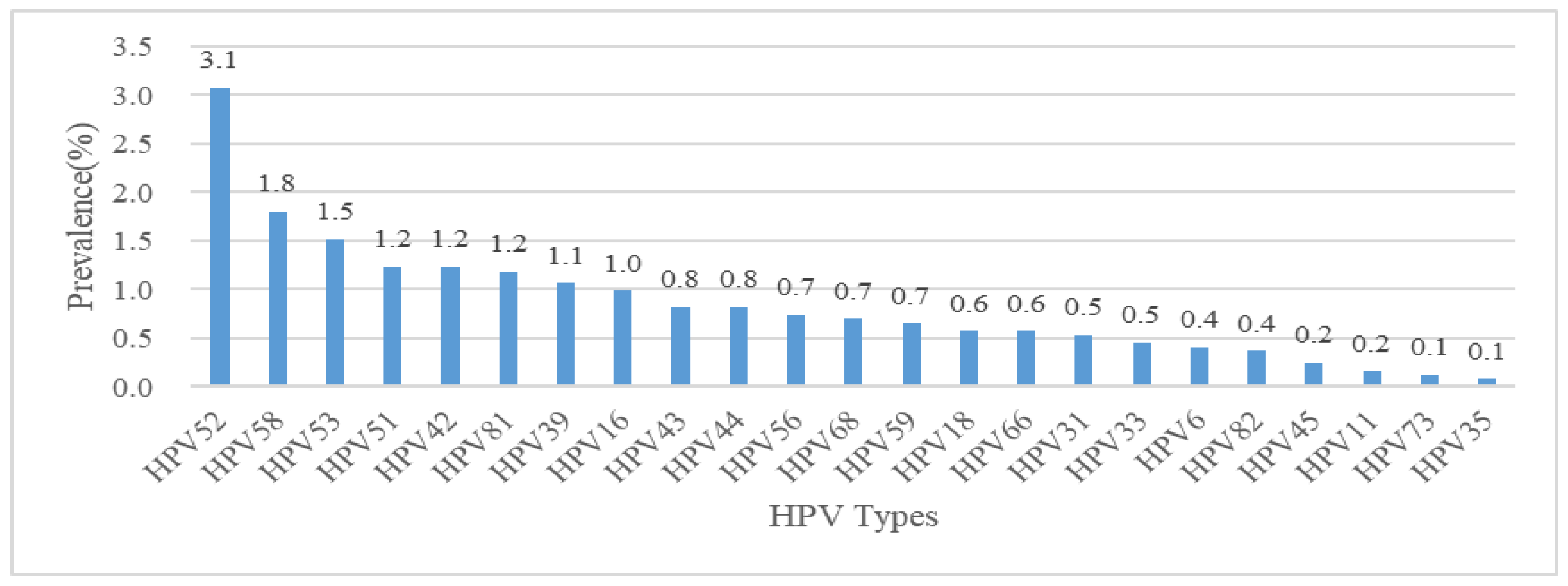
3.1. HPV Infection and Type Distribution
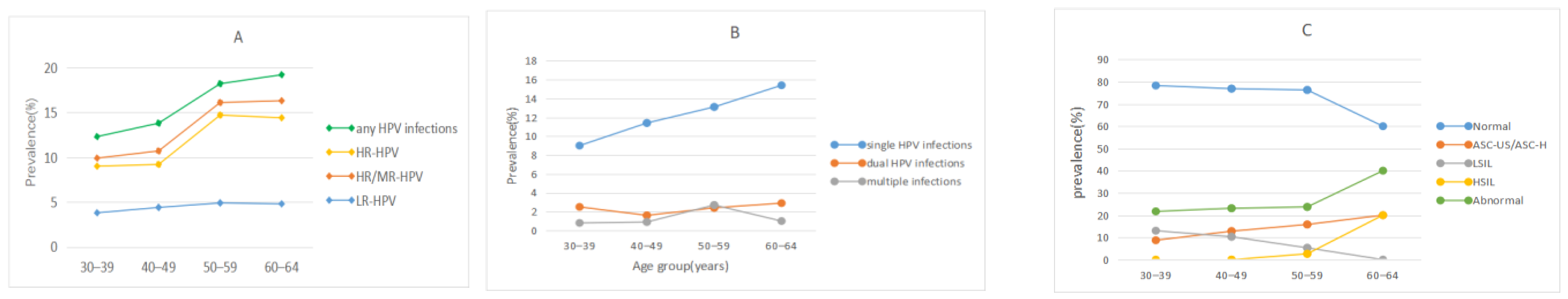
3.2. HPV Infection Status
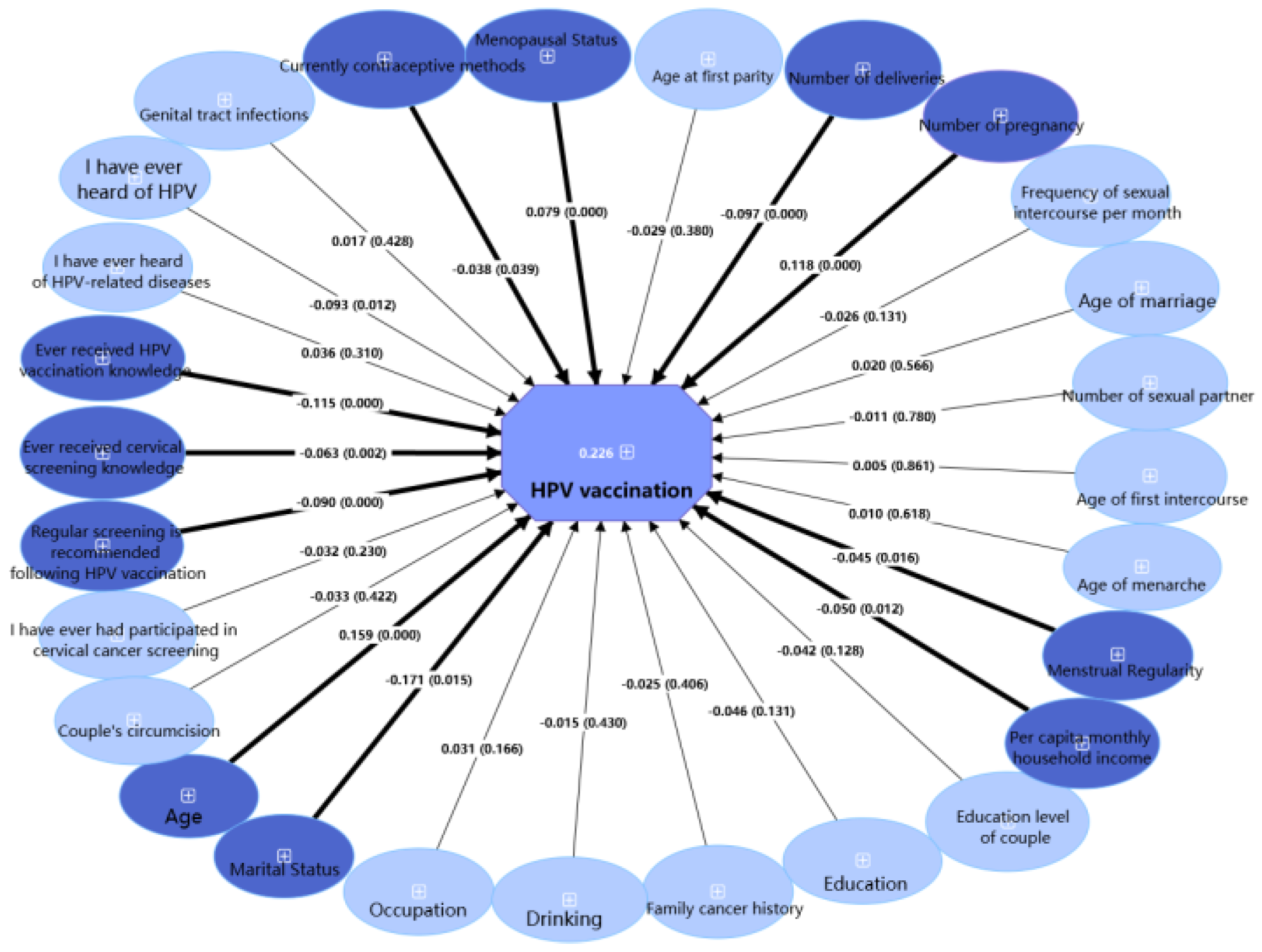
3.3. HPV Vaccine Uptake
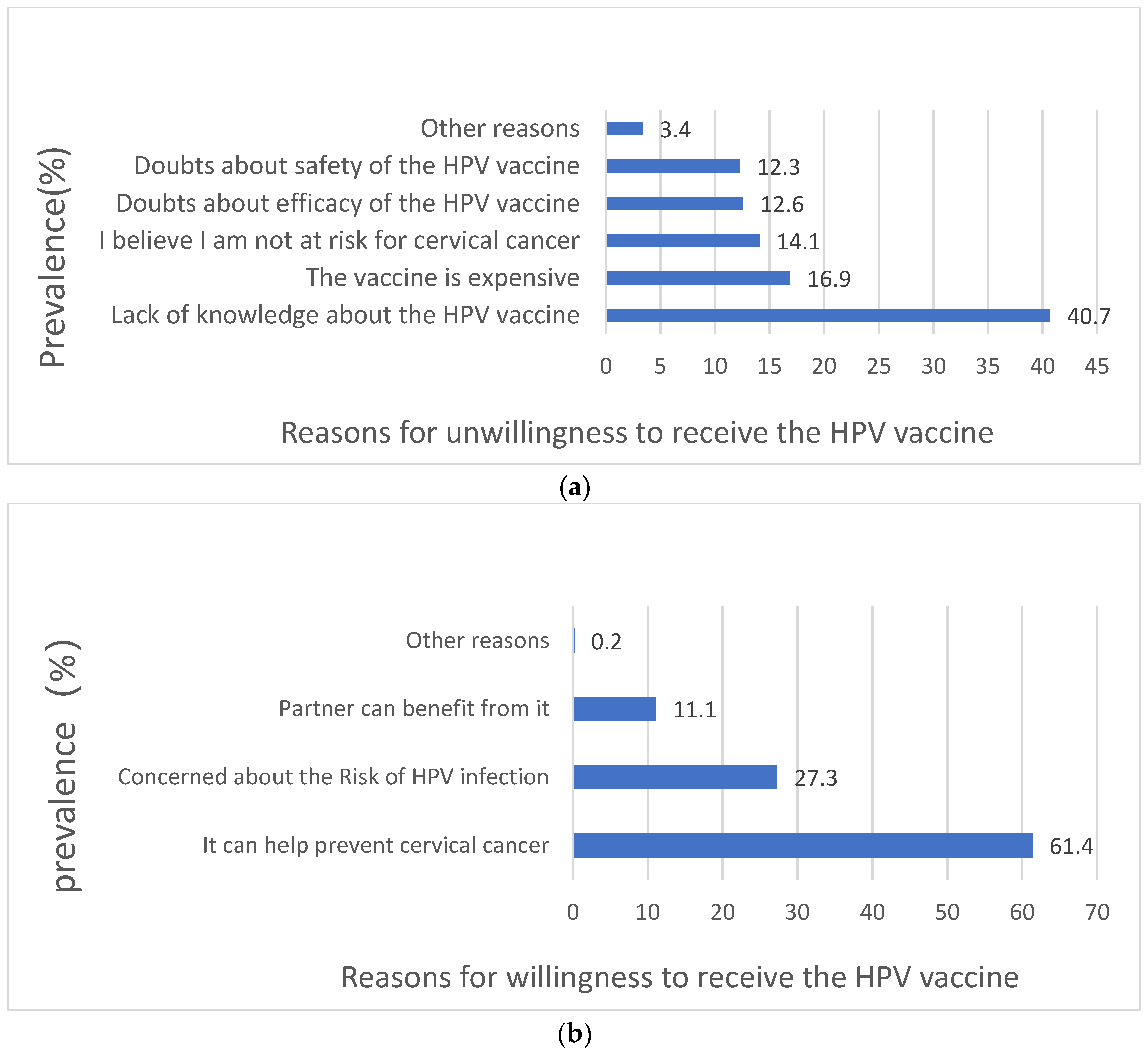
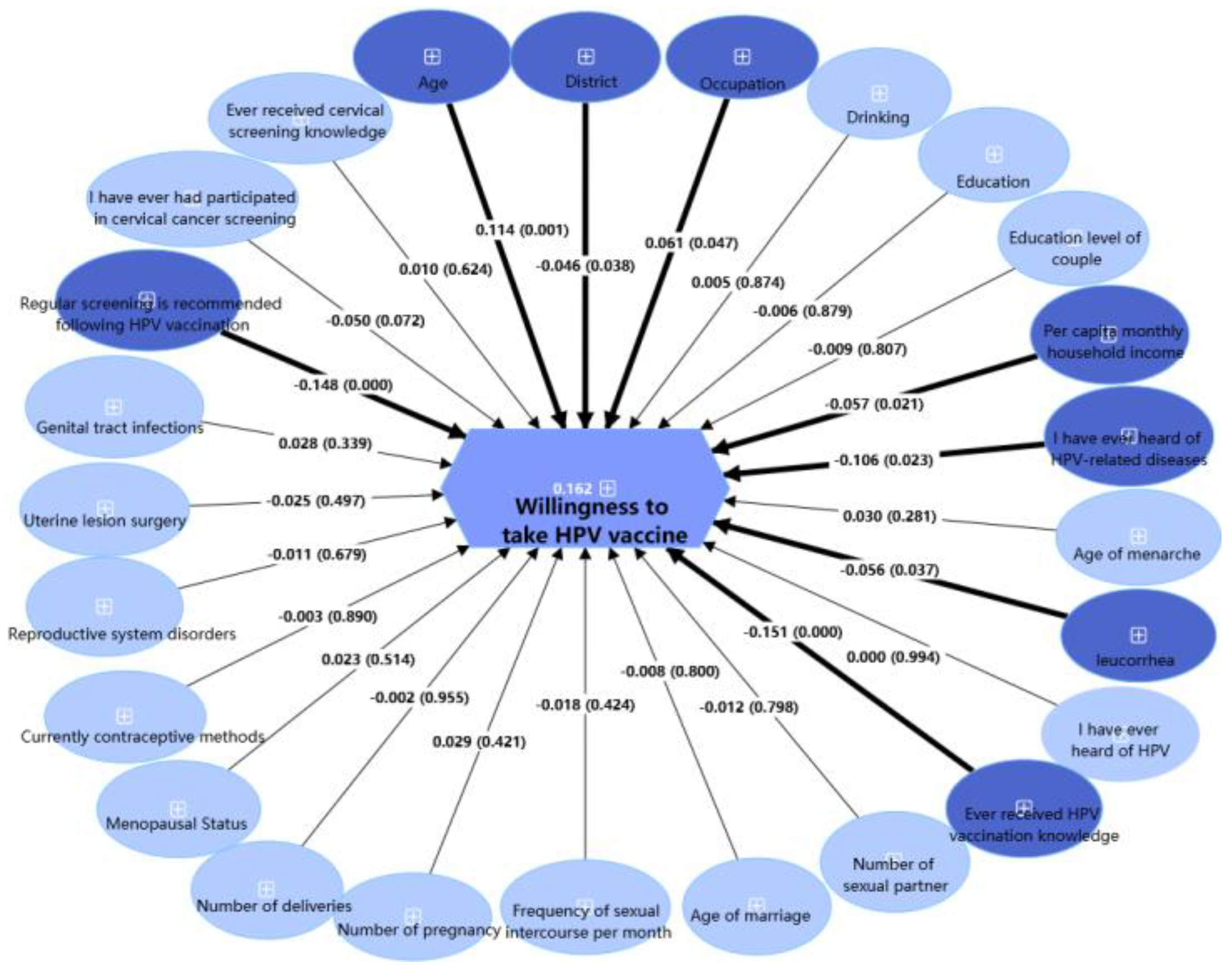
3.4. Willingness to Receive the HPV Vaccination
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer Incidence and Mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/40-all-cancers-excl-non-melanoma-skin-cancer-fact-sheet.pdf (accessed on 17 March 2025).
- Tota, J.E.; Chevarie-Davis, M.; Richardson, L.A.; Devries, M.; Franco, E.L. Epidemiology and Burden of HPV Infection and Related Diseases: Implications for Prevention Strategies. Prev. Med. 2011, 53 (Suppl. S1), S12–S21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhao, Y.; Li, J.; Li, M.; Shi, Y.; Wei, L. Opportunities and Challenges for Human Papillomavirus Vaccination in China. Hum. Vaccines Immunother. 2024, 20, 2329450. [Google Scholar] [CrossRef]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.-C.; et al. Impact of HPV Vaccination and Cervical Screening on Cervical Cancer Elimination: A Comparative Modelling Analysis in 78 Low-Income and Lower-Middle-Income Countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 12 March 2025).
- Hu, S.; Xu, X.; Zhang, Y.; Liu, Y.; Yang, C.; Wang, Y.; Wang, Y.; Yu, Y.; Hong, Y.; Zhang, X.; et al. A Nationwide Post-Marketing Survey of Knowledge, Attitude and Practice toward Human Papillomavirus Vaccine in General Population: Implications for Vaccine Roll-out in Mainland China. Vaccine 2021, 39, 35–44. [Google Scholar] [CrossRef]
- Gong, X.; Xu, J.; He, Y.; Zou, G.; Liu, J. Socioeconomic Inequalities in Human Papillomavirus Knowledge and Vaccine Uptake: Evidence from a Cross-Sectional Study in China. Front. Public Health 2024, 12, 1399192. [Google Scholar] [CrossRef]
- Yuanyue, L.; Baloch, Z.; Shanshan, L.; Yasmeen, N.; Xiaomei, W.; Khan, J.M.; Xueshan, X. Cervical Cancer, Human Papillomavirus Infection, and Vaccine-Related Knowledge: Awareness in Chinese Women. Cancer Control J. Moffitt Cancer Cent. 2018, 25, 1073274818799306. [Google Scholar] [CrossRef]
- Li, M.; Zhao, C.; Zhao, Y.; Li, J.; Wei, L. Immunogenicity, Efficacy, and Safety of Human Papillomavirus Vaccine: Data from China. Front. Immunol. 2023, 14, 1112750. [Google Scholar] [CrossRef]
- An, J.; Liu, Y.; Ma, Y.; Jiao, Y.-Z.; Liang, X.-F.; Jin, N.; Bao, J.; Jiang, N.; Zhang, X.-S. Real-World Data of China: Analysis of HPV Vaccine Coverage and Post-Vaccination Adverse Reaction Monitoring in Western Chinese Provinces from 2018 to 2021. Hum. Vaccines Immunother. 2024, 20, 2315653. [Google Scholar] [CrossRef]
- Qin, S.; Fu, J.X.; Chen, M.Z.; Meng, Y.T.; Xu, C.; Luo, Y. Acceptability of Vaccination against Human Papillomavirus among Women Aged 20 to 45 in Rural Hunan Province, China: A Cross-Sectional Study. Vaccine 2020, 38, 4732–4739. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, F.; Wang, Z.; Chen, H.; Chen, M.; Peng, C.; Chen, K.; Chen, C.; Xiong, H.; Xie, X. An Investigation on Cervical Cancer and Human Papillomavirus Vaccine Knowledge, and Analysis of Influencing Factors for Choosing Domestic or Imported 2vHPV Vaccine among Females in Shenzhen, China. Hum. Vaccines Immunother. 2023, 19, 2225389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gu, B.; Xu, X.; Li, Y.; Cheng, P.; Huo, Y.; Liu, G.; Zhang, X. On Imported and Domestic Human Papillomavirus Vaccines: Cognition, Attitude, and Willingness to Pay in Chinese Medical Students. Front. Public Health 2022, 10, 863748. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Min, K.J.; Lee, Y.J.; Suh, M.; Yoo, C.W.; Lim, M.C.; Choi, J.; Ki, M.; Kim, Y.M.; Kim, J.W.; Kim, J.H.; et al. The Korean Guideline for Cervical Cancer Screening. J. Gynecol. Oncol. 2015, 26, 232–239. [Google Scholar] [CrossRef]
- Saitoh, E.; Saika, K.; Morisada, T.; Aoki, D. Status of Cervical Cancer Screening among Adolescents and Young Adults (AYA) in Japan. Int. J. Clin. Oncol. 2022, 27, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Tachezy, R.; Robova, H. Cervical Cancer: What Is the Optimal Age for Routine Testing? Future Oncol. 2015, 11, 1137–1140. [Google Scholar] [CrossRef]
- Sawaya, G.F.; Sanstead, E.; Alarid-Escudero, F.; Smith-McCune, K.; Gregorich, S.E.; Silverberg, M.J.; Leyden, W.; Huchko, M.J.; Kuppermann, M.; Kulasingam, S. Estimated Quality of Life and Economic Outcomes Associated with 12 Cervical Cancer Screening Strategies: A Cost-Effectiveness Analysis. JAMA Intern. Med. 2019, 179, 867–878. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention. Available online: https://www.who.int/publications/i/item/9789240030824 (accessed on 17 March 2025).
- Wang, L.-H.; Zhao, G.-L. Comprehensive guidelines for cervical cancer prevention and control in China. Chin. J. Woman Child Health Res. 2018, 29, 1–3. [Google Scholar]
- Shenzhen Municipal Bureau of Statistics. The 7th National Population Census Bulletin of Shenzhen. No. 19. 2021. Available online: https://www.sz.gov.cn/zfgb/2021/gb1199/content/post_8806392.html (accessed on 5 August 2024).
- Li, J.; Kang, J.; Mao, Y.; Zheng, P.; Abdullah, A.S.; Wu, G.; Wang, F. Investigating HPV- and HPV Vaccine-Related Knowledge, Perceptions, and Information Sources among Health Care Providers in Three Big Cities in China. Vaccines 2020, 8, 499. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Brasel, K.; Haider, A.; Haukoos, J. Practical Guide to Survey Research. JAMA Surg. 2020, 155, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://aapor.org/wp-content/uploads/2024/03/Standards-Definitions-10th-edition.pdf (accessed on 12 May 2025).
- Zhu, B.; Liu, Y.; Zuo, T.; Cui, X.; Li, M.; Zhang, J.; Yu, H.; Piao, H. The Prevalence, Trends, and Geographical Distribution of Human Papillomavirus Infection in China: The Pooled Analysis of 1.7 Million Women. Cancer Med. 2019, 8, 5373–5385. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Cheng, S.; Liu, D.; Tian, Y.; Hu, F.; Zhang, Z.; Zhu, T.; Su, Z.; Liu, Y.; Wang, S.; et al. Head-to-Head Comparison of 7 High-Sensitive Human Papillomavirus Nucleic Acid Detection Technologies with the SPF10 LiPA-25 System. J. Natl. Cancer Cent. 2022, 2, 148–154. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Pap Test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 271–281. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Tang, Y.; Li, Z.; Wang, S.; Luo, X.; He, T.; Yin, A.; Luo, M. Cervical HPV Infection in Guangzhou, China: An Epidemiological Study of 198,111 Women from 2015 to 2021. Emerg. Microbes Infect. 2023, 12, e2176009. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, F.; Dai, J.; Zhang, T.; Chen, Z.; Zhang, M.; Wu, R.; Kang, X. Prevalence of Cervicovaginal Human Papillomavirus Infection and Genotype Distribution in Shanghai, China. Virol. J. 2022, 19, 146. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, N.; Li, B.; Shang, E.; Wang, J.; Zhang, M.; Yang, X. Prevalence and Genotype Distribution of Human Papillomavirus Infections in Beijing, China between 2016 and 2020. Virol. J. 2023, 20, 11. [Google Scholar] [CrossRef]
- Li, N.; Franceschi, S.; Howell-Jones, R.; Snijders, P.J.F.; Clifford, G.M. Human Papillomavirus Type Distribution in 30,848 Invasive Cervical Cancers Worldwide: Variation by Geographical Region, Histological Type and Year of Publication. Int. J. Cancer 2011, 128, 927–935. [Google Scholar] [CrossRef]
- Zeng, X.-X.; Yan, L.-X.; Huang, X.-X.; He, C.-H.; Liu, W.-G.; Yuan, W.-Q.; Qiu, Y.-P.; Liu, Z.-X. Prevalence and Genotype Distribution of Human Papillomavirus among Hakka Women in China. Ann. Transl. Med. 2016, 4, 276. [Google Scholar] [CrossRef]
- Mai, Q.; Yang, X.; Cheng, H.; Wu, G.; Wu, Z. Prevalence and Genotype Distribution of Human Papillomavirus among Women with Cervical Lesions in Shenzhen City, China. Hum. Vaccines Immunother. 2021, 17, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Pauli, S.; Kops, N.L.; Bessel, M.; Lina Villa, L.; Moreno Alves Souza, F.; Mendes Pereira, G.F.; Neves Hugo, F.; POP-Brazil Study Group; Wendland Hugo, E. Sexual Practices and HPV Infection in Unvaccinated Young Adults. Sci. Rep. 2022, 12, 12385. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.D. HPV Disease Transmission Protection and Control. Microb. Cell 2016, 3, 476–490. [Google Scholar] [CrossRef]
- Jo, S.; Han, S.-Y.; Walters, C.A. Factors Associated with the HPV Vaccination among Korean Americans and Koreans: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 19, 51. [Google Scholar] [CrossRef]
- Rebolj, M.; Pesola, F.; Mathews, C.; Mesher, D.; Soldan, K.; Kitchener, H. The Impact of Catch-up Bivalent Human Papillomavirus Vaccination on Cervical Screening Outcomes: An Observational Study from the English HPV Primary Screening Pilot. Br. J. Cancer 2022, 127, 278–287. [Google Scholar] [CrossRef]
- Steben, M.; Tan Thompson, M.; Rodier, C.; Mallette, N.; Racovitan, V.; DeAngelis, F.; Stutz, M.; Rampakakis, E. A Review of the Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: 10 Years of Clinical Experience in Canada. J. Obstet. Gynaecol. Can. 2018, 40, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Baldur-Felskov, B.; Dehlendorff, C.; Munk, C.; Kjaer, S.K. Early Impact of Human Papillomavirus Vaccination on Cervical Neoplasia--Nationwide Follow-up of Young Danish Women. J. Natl. Cancer Inst. 2014, 106, djt460. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, X.; Zhu, F.; Hong, Y.; Hu, Y.; Zhang, X.; Pan, Q.; Zhang, W.; Zhang, C.; Yang, X.; et al. Efficacy of the AS04-Adjuvanted HPV-16/18 Vaccine in Young Chinese Women with Oncogenic HPV Infection at Baseline: Post-Hoc Analysis of a Randomized Controlled Trial. Hum. Vaccines Immunother. 2021, 17, 955–964. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, W.; Yang, S.; Hu, D.; Bai, L.; Xiang, R.; Zhao, X.; Nie, Y.; Shi, Q.-L. Prevalence of High-Risk Human Papillomavirus Infection, Associated Risk Factors, and Relationship with Cervical Precancerous Lesions in Perimenopausal and Older Women in an Area with High Cervical Cancer Incidence in China. Cureus 2024, 16, e58081. [Google Scholar] [CrossRef]
- Manolescu, L.S.C.; Zugravu, C.; Zaharia, C.N.; Dumitrescu, A.I.; Prasacu, I.; Radu, M.C.; Letiția, G.D.; Nita, I.; Cristache, C.M.; Gales, L.N. Barriers and Facilitators of Romanian HPV (Human Papillomavirus) Vaccination. Vaccines 2022, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rodewald, L.E.; Du, A.H.; Tang, S. Advancing the National Immunization Program in an Era of Achieving Universal Vaccine Coverage in China and Beyond. Infect. Dis. Poverty 2024, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Hocking, J.; Canfell, K.; Ma, X.; Chow, E.P.F.; Xu, X.; Zhang, L.; Zhuang, G. Domestic HPV Vaccine Price and Economic Returns for Cervical Cancer Prevention in China: A Cost-Effectiveness Analysis. Lancet Glob. Health 2020, 8, e1335–e1344. [Google Scholar] [CrossRef] [PubMed]
- Makadzange, E.E.; Peeters, A.; Joore, M.A.; Kimman, M.L. The Effectiveness of Health Education Interventions on Cervical Cancer Prevention in Africa: A Systematic Review. Prev. Med. 2022, 164, 107219. [Google Scholar] [CrossRef]
| HPV Genotype | N (%) (N = 2440) | 30–39 (%) (n = 1142) | 40–49 (%) (n = 704) | 50–59 (%) (n = 490) | 60–64 (%) (n = 104) | X2 | p | p a |
|---|---|---|---|---|---|---|---|---|
| Type of infection | ||||||||
| HPV | 347 (14.2) | 141 (12.3) | 97 (13.8) | 89 (18.2) | 20 (19.2) | 11.784 | <0.05 | <0.001 |
| HR-HPV | 255 (10.5) | 103 (9.0) | 65 (9.2) | 72 (14.7) | 15 (14.4) | 14.796 | <0.05 | <0.001 |
| HR/MR-HPV | 284 (11.6) | 113 (9.9) | 75 (10.7) | 79 (16.1) | 17 (16.3) | 15.860 | <0.05 | <0.001 |
| Lr-HPV | 103 (4.2) | 43 (3.8) | 31 (4.4) | 24 (4.9) | 5 (4.8) | 1.288 | 0.732 | 0.275 |
| Number of HPV infection | ||||||||
| Single | 263 (10.8) | 103 (9.0) | 80 (11.4) | 64 (13.1) | 16 (15.4) | 8.875 | <0.05 | <0.05 |
| Dual | 55 (2.3) | 29 (2.5) | 11 (1.6) | 12 (2.4) | 3 (2.9) | 2.222 | 0.528 | 0.880 |
| Multiple | 29 (1.2) | 9 (0.8) | 6 (0.9) | 13 (2.7) | 1 (1.0) | 11.232 | <0.05 | <0.05 |
| TBS diagnostic | N (%) (N = 128) | 30–39 n (%) (n = 46) | 40–49 n (%) (n = 39) | 50–59 n (%) (n = 38) | 60–64 n (%) (n = 5) | X2 | p | p a |
| Pathological type | ||||||||
| Normal | 98 (76.6) | 36 (78.3) | 30 (76.9) | 29 (76.3) | 3 (60.0) | 0.755 | 0.860 | 0.559 |
| Abnormal | 30 (23.4) | 10 (21.7) | 9 (23.1) | 9 (23.7) | 2 (40.0) | 0.755 | 0.860 | 0.559 |
| ASC-US/ASC-H | 16 (12.5) | 4 (8.7) | 5 (12.8) | 6 (15.8) | 1 (20.0) | 1.249 | 0.741 | 0.268 |
| LSIL | 12 (9.4) | 6 (13.0) | 4 (10.3) | 2 (5.3) | 0 (0.0) | 2.562 | 0.464 | 0.160 |
| HSIL | 2 (1.6) | 0 (0.0) | 0 (0.0) | 1 (2.6) | 1 (20.0) | 6.351 | 0.096 | <0.05 |
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| HPV State | |||||
| Demographic Variables | Overall | Positive (n = 347) | Negative (n = 2093) | χ2 | p |
| Age group | |||||
| 30–39 | 1142 (46.8) | 141 (12.3) | 1001 (87.7) | 11.784 | <0.05 |
| 40–49 | 704 (28.9) | 97 (13.8) | 607 (86.2) | ||
| 50–59 | 490 (20.1) | 89 (18.2) | 401 (81.8) | ||
| 60–64 | 104 (4.3) | 20 (19.2) | 84 (80.8) | ||
| Ethnicity | |||||
| Han | 2323 (95.2) | 323 (13.9) | 2000 (86.1) | 3.988 | <0.05 |
| Other | 117 (4.8) | 24 (20.5) | 93 (79.5) | ||
| District * | |||||
| Eastern | 707 (29.0) | 101 (14.3) | 606 (85.7) | 1.413 | 0.493 |
| Western | 722 (29.6) | 111 (15.4) | 611 (84.6) | ||
| Middle | 1011 (41.4) | 135 (13.4) | 876 (86.6) | ||
| Marital status | |||||
| Single | 45 (1.8) | 12 (26.7) | 33 (73.3) | 15.838 | <0.001 |
| Divorced/widowed | 101 (4.1) | 25 (24.8) | 76 (75.2) | ||
| Married | 2294 (94.0) | 310 (13.5) | 1984 (86.5) | ||
| Occupation | |||||
| Medical and health personnel | 482 (19.8) | 41 (8.5) | 441 (91.5) | 31.907 | <0.001 |
| Non-manual labor | 841 (34.5) | 101 (12.0) | 740 (88.0) | ||
| Manual labor | 1117 (45.8) | 205 (18.4) | 912 (81.6) | ||
| Smoking | |||||
| Never | 2418 (99.1) | 344 (14.2) | 2074 (85.8) | 0.379 | 0.827 |
| Past (abstained ≥3 months) | 11 (0.5) | 2 (18.2) | 9 (81.8) | ||
| Current (over 6 months) | 11 (0.5) | 1 (9.1) | 10 (90.9) | ||
| Drinking | |||||
| Never | 1636 (67.0) | 217 (13.3) | 1419 (86.7) | 5.928 | 0.052 |
| Often (<3 times/week) | 795 (32.6) | 127 (16.0) | 668 (84.0) | ||
| Usually (3–7 times/week) | 9 (0.4) | 3 (33.3) | 6 (66.7) | ||
| Family cancer history | |||||
| No | 2225 (91.2) | 320 (14.4) | 1905 (85.6) | 0.535 | 0.465 |
| Yes | 215 (8.8) | 27 (12.6) | 188 (87.4) | ||
| Education | |||||
| Second school or below | 869 (35.6) | 165 (19.0) | 704 (81.0) | 30.501 | <0.001 |
| Senior and vocational high school | 437 (17.9) | 65 (14.9) | 372 (85.1) | ||
| College or above | 1134 (46.5) | 117 (10.3) | 1017 (89.7) | ||
| Education level of couple | |||||
| Second school or below | 770 (31.6) | 142 (18.4) | 628 (81.6) | 17.520 | <0.001 |
| Senior and vocational high school | 522 (21.4) | 71 (13.6) | 451 (86.4) | ||
| College or above | 1148 (47.0) | 134 (11.7) | 1014 (88.3) | ||
| Per capita monthly household income | |||||
| <yuan 2000 | 829 (34.0) | 142 (17.1) | 679 (83.0) | 16.332 | <0.001 |
| yuan 2000–3999 | 592 (24.3) | 94 (15.9) | 505 (83.9) | ||
| ≥yuan 4000 | 1019 (41.8) | 111 (10.9) | 909 (89.1) | ||
| Obstetric and gynecologicvariables | |||||
| Menstrual regularity | |||||
| No | 546 (22.4) | 84 (15.4) | 462 (84.6) | 0.780 | 0.377 |
| Yes | 1894 (77.6) | 263 (13.9) | 1631 (86.1) | ||
| Dysmenorrhea | |||||
| No | 1711 (70.1) | 244 (14.3) | 1467 (85.7) | 0.007 | 0.932 |
| Yes | 729 (29.9) | 103 (14.1) | 626 (85.9) | ||
| Age of menarche | |||||
| <13 | 531 (21.8) | 73 (13.7) | 458 (86.3) | 0.878 | 0.645 |
| 13-15 | 1548 (63.4) | 217 (14.0) | 1331 (86.0) | ||
| >15 | 361 (14.8) | 57 (15.8) | 304 (84.2) | ||
| Leucorrhea | |||||
| No | 1831 (75.0) | 250 (13.7) | 1581 (86.3) | 1.937 | 0.164 |
| Yes | 609 (25.0) | 97 (15.9) | 512 (84.1) | ||
| Couple’s circumcision | |||||
| Unknown | 215 (8.8) | 36 (16.7) | 179 (83.3) | 1.606 | 0.448 |
| No | 1936 (79.3) | 274 (14.2) | 1662 (85.8) | ||
| Yes | 289 (11.8) | 37 (12.8) | 252 (87.2) | ||
| Age of sexual debut | |||||
| ≤20 | 665 (27.3) | 119 (17.9) | 546 (82.1) | 10.112 | <0.05 |
| >20 | 1775 (72.7) | 228 (12.8) | 1547 (87.2) | ||
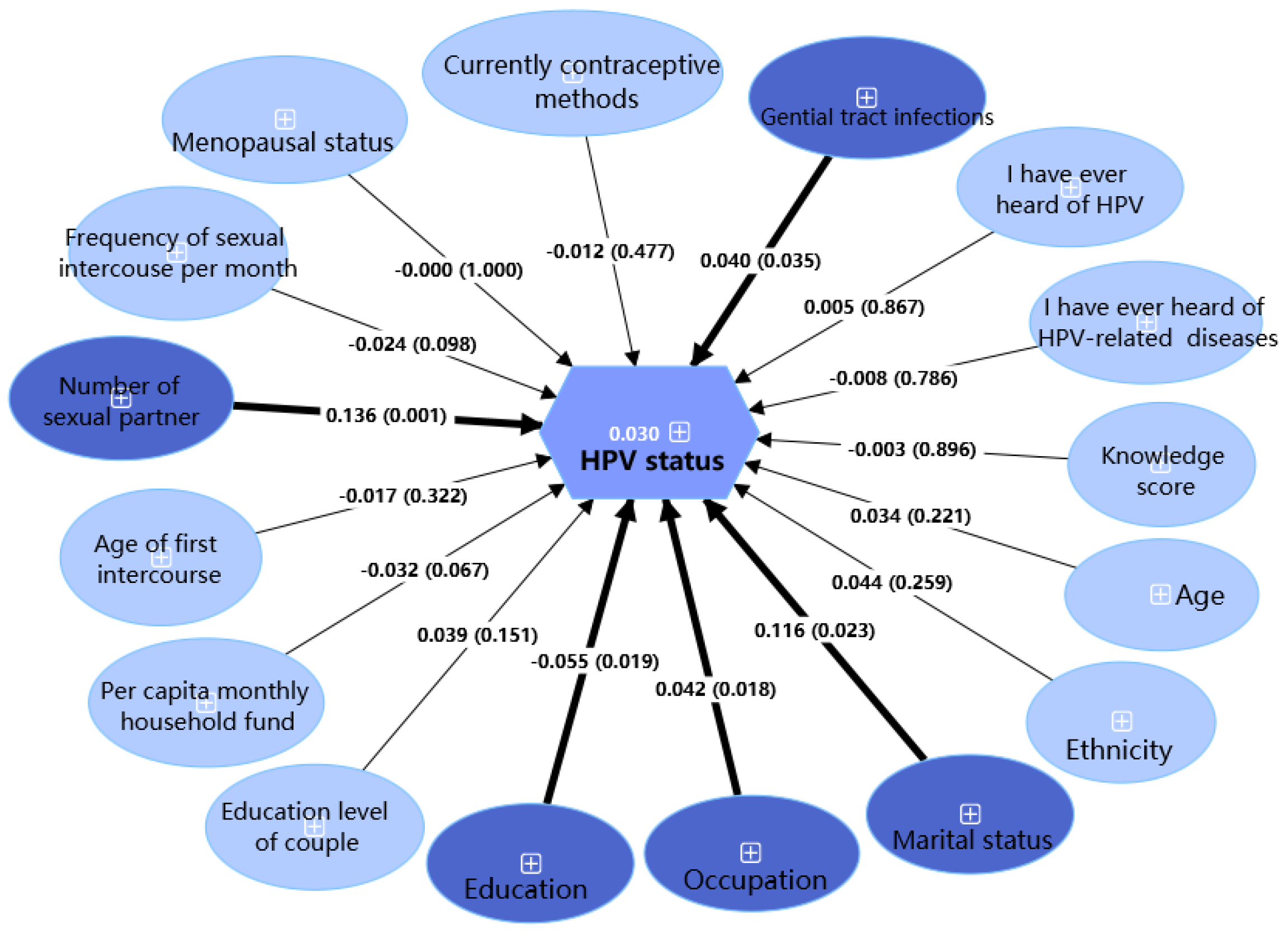
| Number of sexual partners | |||||
| 1 | 1976 (81.0) | 256 (13.0) | 1720 (87.0) | 21.905 | <0.001 |
| 2 | 344 (14.1) | 58 (16.9) | 286 (83.1) | ||
| ≥3 | 120 (4.9) | 33 (27.5) | 87 (72.5) | ||
| Age of marriage | |||||
| ≤20 | 299 (12.5) | 52 (17.4) | 247 (82.6) | 3.302 | 0.069 |
| >20 | 2097 (87.5) | 283 (13.5) | 1814 (86.5) | ||
| Frequency of sexual intercourse per month | |||||
| <4 | 1355 (55.5) | 215 (15.9) | 1140 (84.1) | 6.766 | <0.05 |
| ≥4 | 1085 (44.5) | 132 (12.2) | 953 (87.8) | ||
| Number of pregnancies | |||||
| <2 | 534 (21.9) | 66 (12.4) | 468 (87.6) | 1.942 | 0.163 |
| ≥2 | 1906 (78.1) | 281 (14.7) | 1625 (85.3) | ||
| Number of deliveries | |||||
| <2 | 929 (38.1) | 138 (14.9) | 791 (85.1) | 0.493 | 0.482 |
| ≥2 | 1511 (61.9) | 209 (13.8) | 1302 (86.2) | ||
| Age at first parity | |||||
| ≤20 | 170 (7.3) | 30 (17.6) | 140 (82.4) | 1.932 | 0.165 |
| >20 | 2160 (92.7) | 298 (13.8) | 1863 (86.2) | ||
| Menopausal status | |||||
| No | 1901 (77.9) | 251 (13.2) | 1650 (86.8) | 7.307 | <0.05 |
| Yes | 539 (22.1) | 96 (17.8) | 443 (82.2) | ||
| Prior experience and medical history | |||||
| Current contraceptive methods (like condom, contraceptives pills, sterilization, IUD) | |||||
| No | 854 (35.0) | 142 (16.6) | 712 (83.4) | 6.236 | <0.05 |
| Yes | 1586 (65.0) | 205 (12.9) | 1381 (87.1) | ||
| Ever had reproductive system disorders (like uterine myomas, cervical polyps, cervicitis, endometriosis) | |||||
| No | 1722 (70.6) | 236 (13.7) | 1486 (86.3) | 1.279 | 0.258 |
| Yes | 718 (29.4) | 111 (15.5) | 607 (84.5) | ||
| Ever had uterine surgery (like hysterectomy with cervical preservation, cervical conization, cervical cerclage, polypectomy, LEEP) | |||||
| No | 2118 (86.8) | 300 (14.2) | 1818 (85.8) | 0.043 | 0.836 |
| Yes | 322 (13.2) | 47 (14.6) | 275 (85.4) | ||
| Genital tract infections (like gonococcal, chlamydia trachomatis, mycoplasma, trichomoniasis) | |||||
| No | 1952 (80.0) | 262 (13.4) | 1690 (86.6) | 5.110 | <0.05 |
| Yes | 488 (20.0) | 85 (17.4) | 403 (82.6) | ||
| HPV knowledge | |||||
| I have ever heard of HPV | |||||
| No | 684 (28.0) | 114 (16.7) | 570 (83.3) | 4.659 | <0.05 |
| Yes | 1756 (72.0) | 233 (13.3) | 1523 (86.7) | ||
| I have ever heard of HPV-related diseases, such as genital warts and cervical cancer | |||||
| No | 865 (35.5) | 140 (16.2) | 725 (83.8) | 4.236 | <0.05 |
| Yes | 1575 (64.5) | 207 (13.1) | 1368 (86.9) | ||
| I have ever heard of HPV vaccine | |||||
| No | 487 (20.0) | 79 (16.2) | 408 (83.8) | 1.996 | 0.158 |
| Yes | 1953 (80.0) | 268 (13.7) | 1685 (86.3) | ||
| I have ever received HPV vaccination | |||||
| No | 1736 (71.1) | 253 (14.6) | 1483 (85.4) | 0.613 | 0.434 |
| Yes | 704 (28.9) | 94 (13.4) | 610 (86.6) | ||
| Ever received HPV vaccination knowledge | |||||
| No | 489 (20.0) | 79 (16.2) | 410 (83.8) | 1.875 | 0.171 |
| Yes | 1951 (80.0) | 268 (13.7) | 1683 (86.3) | ||
| Ever received cervical screening knowledge | |||||
| No | 947 (38.8) | 142 (15.0) | 805 (85.0) | 0.759 | 0.384 |
| Yes | 1493 (61.2) | 205 (13.7) | 1288 (86.3) | ||
| Regular screening is recommended following HPV vaccination | |||||
| No | 191 (7.8) | 36 (18.8) | 155 (81.2) | 4.006 | 0.135 |
| Unknown | 526 (21.6) | 77 (14.6) | 449 (85.4) | ||
| Yes | 1723 (70.6) | 234 (13.6) | 1489 (86.4) | ||
| I have ever participated in cervical cancer screening | |||||
| No | 947 (38.8) | 142 (15.0) | 805 (85.0) | 0.765 | 0.682 |
| National (free) | 834 (34.2) | 114 (13.7) | 720 (86.3) | ||
| Self-paid | 659 (27.0) | 91 (13.8) | 568 (86.2) | ||
| Score | |||||
| <5 | 771 (31.6) | 129 (16.7) | 642 (83.3) | 5.822 | <0.05 |
| ≥5 | 1669 (68.4) | 218 (13.1) | 1451 (86.9) | ||
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| HPV Vaccination | χ2 | p | |||
| Demographic Variables | Overall | Yes (n = 704) | No (n = 1736) | ||
| Age Group | |||||
| 30–39 | 1142 (46.8) | 497 (43.5) | 645 (56.5) | 328.545 | <0.001 |
| 40–49 | 704 (28.9) | 195 (27.7) | 509 (72.3) | ||
| 50–59 | 490 (20.1) | 11 (2.2) | 479 (97.8) | ||
| 60–64 | 104 (4.3) | 1 (1.0) | 103 (99.0) | ||
| Ethnicity | |||||
| Han | 2323 (95.2) | 671 (28.9) | 1652 (71.1) | 0.025 | 0.874 |
| Other | 117 (4.8) | 33 (28.2) | 84 (71.8) | ||
| District * | |||||
| Eastern | 707 (29.0) | 226 (32.0) | 481 (68.0) | 5.825 | 0.054 |
| Western | 722 (29.6) | 209 (28.9) | 513 (71.1) | ||
| Middle | 1011 (41.4) | 269 (26.6) | 742 (73.4) | ||
| Marital Status | |||||
| Single | 45 (1.8) | 28 (62.2) | 17 (37.8) | 24.882 | <0.001 |
| Divorced/widowed | 101 (4.1) | 28 (27.7) | 73 (72.3) | ||
| Married | 2294 (94.0) | 648 (28.2) | 1646 (71.8) | ||
| Occupation | |||||
| Medical and health personnel | 482 (19.8) | 222 (46.1) | 260 (53.9) | 211.659 | <0.001 |
| Non-manual labor | 841 (34.5) | 318 (37.8) | 523 (62.2) | ||
| Manual labor | 1117 (45.8) | 164 (14.7) | 953 (85.3) | ||
| Smoking | |||||
| Never | 2418 (99.1) | 697 (28.8) | 1721 (71.2) | 2.088 | 0.352 |
| Past (abstained ≥3 months) | 11 (0.5) | 2 (18.2) | 9 (81.8) | ||
| Current (over 6 months) | 11 (0.5) | 5 (45.5) | 6 (54.5) | ||
| Drinking | |||||
| Never | 1636 (67.0) | 420 (25.7) | 1216 (74.3) | 24.750 | <0.001 |
| Often (<3 times/week) | 795 (32.6) | 282 (35.5) | 513 (64.5) | ||
| Usually (3–7 times/week) | 9 (0.4) | 2 (22.2) | 7 (77.8) | ||
| Family cancer history | |||||
| No | 2225 (91.2) | 2225 (91.2) | 1601 (72.0) | 8.021 | <0.05 |
| Yes | 215 (8.8) | 215 (8.8) | 135 (62.8) | ||
| Education | |||||
| Second school or below | 869 (35.6) | 90 (10.4) | 779 (89.6) | 293.823 | <0.001 |
| Senior and vocational high school | 437 (17.9) | 104 (23.8) | 333 (76.2) | ||
| College or above | 1134 (46.5) | 510 (45.0) | 624 (55.0) | ||
| Education level of couple | |||||
| Second school or below | 770 (31.6) | 77 (10.0) | 693 (90.0) | 263.191 | <0.001 |
| Senior and vocational high school | 522 (21.4) | 125 (23.9) | 397 (6.1) | ||
| College or above | 1148 (47.0) | 502 (43.7) | 646 (56.3) | ||
| Per capita monthly household income | |||||
| <yuan 2000 | 829 (34.0) | 142 (17.1) | 687 (82.9) | 125.623 | <0.001 |
| yuan 2000–3999 | 592 (24.3) | 150 (25.3) | 442 (74.7) | ||
| ≥yuan 4000 | 1019 (41.8) | 412 (40.4) | 607 (59.6) | ||
| Obstetric and gynecologic variables | |||||
| Menstrual regularity | |||||
| No | 546 (22.4) | 110 (20.1) | 436 (79.9) | 25.971 | <0.001 |
| Yes | 1894 (77.6) | 594 (31.4) | 1300 (68.6) | ||
| Dysmenorrhea | |||||
| No | 1711 (70.1) | 487 (28.5) | 1224 (71.5) | 0.423 | 0.515 |
| Yes | 729 (29.9) | 217 (29.8) | 512 (70.2) | ||
| Age of menarche | |||||
| <13 | 531 (21.8) | 197 (37.1) | 334 (62.9) | 47.639 | <0.001 |
| 13–15 | 1548 (63.4) | 450 (29.1) | 1098 (70.9) | ||
| >15 | 361 (14.8) | 57 (15.8) | 304 (84.2) | ||
| Leucorrhea | |||||
| No | 1831 (75.0) | 512 (28.0) | 1319 (72.0) | 2.828 | 0.093 |
| Yes | 609 (25.0) | 192 (31.5) | 417 (68.5) | ||
| Couple’s circumcision | |||||
| Unknown | 215 (8.8) | 68 (31.6) | 147 (68.4) | 19.975 | <0.001 |
| No | 1936 (79.3) | 522 (27.0) | 1414 (73.0) | ||
| Yes | 289 (11.8) | 114 (39.4) | 175 (60.6) | ||
| Age of sexual debut | |||||
| ≤20 | 665 (27.3) | 167 (25.1) | 498 (74.9) | 6.228 | <0.05 |
| >20 | 1775 (72.7) | 537 (30.3) | 1238 (69.7) | ||
| Number of sexual partners | |||||
| 1 | 1976 (81.0) | 534 (27.0) | 1442 (73.0) | 19.628 | <0.001 |
| 2 | 344 (14.1) | 119 (34.6) | 225 (65.4) | ||
| ≥3 | 120 (4.9) | 51 (42.5) | 69 (57.5) | ||
| Age of marriage | |||||
| ≤20 | 299 (12.5) | 43 (14.4) | 256 (85.6) | 32.274 | <0.001 |
| >20 | 2097 (87.5) | 633 (30.2) | 1464 (69.8) | ||
| Frequency of sexual intercourse per month | |||||
| <4 | 1355 (55.5) | 330 (24.4) | 1025 (75.6) | 30.036 | <0.001 |
| ≥4 | 1085 (44.5) | 374 (34.5) | 711 (65.5) | ||
| Number of pregnancies | |||||
| <2 | 534 (21.9) | 224 (41.9) | 310 (58.1) | 57.106 | <0.001 |
| ≥2 | 1906 (78.1) | 480 (25.2) | 1426 (74.8) | ||
| Number of deliveries | |||||
| <2 | 929 (38.1) | 305 (32.8) | 624 (67.2) | 11.568 | <0.001 |
| ≥2 | 1511 (61.9) | 399 (26.4) | 1112 (73.6) | ||
| Age at first parity | |||||
| ≤20 | 170 (7.3) | 24 (14.1) | 146 (85.9) | 17.036 | <0.001 |
| >20 | 2160 (92.7) | 623 (28.8) | 1537 (71.2) | ||
| Menopausal status | |||||
| No | 1901 (77.9) | 687 (36.1) | 1214 (63.9) | 222.571 | <0.001 |
| Yes | 539 (22.1) | 17 (3.2) | 522 (96.8) | ||
| Prior experience and medical history | |||||
| Current contraceptive methods (like condom, contraceptives pills, sterilization, IUD) | |||||
| No | 854 (35.0) | 129 (15.1) | 725 (84.9) | 120.955 | <0.001 |
| Yes | 1586 (65.0) | 575 (36.3) | 1011 (63.7) | ||
| Ever had reproductive system disorders (like uterine myomas, cervical polyps, cervicitis, endometriosis) | |||||
| No | 1722 (70.6) | 488 (28.3) | 1234 (71.7) | 0.751 | 0.386 |
| Yes | 718 (29.4) | 216 (30.1) | 502 (69.9) | ||
| Ever had uterine surgery (like hysterectomy with cervical preservation, cervical conization, cervical cerclage, polypectomy, LEEP) | |||||
| No | 2118 (86.8) | 611 (28.8) | 1507 (71.2) | 0.000 | 0.990 |
| Yes | 322 (13.2) | 93 (28.9) | 229 (71.1) | ||
| Genital tract infections (like gonococcal, chlamydia trachomatis, mycoplasma, trichomoniasis) | |||||
| No | 1952 (80.0) | 544 (27.9) | 1408 (72.1) | 4.600 | <0.05 |
| Yes | 488 (20.0) | 160 (32.8) | 328 (67.2) | ||
| HPV knowledge | |||||
| I have ever heard of HPV | |||||
| No | 684 (28.0) | 50 (7.3) | 634 (92.7) | 214.868 | <0.001 |
| Yes | 1756 (72.0) | 654 (37.2) | 1102 (62.8) | ||
| I have ever heard of HPV-related diseases, such as genital warts and cervical cancer | |||||
| No | 865 (35.5) | 112 (12.9) | 753 (87.1) | 165.128 | <0.001 |
| Yes | 1575 (64.5) | 592 (37.6) | 983 (62.4) | ||
| Ever received HPV vaccination knowledge | |||||
| No | 489 (20.0) | 1 (0.2) | 488 (99.8) | 244.504 | <0.001 |
| Yes | 1951 (80.0) | 703 (36.0) | 1248 (64.0) | ||
| Ever received cervical screening knowledge | |||||
| No | 947 (38.8) | 184 (19.4) | 763 (80.6) | 66.940 | <0.001 |
| Yes | 1493 (61.2) | 520 (34.8) | 973 (65.2) | ||
| Regular screening is recommended following HPV vaccination | |||||
| No | 191 (7.8) | 23 (12.0) | 168 (88.0) | 120.602 | <0.001 |
| Unknown | 526 (21.6) | 72 (13.7) | 454 (86.3) | ||
| Yes | 1723 (70.6) | 609 (35.3) | 1114 (64.7) | ||
| I have ever participated in cervical cancer screening | |||||
| No | 947 (38.8) | 184 (19.4) | 763 (80.6) | 87.562 | <0.001 |
| National (free) | 834 (34.2) | 251 (30.1) | 583 (69.9) | ||
| Self-paid | 659 (27.0) | 269 (40.8) | 390 (59.2) | ||
| HPV state | |||||
| Negative | 2093 (85.8) | 610 (29.1) | 1483 (70.9) | 0.613 | 0.434 |
| Positive | 347(14.2) | 94(27.1) | 253(72.9) | ||
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| Intention to Take HPV Vaccination | |||||
| Demographic Variables | Overall | Yes (n = 1052) | No (n = 684) | c2 | p |
| Age group | |||||
| 30–39 | 645 (37.2) | 489 (75.8) | 156 (24.2) | 114.779 | <0.001 |
| 40–49 | 509 (29.3) | 294 (57.8) | 215 (42.2) | ||
| 50–59 | 479 (27.6) | 223 (46.6) | 256 (53.4) | ||
| 60–64 | 103 (5.9) | 46 (44.7) | 57 (55.3) | ||
| Ethnicity | |||||
| Han | 1652 (95.2) | 1004 (60.8) | 648 (39.2) | 0.442 | 0.506 |
| Other | 84 (4.8) | 48 (57.1) | 36 (42.9) | ||
| District * | |||||
| Eastern | 481 (27.7) | 270 (56.1) | 211 (43.9) | ||
| Western | 513 (29.6) | 332 (64.7) | 181 (35.3) | 7.663 | <0.05 |
| Middle | 742 (42.7) | 450 (60.6) | 292 (39.4) | ||
| Marital Status | |||||
| Single | 17 (1.0) | 11 (64.7) | 6 (35.3) | ||
| Divorced/widowed | 73 (4.2) | 38 (52.1) | 35 (47.9) | 2.430 | 0.297 |
| Married | 1646 (94.8) | 1003 (60.9) | 643 (39.1) | ||
| Occupation | |||||
| Medical and health personnel | 260 (15.0) | 192 (73.8) | 68 (26.2) | ||
| Non-manual labor | 523 (30.1) | 377 (72.1) | 146 (27.9) | 87.256 | |
| Manual labor | 953 (54.9) | 483 (50.7) | 470 (49.3) | <0.001 | |
| Smoking | |||||
| Never | 1721 (99.1) | 1042 (60.5) | 679 (39.5) | ||
| Past (abstained ≥3 months) | 9 (0.5) | 6 (66.7) | 3 (33.3) | 0.233 | 0.890 |
| Current (Over 6 months) | 6 (0.3) | 4 (66.7) | 2 (33.3) | ||
| Drinking | |||||
| Never | 1216 (70.0) | 709 (58.3) | 507 (41.7) | ||
| Often (<3 times/week) | 513 (29.6) | 340 (66.3) | 173 (33.7) | 10.527 | <0.05 |
| Usually (3–7 times/week) | 7 (0.4) | 3 (42.9) | 4 (57.1) | ||
| Family cancer history | |||||
| No | 1601 (92.2) | 960 (60.0) | 641 (40.0) | ||
| Yes | 135 (7.8) | 92 (68.1) | 43 (31.9) | 3.494 | 0.062 |
| Education | |||||
| Second school or below | 779 (44.9) | 379 (48.7) | 400 (51.3) | 117.181 | <0.001 |
| Senior and vocational high school | 333 (19.2) | 193 (58.0) | 140 (42.0) | ||
| College or above | 624 (35.9) | 480 (76.9) | 144 (23.1) | ||
| Education level of couple | |||||
| Second school or below | 693 (39.9) | 348 (50.2) | 345 (49.8) | 89.647 | <0.001 |
| Senior and vocational high school | 397 (22.9) | 221 (55.7) | 176 (44.3) | ||
| College or above | 646 (37.2) | 483 (74.8) | 163 (25.2) | ||
| Per capita monthly household income | |||||
| <yuan 2000 | 687 (39.6) | 350 (50.9) | 337 (49.1) | 60.918 | <0.001 |
| yuan 2000–3999 | 442 (25.5) | 264 (59.7) | 178 (40.3) | ||
| ≥yuan 4000 | 607 (35.0) | 438 (72.2) | 169 (27.8) | ||
| Obstetric and gynecologic variables | |||||
| Menstrual regularity | |||||
| No | 436 (25.1) | 250 (57.3) | 186 (42.7) | 2.591 | 0.107 |
| Yes | 1300 (74.9) | 802 (61.7) | 498 (38.3) | ||
| Dysmenorrhea | |||||
| No | 1224 (70.5) | 726 (59.3) | 498 (40.7) | 2.872 | 0.090 |
| Yes | 512 (29.2) | 326 (63.7) | 186 (36.3) | ||
| Age of menarche | |||||
| <13 | 334 (19.2) | 226 (67.7) | 108 (32.3) | 24.589 | <0.001 |
| 13–15 | 1098 (63.2) | 677 (61.7) | 421 (38.3) | ||
| >15 | 304 (17.5) | 149 (49.0) | 155 (51.0) | ||
| Leucorrhea | |||||
| No | 1319 (76.0) | 779 (59.1) | 540 (40.9) | 5.448 | <0.05 |
| Yes | 417 (24.0) | 273 (65.5) | 144 (34.5) | ||
| Couple’s circumcision | |||||
| Unknown | 147 (8.5) | 87 (59.2) | 60 (40.8) | 3.830 | 0.147 |
| No | 1414 (81.5) | 847 (59.9) | 567 (40.1) | ||
| Yes | 175 (10.1) | 118 (67.4) | 57 (32.6) | ||
| Age of sexual debut | |||||
| ≤20 | 498 (28.7) | 288 (57.8) | 210 (42.2) | 2.240 | 0.134 |
| >20 | 1238 (71.3) | 764 (61.7) | 474 (38.3) | ||
| Number of sexual partners | |||||
| 1 | 1442 (83.1) | 851 (59.0) | 591 (41.0) | 10.793 | <0.05 |
| 2 | 225 (13.0) | 149 (66.2) | 76 (33.8) | ||
| ≥3 | 69 (4.0) | 52 (75.4) | 17 (24.6) | ||
| Age of marriage | |||||
| ≤20 | 256 (14.9) | 125 (48.8) | 131 (51.2) | 17.218 | <0.001 |
| >20 | 1464 (85.1) | 916 (62.6) | 548 (37.4) | ||
| Frequency of sexual intercourse per month | |||||
| <4 | 1025 (59.0) | 589 (57.5) | 436 (42.5) | 10.306 | <0.05 |
| ≥4 | 711 (41.0) | 463 (65.1) | 248 (34.9) | ||
| Number of pregnancies | |||||
| <2 | 310 (17.9) | 219 (70.6) | 91 (29.4) | 15.952 | <0.001 |
| ≥2 | 1426 (82.1) | 833 (58.4) | 593 (41.6) | ||
| Number of deliveries | |||||
| <2 | 624 (35.9) | 419 (67.1) | 205 (32.9) | 17.495 | <0.001 |
| ≥2 | 1112 (64.1) | 633 (56.9) | 479 (43.1) | ||
| Age at first parity | |||||
| ≤20 | 146 (8.7) | 79 (54.1) | 67 (45.9) | 2.517 | 0.113 |
| >20 | 1537 (91.3) | 935 (60.8) | 602 (39.2) | ||
| Menopausal Status | |||||
| No | 1214 (69.9) | 806 (66.4) | 408 (33.6) | 56.746 | <0.001 |
| Yes | 522 (30.1) | 246 (47.1) | 276 (52.9) | ||
| Prior experience and medical history | |||||
| Current contraceptive methods (like condom, contraceptives pills, sterilization, IUD) | |||||
| No | 725 (41.8) | 388 (53.5) | 337 (46.5) | 26.149 | <0.001 |
| Yes | 1011 (58.2) | 664 (65.7%) | 347 (34.3) | ||
| Ever had reproductive system disorders (like uterine myomas, cervical polyps, cervicitis, endometriosis) | |||||
| No | 1234 (71.1) | 721 (58.4) | 513 (41.6) | 8.425 | <0.05 |
| Yes | 502 (28.9) | 331 (65.9) | 171 (34.1) | ||
| Ever had uterine surgery (like hysterectomy with cervical preservation, cervical conization, cervical cerclage, polypectomy, LEEP) | |||||
| No | 1507 (86.8) | 897 (59.5) | 610 (40.5) | 5.548 | <0.05 |
| Yes | 229 (13.2) | 155 (67.7) | 74 (32.3) | ||
| Genital tract infections (like gonococcal, chlamydia trachomatis, mycoplasma, trichomoniasis) | |||||
| No | 1408 (81.1) | 835 (59.3) | 573 (40.7) | 5.235 | <0.05 |
| Yes | 328 (18.9) | 217 (66.2) | 111 (33.8) | ||
| HPV knowledge | |||||
| I have ever heard of HPV | |||||
| No | 634 (36.5) | 268 (42.3) | 366 (57.7) | 140.509 | <0.001 |
| Yes | 1102 (63.5) | 784 (71.1) | 318 (28.9) | ||
| I have ever heard of HPV-related diseases, such as genital warts and cervical cancer | |||||
| No | 753 (43.4) | 337 (44.8) | 416 (55.2) | 139.827 | <0.001 |
| Yes | 983 (56.6) | 715 (72.7) | 268 (27.3) | ||
| Ever received HPV vaccination knowledge | |||||
| No | 488 (28.1) | 178 (36.5) | 310 (63.5) | 165.451 | <0.001 |
| Yes | 1248 (71.9) | 874 (70.0) | 374 (30.0) | ||
| Ever received cervical screening knowledge | |||||
| No | 763 (44.0) | 433 (56.7) | 330 (43.3) | 8.448 | <0.05 |
| Yes | 973 (56.0) | 619 (63.6) | 354 (36.4) | ||
| Regular screening is recommended following HPV vaccination | |||||
| No | 168 (9.7) | 48 (28.6) | 120 (71.4) | 137.674 | <0.001 |
| Unknown | 454 (26.2) | 224 (49.3) | 230 (50.7) | ||
| Yes | 1114 (64.2) | 780 (70.0) | 334 (30.0) | ||
| I have ever participated in cervical cancer screening | |||||
| No | 763 (44.0) | 433 (56.7) | 330 (43.3) | 24.462 | <0.001 |
| National (free) | 583 (33.6) | 341 (58.5) | 242 (41.5) | ||
| Self-paid | 390 (22.5) | 278 (71.3) | 112 (28.7) | ||
| HPV state | |||||
| Negative | 1483 (85.4) | 910 (61.4) | 573 (38.6) | 2.481 | 0.115 |
| Positive | 253 (14.6) | 142 (56.1) | 111 (43.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, Z.; Zhu, M.; Chen, Z.; Ni, W.; Lai, L.; Lin, B.; Jiang, L.; Jing, Y.; Fan, J. HPV Infection Prevalence, Vaccination-Related Knowledge, Attitudes, and Barriers Among Women Aged 30–64 in Shenzhen, China: A Cross-Sectional Study. Vaccines 2025, 13, 561. https://doi.org/10.3390/vaccines13060561
Ouyang Z, Zhu M, Chen Z, Ni W, Lai L, Lin B, Jiang L, Jing Y, Fan J. HPV Infection Prevalence, Vaccination-Related Knowledge, Attitudes, and Barriers Among Women Aged 30–64 in Shenzhen, China: A Cross-Sectional Study. Vaccines. 2025; 13(6):561. https://doi.org/10.3390/vaccines13060561
Chicago/Turabian StyleOuyang, Zhongai, Minting Zhu, Zhijian Chen, Weigui Ni, Lijuan Lai, Bingyi Lin, Long Jiang, Yi Jing, and Jingjie Fan. 2025. "HPV Infection Prevalence, Vaccination-Related Knowledge, Attitudes, and Barriers Among Women Aged 30–64 in Shenzhen, China: A Cross-Sectional Study" Vaccines 13, no. 6: 561. https://doi.org/10.3390/vaccines13060561
APA StyleOuyang, Z., Zhu, M., Chen, Z., Ni, W., Lai, L., Lin, B., Jiang, L., Jing, Y., & Fan, J. (2025). HPV Infection Prevalence, Vaccination-Related Knowledge, Attitudes, and Barriers Among Women Aged 30–64 in Shenzhen, China: A Cross-Sectional Study. Vaccines, 13(6), 561. https://doi.org/10.3390/vaccines13060561




