Maternal Vaccination as an Integral Part of Life-Course Immunization: A Scoping Review of Uptake, Barriers, Facilitators, and Vaccine Hesitancy for Antenatal Vaccination in Ireland
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Statement of Research Question
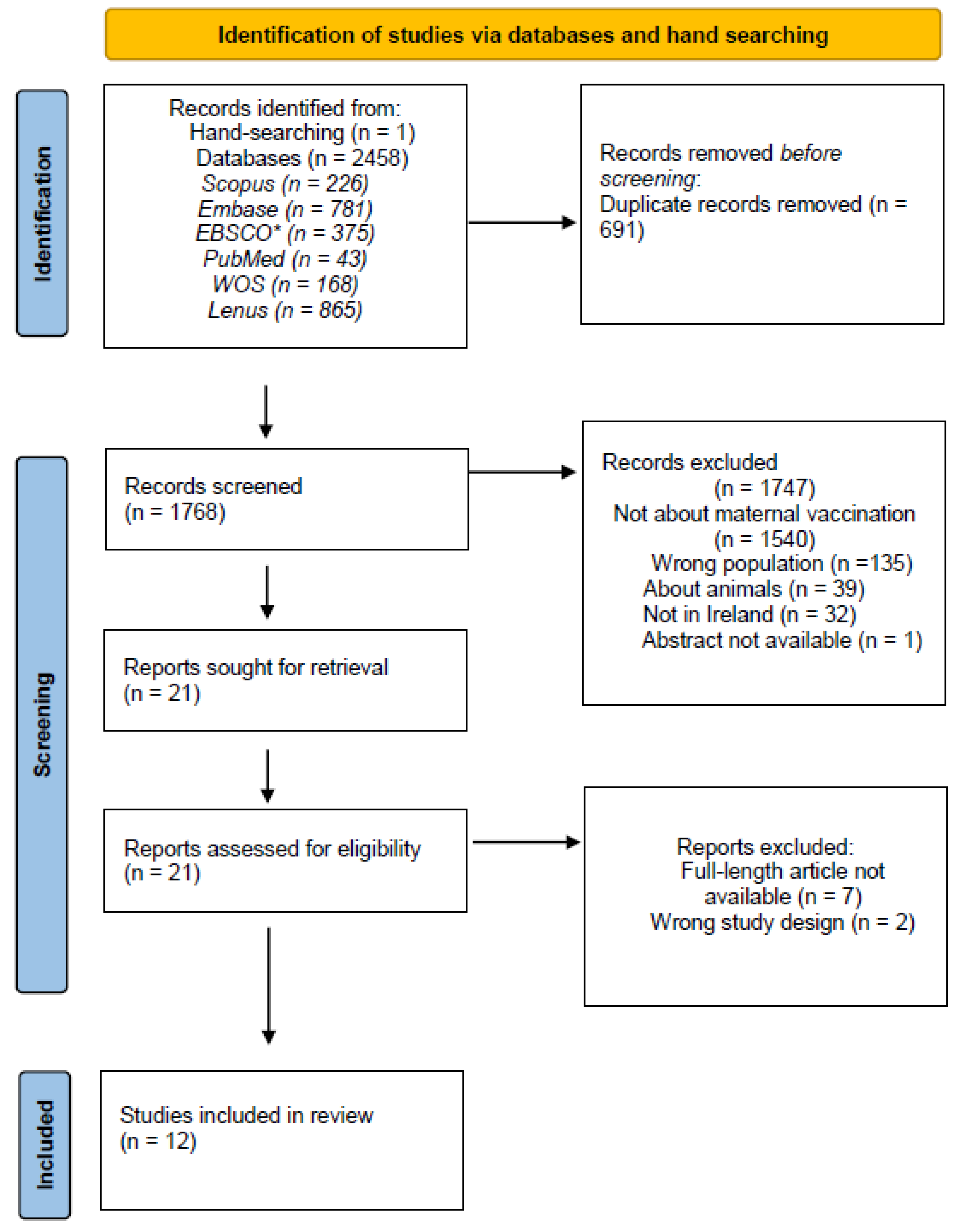
2.3. Identification of Relevant Studies
2.4. Selection of Relevant Studies
2.5. Charting the Data
2.6. Collating, Summarizing, and Reporting the Results
3. Results
3.1. Study Characteristics
3.2. Sociodemographic Characteristics Associated with Maternal Vaccination
3.3. The 5A Factors Associated with Maternal Vaccination
3.4. Acceptance
3.4.1. Individual Characteristics
3.4.2. Perception of Illness
3.4.3. Social Context of HCPs
3.4.4. Vaccine
3.5. Affordability
3.6. Access
3.6.1. Convenience
3.6.2. Location of Vaccination
3.6.3. Contact with Healthcare Systems
3.7. Awareness
3.7.1. Women’s Knowledge of Vaccines and Vaccine Schedule
3.7.2. HCPs’ Knowledge of Vaccines and Vaccine Schedule
3.7.3. Availability of Information
3.8. Activation
3.8.1. Prompts and Reminders
3.8.2. Workplace Policies
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. Newborn Mortality; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/newborn-mortality (accessed on 25 June 2024).
- Dattani, S.; Spooner, F.; Ritchie, H.; Roser, M. Child and Infant Mortality. 2023. Available online: https://ourworldindata.org/child-mortality (accessed on 25 June 2024).
- Jones, C.; Heath, P. Antenatal immunization. Hum. Vaccines Immunother. 2014, 10, 2118–2122. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Klein, S. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef]
- Sappenfield, E.; Jamieson, D.; Kourtis, A. Pregnancy and susceptibility to infectious diseases. Infect. Dis. Obstet. Gynaecol. 2013, 2013, 752852. [Google Scholar] [CrossRef]
- Strunk, T.; Currie, A.; Richmond, P.; Simmer, K.; Burgner, D. Innate immunity in human newborn infants: Prematurity means more than immaturity. J. Matern. Fetal Neonatal Med. 2010, 24, 25–31. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic immune system development in newborn children. Cell 2018, 174, 1277–1292. [Google Scholar] [CrossRef]
- Jennewein, M.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 2017, 9, 605–613. [Google Scholar] [CrossRef]
- World Health Organization. Vaccines against influenza WHO position paper—November 2012. Weekly Epidemiological Record. Relev. épidémiologique Hebd. 2012, 87, 461–476. [Google Scholar]
- World Health Organization. Pertussis vaccines: WHO position paper, August 2015 -recommendations. Wkly. Epidemiol. Rec. Relev. épidémiologique Hebd. 2016, 34, 1423–1425. [Google Scholar] [CrossRef]
- Etti, M.; Calvert, A.; Galiza, E.; Lim, S.; Khalil, A.; Le Doare, K.; Heath, P.T. Maternal vaccination: A review of current evidence and recommendations. Am. J. Obstet. Gynecol. 2022, 226, 459–474. [Google Scholar] [CrossRef]
- Neuzil, K.; Reed, G.; Mitchel, E.; Simonsen, L.; Griffin, M. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am. J. Epidemiol. 1998, 148, 1094–1102. [Google Scholar] [CrossRef]
- Mertz, D.; Geraci, J.; Winkup, J.; Gessner, B.; Ortiz, J.; Loeb, M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta-analysis of observational studies. Vaccine 2017, 35, 521–528. [Google Scholar] [CrossRef]
- Wang, R.; Yan, W.; Du, M.; Tao, L.; Liu, J. The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of Cohort studies. Int. J. Infect. Dis. 2021, 105, 567–578. [Google Scholar] [CrossRef]
- Rasmussen, S.; Jamieson, D.; Uyeki, T. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynaecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef]
- Bhat, N.; Wright, J.G.; Broder, K.R.; Murray, E.L.; Greenberg, M.E.; Glover, M.J.; Likos, A.M.; Posey, D.L.; Klimov, A.; Lindstrom, S.E.; et al. Influenza-associated deaths among children in the United States, 2003–2004. N. Engl. J. Med. 2005, 353, 2559–2567. [Google Scholar] [CrossRef]
- Poehling, K.A.; Edwards, K.M.; Weinberg, G.A.; Szilagyi, P.; Staat, M.A.; Iwane, M.K.; Bridges, C.B.; Grijalva, C.G.; Zhu, Y.; Bernstein, D.I.; et al. The underrecognized burden of influenza in young children. N. Engl. J. Med. 2006, 355, 31–40. [Google Scholar] [CrossRef]
- MacDonald, N.; Bortolussi, R. Protecting young babies from influenza. Paediatr. Child Health 2009, 14, 612–614. [Google Scholar] [CrossRef]
- Tan, T.; Dalby, T.; Forsyth, K.; Halperin, S.A.; Heininger, U.; Hozbor, D.; Plotkin, S.; Ulloa-Gutierrez, R.; von König, C.H.W. Pertussis across the Globe. Paediatr. Infect. Dis. J. 2015, 34, e222–e232. [Google Scholar] [CrossRef]
- Healy, M. Pertussis vaccination in pregnancy. Hum. Vaccines Immunother. 2016, 12, 1972–1981. [Google Scholar] [CrossRef]
- Stefanelli, P.; Buttinelli, G.; Vacca, P.; Tozzi, A.E.; Midulla, F.; Carsetti, R.; Fedele, G.; Villani, A.; Concato, C. Severe pertussis infection in infants less than 6 months of age: Clinical manifestations and molecular characterization. Hum. Vaccines Immunother. 2017, 13, 1073–1077. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Zhang, Y.; Xu, L.; Miao, M.; Yang, H.; Liu, Y.; He, S.; Pang, L. Analysis of clinical characteristics of severe pertussis in infants and children: A retrospective study. BMC Pediatr. 2021, 21, 65. [Google Scholar] [CrossRef]
- Bisgard, K.M.; Pascual, F.B.; Ehresmann, K.R.; Miller, C.A.; Cianfrini, C.; Jennings, C.E.; Rebmann, C.A.; Gabel, J.; Schauer, S.L.; Lett, S.M. Infant pertussis. Pediatr. Infect. Dis. J. 2004, 23, 985–989. [Google Scholar] [CrossRef]
- Wendelboe, A.M.; Njamkepo, E.; Bourillon, A.; Floret, D.D.; Gaudelus, J.; Gerber, M.; Grimprel, E.; Greenberg, D.; Halperin, S.; Liese, J.; et al. Transmission of bordetella pertussis to young infants. Pediatr. Infect. Dis. J. 2007, 26, 2939. [Google Scholar] [CrossRef]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-COV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- Philip, R.K.; Attwell, K.; Breuer, T.; Di Pasquale, A.; Lopalco, P.L. Life-course immunization as a gateway to health. Expert Rev. Vaccines 2018, 17, 851–864. [Google Scholar] [CrossRef]
- Philip, R.K.; Di Pasquale, A. Health Care Professionals’ Perspectives on life-course immunization: A qualitative survey from a european conference. Vaccines 2020, 8, 185. [Google Scholar] [CrossRef]
- World Health Organisation. Ensuring Immunization Through the Life Course; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/laos/ensuring-immunization-through-the-life-course (accessed on 25 June 2024).
- Pan American Heath Organisation (PAHO). Building Better Immunity: A Life Course Approach to Healthy Longevity; Pan American Health Organisation (PAHO): Washington, DC, USA, 2024; Available online: https://www.paho.org/en/documents/building-better-immunity-life-course-approach-healthy-longevity (accessed on 4 February 2024).
- Doherty, T.M.; Di Pasquale, A.; Finnegan, G.; Lele, J.; Philip, R.K. Sustaining the momentum for adult vaccination post-covid-19 to leverage the global uptake of life-course immunisation: A scoping review and call to action. Int. J. Infect. Dis. 2024, 142, 106963. [Google Scholar] [CrossRef]
- Royal College of Physicians of Ireland. NIAC Immunisation Guidelines. Chapter 11. Influenza. Available online: https://www.hiqa.ie/reports-and-publications/niac-immunisation-guideline/chapter-11-influenza (accessed on 26 June 2024).
- Royal College of Physicians of Ireland. NIAC immunisation guidelines. Chapter 15. Pertussis. Available online: https://www.hiqa.ie/sites/default/files/NIAC/Immunisation_Guidelines/Chapter_15_Pertussis.pdf (accessed on 26 June 2024).
- Royal College of Physicians of Ireland. NIAC Immunisation Guidelines Chapter, 0.5.A. COVID-19. Available online: https://www.hiqa.ie/sites/default/files/NIAC/Immunisation_Guidelines/Chapter_05a_COVID-19.pdf (accessed on 26 June 2024).
- Tamma, P.D.; Ault, K.A.; del Rio, C.; Steinhoff, M.C.; Halsey, N.A.; Omer, S.B. Safety of influenza vaccination during pregnancy. Am. J. Obstet. Gynaecol. 2009, 201, 547–552. [Google Scholar] [CrossRef]
- Kharbanda, E.O.; Vazquez-Benitez, G.; Lipkind, H.; Naleway, A.; Lee, G.; Nordin, J.D. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet. Gynaecol. 2013, 122, 659–667. [Google Scholar] [CrossRef]
- Nordin, J.D.; Kharbanda, E.O.; Benitez, G.V.; Nichol, K.; Lipkind, H.; Naleway, A.; Lee, G.M.; Hambidge, S.; Shi, W.; Olsen, A. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet. Gynaecol. 2013, 121, 519–525. [Google Scholar] [CrossRef]
- Griffin, J.B.; Yu, L.; Watson, D.; Turner, N.; Walls, T.; Howe, A.S.; Jiang, Y.; Petousis-Harris, H. Pertussis immunisation in pregnancy safety (PIPS) study: A retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine. Vaccine 2018, 36, 5173–5179. [Google Scholar] [CrossRef]
- Health Service Executive and Health Protection Surveillance Centre. Surveillance Reports; Health Service Executive and Health Protection Surveillance Centre: Dublin, Ireland, 2012; Available online: https://www.hpsc.ie/a-z/outbreaks/surveillancereports/ (accessed on 26 June 2024).
- Health Protection Surveillance Centre. Surveillance Reports on Outbreaks; Health Service Executive and Health Protection Surveil-lance Centre: Dublin, Ireland, 2013; Available online: https://www.hpsc.ie/a-z/outbreaks/ (accessed on 26 June 2024).
- Health Protection Surveillance Centre. Annual Epidemiological Report 2016; Health Protection Surveillance Centre: Dublin, Ireland, 2016; Available online: https://www.hpsc.ie/abouthpsc/annualreports/annualepidemiologicalreports1999-2016/HSE%20HPSC%20Annual%20Report%20ready%202016%20update%20to%20HPV%20target%20rate%2013112019.pdf (accessed on 26 June 2024).
- Geoghegan, S.; Shuster, S.; Butler, K.M.; Feemster, K.A. Understanding barriers and facilitators to maternal immunization: A systematic narrative synthesis of the published literature. Matern. Child Health J. 2022, 26, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Robinson, K.; Vallée-Tourangeau, G. The 5As: A practical taxonomy for the determinants of vaccine uptake. Vaccine 2016, 34, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. Prisma extension for scoping reviews (PRISMA-SCR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The Prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Barrett, T.; McEntee, E.; Drew, R.; O’reilly, F.; O’carroll, A.; O’shea, A.; Cleary, B. Influenza vaccination in pregnancy: Vaccine uptake, maternal and healthcare providers’ knowledge and attitudes. A quantitative study. BJGP Open 2018, 2. [Google Scholar] [CrossRef]
- O’Connell, A.; Tummon, A.; Coleman, K.; Jordan, A.; McCormack, J.; Kelly, M.E. Antenatal Pertussis Vaccination: Why are General Practitioners Reluctant? A Mixed Methods Study Setting. Ir. Med. J. 2017, 110, 634. [Google Scholar]
- Ugezu, C.; Essajee, M. Exploring patients’ awareness and healthcare professionals’ knowledge and attitude to pertussis and influenza vaccination during the antenatal periods in Cavan Monaghan General Hospital. Hum. Vaccines Immunother. 2018, 14, 978–983. [Google Scholar] [CrossRef]
- Cleary, B.J.; Rice, Ú.; Eogan, M.; Metwally, N.; McAuliffe, F. 2009 A/H1N1 influenza vaccination in pregnancy: Uptake and pregnancy outcomes—A historical cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, S.; Stephens, L.C.; Feemster, K.A.; Drew, R.J.; Eogan, M.; Butler, K.M. “this choice does not just affect me.” attitudes of pregnant women toward covid-19 vaccines: A mixed-methods study. Hum. Vaccines Immunother. 2021, 17, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Deleau, D.; Brophy, C.; McAuliffe, F.M.; Mahoney, R. Uptake of the Influenza Vaccination in Pregnancy. Ir. Med. J. 2016, 109, 449. [Google Scholar] [PubMed]
- Quattrocchi, A.; Mereckiene, J.; Fitzgerald, M.; Cotter, S. Determinants of influenza and pertussis vaccine uptake in pregnant women in Ireland: A cross-sectional survey in 2017/18 influenza season. Vaccine 2019, 37, 6390–6396. [Google Scholar] [CrossRef]
- Ceulemans, M.; Foulon, V.; Panchaud, A.; Winterfeld, U.; Pomar, L.; Lambelet, V.; Cleary, B.; O’shaughnessy, F.; Passier, A.; Richardson, J.L.; et al. Vaccine willingness and impact of the COVID-19 pandemic on women’s perinatal experiences and practices—A multinational, cross-sectional study covering the first wave of the pandemic. Int. J. Environ. Res. Public Health 2021, 18, 3367. [Google Scholar] [CrossRef]
- Hallissey, R.; O’Connell, A.; Warren, M. Factors that Influence Uptake of Vaccination in Pregnancy. Ir. Med J. 2018, 111, 713. [Google Scholar]
- Maisa, A.; Milligan, S.; Quinn, A.; Boulter, D.; Johnston, J.; Treanor, C.; Bradley, D.T. Vaccination against pertussis and influenza in pregnancy: A qualitative study of barriers and facilitators. PublicHealth 2018, 162, 111–117. [Google Scholar] [CrossRef]
- O'Shea, A.; Cleary, B.; McEntee, E.; Barrett, T.; O'Carroll, A.; Drew, R.; O’Reilly, F. To vaccinate or not to vaccinate? women’s perception of vaccination in pregnancy: A qualitative study. BJGP Open 2018, 2, bjgpopen18X101457. [Google Scholar] [CrossRef]
- Luteijn, J.M.; Dolk, H.; Marnoch, G.J. Differences in pandemic influenza vaccination policies for pregnant women in Europe. BMC Public Health 2011, 11, 819. [Google Scholar] [CrossRef]
- Staines, A.; Balanda, K.P.; Barron, S.; Corcoran, Y.; Fahy, L.; Gallagher, L.; Greally, T.; Kilroe, J.; Mohan, C.M.; Matthews, A.; et al. Child health care in Ireland. J. Pediatr. 2016, 177, S87–S106. [Google Scholar] [CrossRef]
- Health Service Executive. Tdap Vaccination During Pregnancy. 2020. Available online: https://www.hse.ie/eng/health/immunisation/hcpinfo/othervaccines/pertussis/ (accessed on 26 June 2024).
- Geoghegan, S.; O’Callaghan, K.P.; Offit, P.A. Vaccine safety: Myths and misinformation. Front. Microbiol. 2020, 11, 372. [Google Scholar] [CrossRef]
- Royal College of Physicians of Ireland. NIAC Immunisation Guidelines. Chapter 04. Immunisation and Health Information for Health Care Workers and Others in at Risk Populations. Available online: https://rcpi.access.preservica.com/uncategorized/ (accessed on 26 June 2024).
- Kilich, E.; Dada, S.; Francis, M.R.; Tazare, J.; Chico, R.M.; Paterson, P.; Larson, H.J. Factors that influence vaccination decision-making among pregnant women: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0234827. [Google Scholar] [CrossRef]
- Cronin, A.; Ibrahim, N. A scoping review of literature exploring factors affecting vaccine uptake within Roma communities across Europe. Expert Rev. Vaccines 2022, 21, 1429–1442. [Google Scholar] [CrossRef]
- Gauld, N.; Martin, S.; Sinclair, O.; Petousis-Harris, H.; Dumble, F.; Grant, C.C. A qualitative study of views and experiences of women and health care professionals about free maternal vaccinations administered at community pharmacies. Vaccines 2020, 8, 152. [Google Scholar] [CrossRef]
- Llamas, A.; Amirthalingam, G.; Andrews, N.; Edelstein, M. Delivering prenatal pertussis vaccine through maternity services in England: What is the impact on vaccine coverage? Vaccine 2020, 38, 5332–5336. [Google Scholar] [CrossRef]
- Howe, A.S.; Gauld, N.J.; Cavadino, A.Y.; Petousis-Harris, H.; Dumble, F.; Sinclair, O.; Grant, C.C. Increasing uptake of maternal pertussis vaccinations through funded administration in community pharmacies. Vaccines 2022, 10, 150. [Google Scholar] [CrossRef]
- Gesser-Edelsburg, A.; Shir-Raz, Y.; Hayek, S.; Aassaraf, S.; Lowenstein, L. Despite awareness of recommendations, why do health care workers not immunize pregnant women? Am. J. Infect. Control. 2017, 45, 436–439. [Google Scholar] [CrossRef]
- Regan, A.K.; Hauck, Y.; Nicolaou, L.; Engelbrecht, D.; Butt, J.; Mak, D.B.; Priest, R.; Cukierman, R.; Effler, P.V. Midwives’ knowledge, attitudes and learning needs regarding antenatal vaccination. Midwifery 2018, 62, 199–204. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on the Evaluation of Vaccine Purchase Financing in the United States. Financing Vaccines in the 21st Century: Assuring Access and Availability; National Academies Press (US): Washington, DC, USA, 2003. Available online: https://pubmed.ncbi.nlm.nih.gov/25057673/ (accessed on 26 June 2024). [CrossRef]
- MacDougall, D.M.; Halperin, S.A. Improving rates of maternal immunization: Challenges and opportunities. Hum. Vaccines Immunother. 2015, 12, 857–865. [Google Scholar] [CrossRef]
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 13–22. [Google Scholar] [CrossRef]
- McCormack, S.; Thompson, C.; Nolan, M.; Imcha, M.; Dee, A.; Saunders, J.; Philip, R.K. Maternal awareness, acceptability and willingness towards respiratory syncytial virus (RSV) vaccination during pregnancy in Ireland. Immun. Inflamm. Dis. 2024, 12, e1257. [Google Scholar] [CrossRef] [PubMed]
- McCarron, S.A.; Bradley, D.T.; Hart, N.D. A scoping review of the reasons for and approaches to non-uptake of pertussis and influenza vaccinations in pregnant women in the United Kingdom and Ireland. BMC Pregnancy Childbirth 2023, 23, 857. [Google Scholar] [CrossRef] [PubMed]
- Razai, M.S.; Mansour, R.; Ravindran, P.; Freeman, S.; Mason-Apps, C.; Morris, J.; Majeed, A.; Ussher, M.; Hargreaves, S.; Oakeshott, P. Facilitators and barriers to vaccination uptake in pregnancy: A qualitative systematic review. PLoS ONE 2024, 19, e0298407. [Google Scholar] [CrossRef]
- Kurasawa, K. Maternal vaccination—Current status, challenges, and opportunities. J. Obs. Gynaecol. Res. 2023, 49, 493–509. [Google Scholar] [CrossRef]
- Meaney-Delman, D.; Carroll, S.; Polen, K.; Jatlaoui, T.C.; Meyer, S.; Oliver, S.; Gee, J.; Shimabukuro, T.; Razzaghi, H.; Riley, L.; et al. Planning for the future of maternal immunization: Building on lessons learned from the COVID-19 pandemic. Vaccine 2024, 42, 125644. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Population: pregnant women or women of reproductive age | Studies conducted outside of Ireland. |
| Concept: factors influencing maternal vaccination | Studies focusing solely on non-pregnant women. |
| Context: Studies conducted in Ireland. | Animal studies, editorials, opinions, commentaries. |
| Publication types: peer-reviewed articles, freely accessible articles. | Studies not written in English. |
| Methodological approaches: quantitative, qualitative, mixed-method studies. | Studies with insufficient data or inadequate reporting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanni, A.; Ibrahim, N.; Tilley, D.; Bontha, S.; McMorrow, A.; Philip, R.K. Maternal Vaccination as an Integral Part of Life-Course Immunization: A Scoping Review of Uptake, Barriers, Facilitators, and Vaccine Hesitancy for Antenatal Vaccination in Ireland. Vaccines 2025, 13, 557. https://doi.org/10.3390/vaccines13060557
Sanni A, Ibrahim N, Tilley D, Bontha S, McMorrow A, Philip RK. Maternal Vaccination as an Integral Part of Life-Course Immunization: A Scoping Review of Uptake, Barriers, Facilitators, and Vaccine Hesitancy for Antenatal Vaccination in Ireland. Vaccines. 2025; 13(6):557. https://doi.org/10.3390/vaccines13060557
Chicago/Turabian StyleSanni, Adeyinka, Nuha Ibrahim, Dorothea Tilley, Sandra Bontha, Amy McMorrow, and Roy K. Philip. 2025. "Maternal Vaccination as an Integral Part of Life-Course Immunization: A Scoping Review of Uptake, Barriers, Facilitators, and Vaccine Hesitancy for Antenatal Vaccination in Ireland" Vaccines 13, no. 6: 557. https://doi.org/10.3390/vaccines13060557
APA StyleSanni, A., Ibrahim, N., Tilley, D., Bontha, S., McMorrow, A., & Philip, R. K. (2025). Maternal Vaccination as an Integral Part of Life-Course Immunization: A Scoping Review of Uptake, Barriers, Facilitators, and Vaccine Hesitancy for Antenatal Vaccination in Ireland. Vaccines, 13(6), 557. https://doi.org/10.3390/vaccines13060557







