The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review
Abstract
1. Introduction
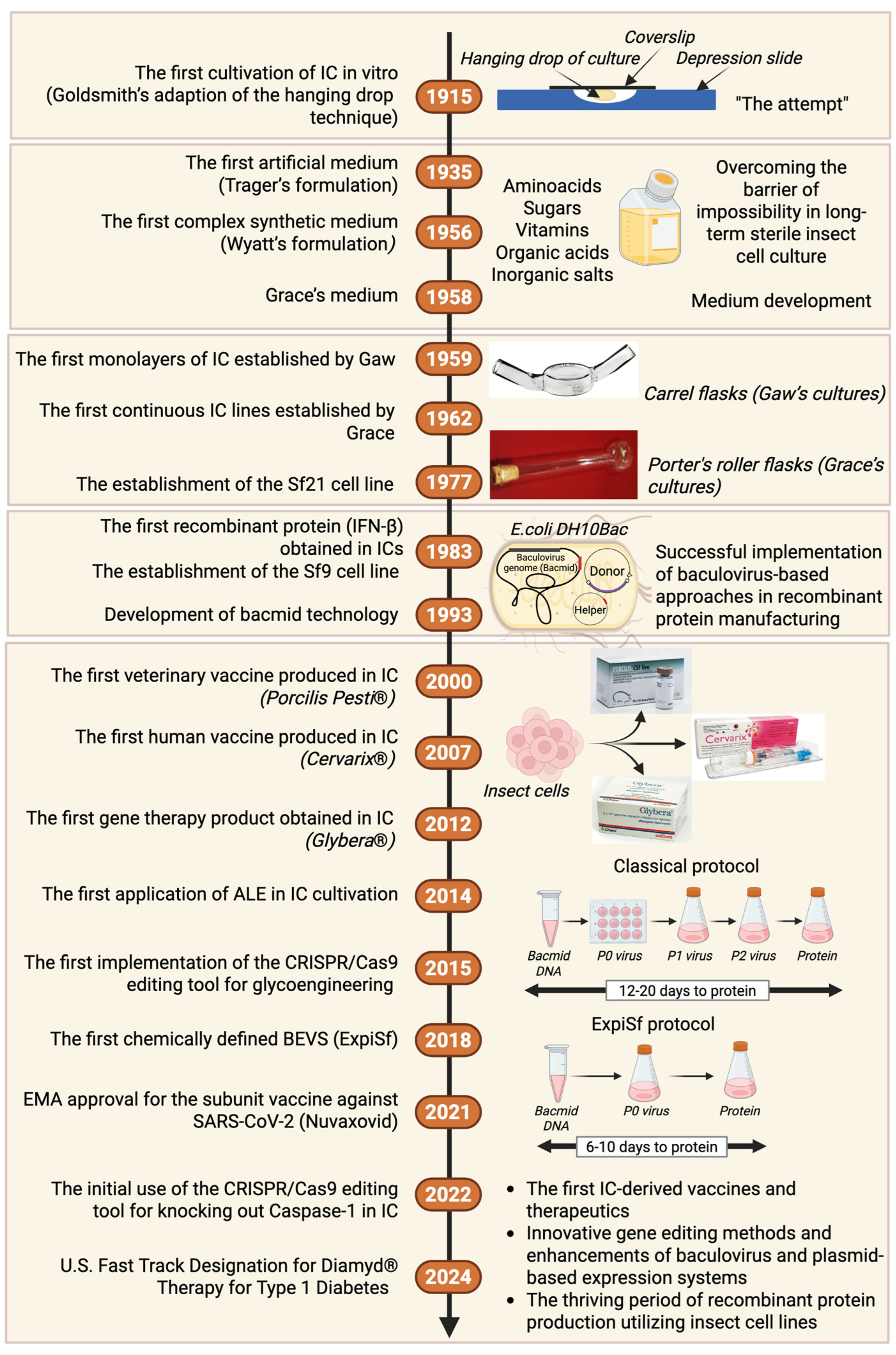
2. History of Insect Cell Culture Development and Their Initial Utilization in Protein Recombination Studies
3. Different Approaches for Recombinant Molecule Expression in Insect Cell Lines
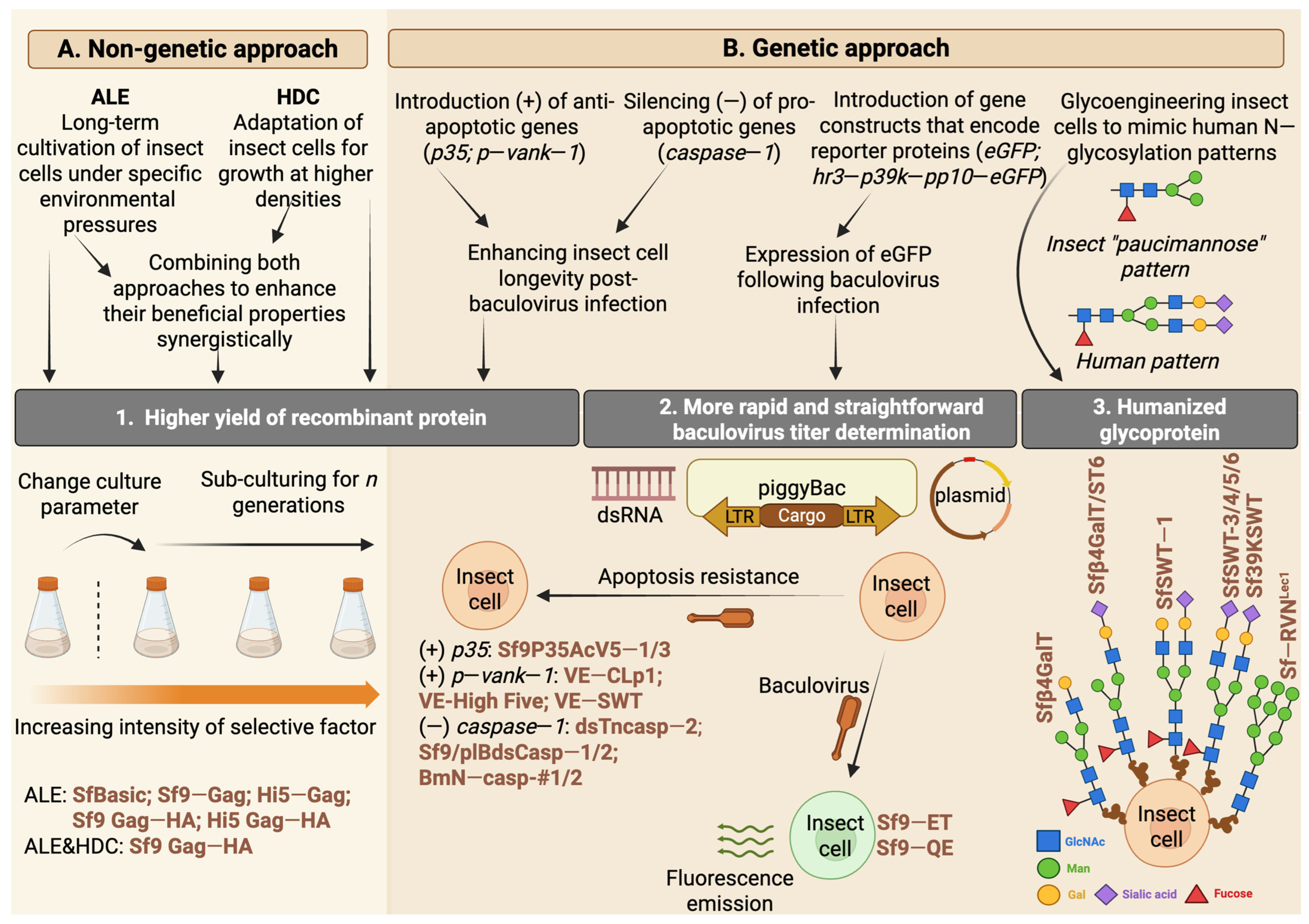
4. Strategies for Enhancement of Biomanufacturing with Insect Cell Lines
4.1. Reducing Cell Debris Contamination and Enhancing Glycosylation Patterns—Baculovirus Modification Techniques
4.2. Enhancing the Survival of Insect Cell Cultures Following Baculovirus Infection
4.3. Improving the Ease of Working with Insect Cell BEVSs
4.4. The Implementation of the Adaptive Laboratory Evolution Approach in the Cultivation of Insect Cells
4.5. Glycoengineering of Insect Cell Lines
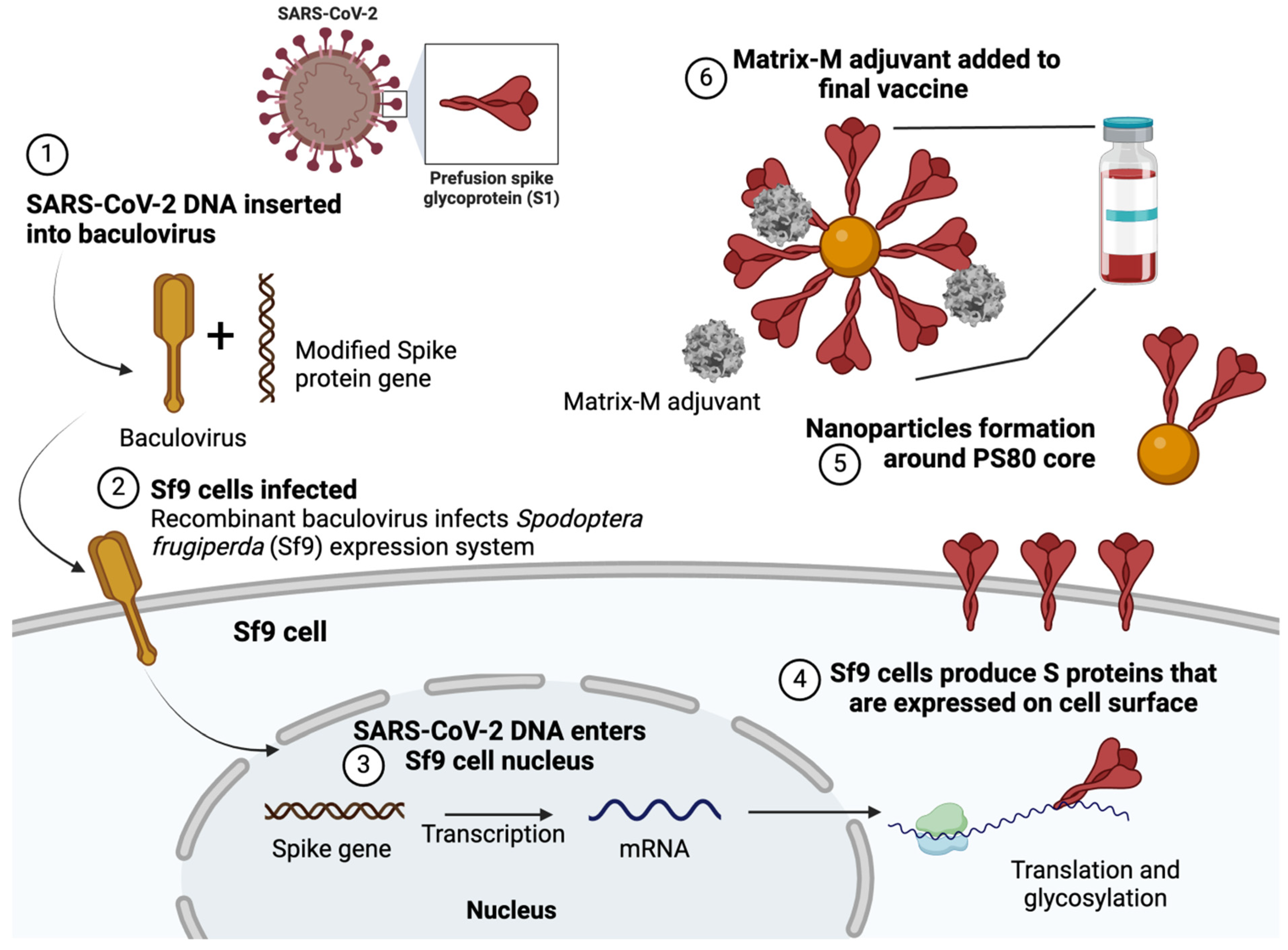
5. The Application of BEVS Technology in the Production of Vaccines and Therapeutics
6. Pros and Cons of BEVSs in the Production of Recombinant Proteins and Vaccines
7. Limitations and Challenges in Implementing Insect Expression Systems for Biopharmaceutical Production
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Pham, P.V. Medical Biotechnology: Techniques and Applications. In Omics Technologies and Bio-Engineering: Towards Improving Quality of Life; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 449–469. [Google Scholar] [CrossRef]
- Singh, M.; Gupta, S.; Rawat, A.K.; Singh, S.K. Advances in Heterologous Protein Expression Strategies in Yeast and Insect Systems. In Advances in Protein Molecular and Structural Biology Methods; Academic Press: Cambridge, MA, USA, 2022; pp. 13–30. [Google Scholar] [CrossRef]
- Tokmakov, A.A.; Kurotani, A.; Takagi, T.; Toyama, M.; Shirouzu, M.; Fukami, Y.; Yokoyama, S. Multiple Post-Translational Modifications Affect Heterologous Protein Synthesis. J. Biol. Chem. 2012, 287, 27106. [Google Scholar] [CrossRef] [PubMed]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant Protein Production in Yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar] [CrossRef]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Harrison, R.G. Observations on the Living Developing Nerve Fiber. Proc. Soc. Exp. Biol. Med. 1907, 4, 140–143. [Google Scholar] [CrossRef]
- Jay, V. The Legacy of Robert Koch. Arch. Pathol. Lab. Med. 2001, 125, 1148–1149. [Google Scholar] [CrossRef]
- Carrel, A. On the permanent life of tissues outside of the organism. J. Exp. Med. 1912, 15, 516. [Google Scholar] [CrossRef]
- Carrel, A.; Burrows, M.T. Cultivation of tissues in vitro and its technique. J. Exp. Med. 1911, 13, 387. [Google Scholar] [CrossRef]
- Goldschmidt, R. Some Experiments on Spermatogenesis in Vitro. Proc. Natl. Acad. Sci. USA 1915, 1, 220–222. [Google Scholar] [CrossRef][Green Version]
- Day, M.F.; Grace, T.D.C. Culture of Insect Tissues. Annu. Rev. Entomol. 1959, 4, 17–38. [Google Scholar] [CrossRef]
- Glaser, R.W. The Growth of Insect Blood Cells in Vitro. Psyche 1917, 24, 1–6. [Google Scholar] [CrossRef]
- Trager, W. Cultivation of the virus of grasserie in silkworm tissue cultures. J. Exp. Med. 1935, 61, 501. [Google Scholar] [CrossRef] [PubMed]
- Trager, W. Multiplication of the Virus of Equine Encephalomyelitis in Surviving Mosquito Tissues. Am. J. Trop. Med. 1938, 1–18, 387–393. [Google Scholar] [CrossRef]
- Wyatt, S.S. Culture in vitro of tissue from the silkworm, Bombyx mori L. J. Gen. Physiol. 1956, 39, 841. [Google Scholar] [CrossRef]
- Gaw, Z.Y.; Liu, N.; Zia, T.U. Tissue Culture Methods for Cultivation of Virus Grasserie. Acta Virol. 1959, 3, 55–60. [Google Scholar]
- Grace, T.D.C. Establishment of Four Strains of Cells from Insect Tissues Grown in Vitro. Nature 1962, 195, 788–789. [Google Scholar] [CrossRef]
- Grace, T.D.C. Development of Insect Cell Culture. In Invertebrate Cell Culture Applications; Maramorosch, K., Ed.; Academic Press: New York, NY, USA, 1982; pp. 1–8. [Google Scholar]
- Grace, T.D.C. Establishment of a Line of Mosquito (Aedes aegypti L.) Cells Grown in Vitro. Nature 1966, 211, 366–367. [Google Scholar] [CrossRef]
- Grace, T.D.C. Effect of Various Substances on Growth of Silkworm Tissues in Vitro. Aust. J. Biol. Sci. 1958, 2, 407–417. [Google Scholar] [CrossRef]
- The Rockefeller University. Keith Porter’s Roller Flasks for Tissue Culture. Unseen World Exhibit. Available online: https://digitalcommons.rockefeller.edu/unseen-world/15/ (accessed on 27 February 2025).
- National Museum of American History. Carrel Flask. Available online: https://americanhistory.si.edu/collections/object/nmah_1111991 (accessed on 27 February 2025).
- MSD Animal Health. Porcilis® PCV. Available online: https://www.msd-animal-health.ph/products/porcilis-csf-live/ (accessed on 20 May 2025).
- MIMS Indonesia. Cervarix. Available online: https://www.mims.com/indonesia/drug/info/cervarix?type=full (accessed on 27 February 2025).
- Fierce Pharma. Can Other Gene Therapy Developers Avoid Glybera’s Fate? Available online: https://www.fiercepharma.com/pharma/glybera-s-a-flop-how-can-other-gene-therapy-developers-avoid-fate (accessed on 27 February 2025).
- Thermo Fisher Scientific. ExpiSfTM Expression System Starter Kit. Available online: https://www.thermofisher.com/order/catalog/product/A38841 (accessed on 27 February 2025).
- Chiu, R.J.; Black, L.M. Monolayer Cultures of Insect Cell Lines and Their Inoculation with a Plant Virus. Nature 1967, 215, 1076–1078. [Google Scholar] [CrossRef]
- Vail, P.V.; Sutter, G.; Jay, D.L.; Gough, D. Reciprocal Infectivity of Nuclear Polyhedrosis Viruses of the Cabbage Looper and Alfalfa Looper. J. Invertebr. Pathol. 1971, 17, 383–388. [Google Scholar] [CrossRef]
- Jackson, D.A.; Symons, R.H.; Berg, P. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.Y.; Boyer, H.W.; Helling, R.B. Construction of Biologically Functional Bacterial Plasmids In Vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240. [Google Scholar] [CrossRef] [PubMed]
- Itakura, K.; Hirose, T.; Crea, R.; Riggs, A.D.; Heyneker, H.L.; Bolivar, F.; Boyer, H.W. Expression in Escherichia coli of a Chemically Synthesized Gene for the Hormone Somatostatin. Science (1979) 1977, 198, 1056–1063. [Google Scholar] [CrossRef]
- Vaughn, J.L.; Goodwin, R.H.; Tompkins, G.J.; McCawley, P. The Establishment of Two Cell Lines from the Insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 1977, 13, 213–217. [Google Scholar] [CrossRef]
- Goodwin, R.H.; Vaughn, J.L.; Adams, J.R.; Louloudes, S.J. Replication of a Nuclear Polyhedrosis Virus in an Established Insect Cell Line. J. Invertebr. Pathol. 1970, 16, 284–288. [Google Scholar] [CrossRef]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of Human Beta Interferon in Insect Cells Infected with a Baculovirus Expression Vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar]
- Smith, G.E.; Fraser, M.J.; Summers, M.D. Molecular Engineering of the Autographa californica Nuclear Polyhedrosis Virus Genome: Deletion Mutations Within the Polyhedrin Gene. J. Virol. 1983, 46, 584. [Google Scholar] [CrossRef]
- Goeddel, D.V.; Shepard, H.M.; Elizabeth, Y.; Leung, D.; Crea, R.; Sloma, A.; Pestka, S. Synthesis of Human Fibroblast Interferon by E. coli. Nucleic Acids Res. 1980, 8, 4057–4074. [Google Scholar] [CrossRef][Green Version]
- Mitrani Rosenbaum, S.; Maroteaux, L.; Mory, Y.; Revel, M.; Howley, P.M. Inducible Expression of the Human Interferon P, Gene Linked to a Bovine Papilloma Virus DNA Vector and Maintained Extrachromosomally in Mouse Cells. Mol. Cell. Biol. 1983, 3, 233–240. [Google Scholar]
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N.; et al. Genetic Engineering of Baculovirus-Insect Cell System to Improve Protein Production. Front. Bioeng. Biotechnol. 2022, 10, 994743. [Google Scholar] [CrossRef]
- ATCC. Sf9 CRL-1711TM Cell Line. Available online: https://www.atcc.org/products/crl-1711 (accessed on 27 February 2025).
- Wickham, T.J.; Davis, T.; Granados, R.R.; Shuler, M.L.; Wood, H.A. Screening of Insect Cell Lines for the Production of Recombinant Proteins and Infectious Virus in the Baculovirus Expression System. Biotechnol. Prog. 1992, 8, 391–396. [Google Scholar] [CrossRef]
- Maghodia, A.B.; Geisler, C.; Jarvis, D.L. A New Nodavirus-Negative T. Ni Cell Line for Baculovirus-Mediated Protein Production. Biotechnol. Bioeng. 2020, 117, 3248. [Google Scholar] [CrossRef] [PubMed]
- Cellosaurus. Search Results for “Insect Cell Line”. Available online: https://www.cellosaurus.org/search?query=insect+cell+line (accessed on 27 February 2025).
- He, X.; Lu, L.; Huang, P.; Yu, B.; Peng, L.; Zou, L.; Ren, Y. Insect Cell-Based Models: Cell Line Establishment and Application in Insecticide Screening and Toxicology Research. Insects 2023, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Fabre, L.L.; Arrías, P.N.; Masson, T.; Pidre, M.L.; Romanowski, V. Baculovirus-Derived Vectors for Immunization and Therapeutic Applications. In Emerging and Reemerging Viral Pathogens; Ennaji, M.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; p. 197. [Google Scholar] [CrossRef]
- Calles, K.; Svensson, I.; Lindskog, E.; Häggström, L. Effects of Conditioned Medium Factors and Passage Number on Sf9 Cell Physiology and Productivity. Biotechnol. Prog. 2006, 22, 394–400. [Google Scholar] [CrossRef]
- Cox, M.M.J. Innovations in the Insect Cell Expression System for Industrial Recombinant Vaccine Antigen Production. Vaccines 2021, 9, 1504. [Google Scholar] [CrossRef]
- Yee, C.M.; Zak, A.J.; Hill, B.D.; Wen, F. The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics. Ind. Eng. Chem. Res. 2018, 57, 10061. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The Baculovirus Expression Vector System: A Commercial Manufacturing Platform for Viral Vaccines and Gene Therapy Vectors. Biotechnol. J. 2015, 10, 702. [Google Scholar] [CrossRef]
- Luckow, V.A.; Lee, S.C.; Barry, G.F.; Olins’, P.O. Efficient Generation of Infectious Recombinant Baculoviruses by Site-Specific Transposon-Mediated Insertion of Foreign Genes into a Baculovirus Genome Propagated in Escherichia coli. J. Virol. 1993, 67, 4566. [Google Scholar] [CrossRef]
- Mamnoon, B.; Farivar, T.N.; Kamyab, A.R.; Ilghari, D.; Khamesipour, A.; Arzenani, M.K. Quality Control of Widely Used Therapeutic Recombinant Proteins by a Novel Real-Time PCR Approach. Iran. Biomed. J. 2016, 20, 56. [Google Scholar] [CrossRef]
- Oxford Expression TechnologiesTM. FlashBACTM Protocol. Available online: https://www.oetltd.com/product-page/flashbac-fb (accessed on 27 February 2025).
- Kitts, P.A.; Ayres, M.D.; Possee, R.D. Linearization of Baculovirus DNA Enhances the Recovery of Recombinant Virus Expression Vectors. Nucleic Acids Res. 1990, 18, 5667–5672. [Google Scholar] [CrossRef]
- Sigma-Aldrich. DiamondBacTM Baculovirus DNA Protocol. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/233/703/d6192bul.pdf?srsltid=AfmBOoq5IMjfA_EL6hDmCZZVuXSnOETDHO1y7YKwxDj1W0gTj6PHCojT (accessed on 27 February 2025).
- Thermo Fisher Scientific. PIB/V5-His Vector Kit Protocol. Available online: https://www.thermofisher.com/order/catalog/product/V802001 (accessed on 27 February 2025).
- Thermo Fisher Scientific. PIZ/V5-His Vector Kit Protocol. Available online: https://www.thermofisher.com/order/catalog/product/V800001 (accessed on 27 February 2025).
- Thermo Fisher Scientific. PMIB/V5-His Vector Kit Protocol. Available online: https://www.thermofisher.com/order/catalog/product/V803001 (accessed on 27 February 2025).
- Thermo Fisher Scientific. PIZT/V5-His Vector Kit Protocol. Available online: https://www.thermofisher.com/order/catalog/product/V801001 (accessed on 27 February 2025).
- Yang, Y.; Zhang, B.; Yong, J.; James, P.; Xu, Z.P.; Mitter, N.; Mahony, T.J.; Mody, K.T. The Use of Cell and Larval Assays to Identify Target Genes for RNA Interference-Meditated Control of the Australian Sheep Blowfly (Lucilia cuprina). Pest Manag. Sci. 2024, 80, 4686–4698. [Google Scholar] [CrossRef]
- Kaipa, J.M.; Krasnoselska, G.; Owens, R.J.; van den Heuvel, J. Screening of Membrane Protein Production by Comparison of Transient Expression in Insect and Mammalian Cells. Biomolecules 2023, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, J.; Mitra, K.; Giri, L. Nonviral Platform for Expression of Recombinant Protein in Insect Cells. Methods Mol. Biol. 2024, 2829, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, M.; Fritz, M.H.Y.; Bhuju, S.; Jarek, M.; Schürig, M.; Geffers, R.; Benes, V.; Besir, H.; Van Den Heuvel, J.; Li, Y. Genomic Analysis and Isolation of RNA Polymerase II Dependent Promoters from Spodoptera Frugiperda. PLoS ONE 2015, 10, e0132898. [Google Scholar] [CrossRef]
- Lampinen, V.; Gröhn, S.; Lehmler, N.; Jartti, M.; Hytönen, V.P.; Schubert, M.; Hankaniemi, M.M. Production of Norovirus-, Rotavirus-, and Enterovirus-like Particles in Insect Cells Is Simplified by Plasmid-Based Expression. Sci. Rep. 2024, 14, 14874. [Google Scholar] [CrossRef]
- Yovcheva, M.; Thompson, K.W. Optimized Workflow from Gene to Product. Methods Mol. Biol. 2024, 2829, 49–66. [Google Scholar] [CrossRef]
- Mi, Y.; Xie, T.; Zhu, B.; Tan, J.; Li, X.; Luo, Y.; Li, F.; Niu, H.; Han, J.; Lv, W.; et al. Production of SARS-CoV-2 Virus-Like Particles in Insect Cells. Vaccines 2021, 9, 554. [Google Scholar] [CrossRef]
- Kurasawa, J.H.; Park, A.; Sowers, C.R.; Halpin, R.A.; Tovchigrechko, A.; Dobson, C.L.; Schmelzer, A.E.; Gao, C.; Wilson, S.D.; Ikeda, Y. Chemically Defined, High-Density Insect Cell-Based Expression System for Scalable AAV Vector Production. Mol. Ther. Methods Clin. Dev. 2020, 19, 330–340. [Google Scholar] [CrossRef]
- Kim, K.; Hahn, T.W. Evaluation of Novel Recombinant Porcine Circovirus Type 2d (PCV2d) Vaccine in Pigs Naturally Infected with PCV2d. Vaccine 2021, 39, 529–535. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Chroboczek, J. Virus-like Particles as Drug Delivery Vectors. Acta Biochim. Pol. 2016, 63, 469–473. [Google Scholar] [CrossRef]
- Ruzzi, F.; Semprini, M.S.; Scalambra, L.; Palladini, A.; Angelicola, S.; Cappello, C.; Pittino, O.M.; Nanni, P.; Lollini, P.L. Virus-like Particle (VLP) Vaccines for Cancer Immunotherapy. Int. J. Mol. Sci. 2023, 24, 12963. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, H.; Deng, F. Advances and Challenges in Enveloped Virus-like Particle (VLP)-Based Vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar] [CrossRef]
- Badruzzaman, A.T.M.; Cheng, Y.; Aloysius, I.; De Lejarazu, R.O.; Sanz Muñoz, I.; Badruzzaman, A.T.M.; Cheng, Y.-C.; Sung, W.-C.; Lee, M.-S. Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice. Vaccines 2024, 12, 667. [Google Scholar] [CrossRef]
- de Mello, R.G.; Bernardino, T.C.; Guardalini, L.G.O.; Astray, R.M.; Antoniazzi, M.M.; Jared, S.G.S.; Núñez, E.G.F.; Jorge, S.A.C. Zika Virus-like Particles (VLPs) Produced in Insect Cells. Front. Pharmacol. 2023, 14, 1181566. [Google Scholar] [CrossRef]
- Bissett, S.L.; Roy, P. Multi-Gene Recombinant Baculovirus Expression Systems: From Inception to Contemporary Applications. Viruses 2024, 16, 492. [Google Scholar] [CrossRef]
- Kim, H.S.; Moon, H.J.; Choi, J.B.; Han, B.K.; Woo, S.D. Efficient Production of Enterovirus 71 (EV71) Virus-like Particles by Controlling Promoter Strength in Insect Cells. Viruses 2024, 16, 834. [Google Scholar] [CrossRef]
- Matsuda, T.; Tanijima, T.; Hirose, A.; Masumi-Koizumi, K.; Katsuda, T.; Yamaji, H. Production of Influenza Virus-like Particles Using Recombinant Insect Cells. Biochem. Eng. J. 2020, 163, 107757. [Google Scholar] [CrossRef]
- Bruder, M.R.; Aucoin, M.G. Utility of Alternative Promoters for Foreign Gene Expression Using the Baculovirus Expression Vector System. Viruses 2022, 14, 2670. [Google Scholar] [CrossRef]
- Van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty Years of Baculovirus-Insect Cell Protein Expression: From Dark Horse to Mainstream Technology. J. Gen. Virol. 2015, 96, 6–23. [Google Scholar] [CrossRef]
- Hodgson, J.J.; Krell, P.J.; Passarelli, A.L. Mature Viral Cathepsin Is Required for Release of Viral Occlusion Bodies from Autographa californica Multiple Nucleopolyhedrovirus-Infected Cells. Virology 2021, 556, 23–32. [Google Scholar] [CrossRef]
- Kaba, S.A.; Salcedo, A.M.; Wafula, P.O.; Vlak, J.M.; Van Oers, M.M. Development of a Chitinase and V-Cathepsin Negative Bacmid for Improved Integrity of Secreted Recombinant Proteins. J. Virol. Methods 2004, 122, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Brown, H.L.; Hawes, C.R.; Lee, B.Y.; Min, M.-K.; King, L.A.; Possee, R.D. Localization of a Baculovirus-Induced Chitinase in the Insect Cell Endoplasmic Reticulum. J. Virol. 1998, 72, 10207–10212. [Google Scholar] [CrossRef]
- Berger, I.; Garzoni, F.; Chaillet, M.; Haffke, M.; Gupta, K.; Aubert, A. The MultiBac Protein Complex Production Platform at the EMBL. J. Vis. Exp. 2013, 77, 50159. [Google Scholar] [CrossRef]
- Crocker, H.L. Baculoviral Nanosystems for DNA Delivery and Energy Sensing. Ph.D. Thesis, University of Bristol, Bristol, UK, 2020. [Google Scholar]
- Expression Systems BestBacTM Protocol. Available online: https://expressionsystems.com/wp-content/uploads/2017/03/BestBac-IFU-Rev-2.pdf (accessed on 27 February 2025).
- Zhang, X.; He, A.; Zong, Y.; Tian, H.; Zhang, Z.; Zhao, K.; Xu, X.; Chen, H. Improvement of Protein Production in Baculovirus Expression Vector System by Removing a Total of 10 Kb of Nonessential Fragments from Autographa californica Multiple Nucleopolyhedrovirus Genome. Front. Microbiol. 2023, 14, 1171500. [Google Scholar] [CrossRef]
- Martínez-Solís, M.; Gómez-Sebastián, S.; Escribano, J.M.; Jakubowska, A.K.; Herrero, S. A Novel Baculovirus-Derived Promoter with High Activity in the Baculovirus Expression System. PeerJ 2016, 2016, e2183. [Google Scholar] [CrossRef]
- Lee, J.H.; Gwak, W.S.; Bae, S.M.; Choi, J.B.; Han, B.K.; Woo, S.D. Increased Productivity of the Baculovirus Expression Vector System by Combining Enhancing Factors. J. Asia Pac. Entomol. 2018, 21, 1079–1084. [Google Scholar] [CrossRef]
- Kim, H.S.; Gwak, W.S.; Bae, J.S.; Kim, T.H.; Choi, J.B.; Han, B.K.; Woo, S.D. Effects of Repeated Burst Sequences on the P10 Promoter Activity of Bombyx mori Nucleopolyhedrovirus. J. Asia Pac. Entomol. 2021, 24, 7–13. [Google Scholar] [CrossRef]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Encyclopedia of Cell Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; Volume 2, pp. 487–494. [Google Scholar]
- Wagner, R.; Liedtke, S.; Kretzschmar, E.; Geyer, H.; Geyer, R.; Klenk, H.D. Elongation of the N-Glycans of Fowl Plague Virus Hemagglutinin Expressed in Spodoptera frugiperda (Sf9) Cells by Coexpression of Human Beta 1,2-N-Acetylglucosaminyltransferase I. Glycobiology 1996, 6, 165–175. [Google Scholar] [CrossRef]
- Hollister, J.R.; Shaper, J.H.; Jarvis, D.L. Stable Expression of Mammalian Beta 1,4-Galactosyltransferase Extends the N-Glycosylation Pathway in Insect Cells. Glycobiology 1998, 8, 473–480. [Google Scholar] [CrossRef]
- Palmberger, D.; Wilson, I.B.H.; Berger, I.; Grabherr, R.; Rendic, D. SweetBac: A New Approach for the Production of Mammalianised Glycoproteins in Insect Cells. PLoS ONE 2012, 7, e34226. [Google Scholar] [CrossRef]
- Palmberger, D.; Klausberger, M.; Berger, I.; Grabherr, R. MultiBac Turns Sweet. Bioengineered 2013, 4, 78. [Google Scholar] [CrossRef] [PubMed]
- Gleixner, J.; Kopanchuk, S.; Grätz, L.; Tahk, M.J.; Laasfeld, T.; Veikšina, S.; Höring, C.; Gattor, A.O.; Humphrys, L.J.; Müller, C.; et al. Illuminating Neuropeptide Y Y4 Receptor Binding: Fluorescent Cyclic Peptides with Subnanomolar Binding Affinity as Novel Molecular Tools. ACS Pharmacol. Transl. Sci. 2024, 7, 1142–1168. [Google Scholar] [CrossRef] [PubMed]
- George Kamita, S.; Majima, K.; Maeda, S. Identification and Characterization of the P35 Gene of Bombyx mori Nuclear Polyhedrosis Virus That Prevents Virus-Induced Apoptosis. J. Virol. 1993, 67, 455. [Google Scholar] [CrossRef]
- Cartier, J.L.; Hershberger, P.A.; Friesen, P.D. Suppression of Apoptosis in Insect Cells Stably Transfected with Baculovirus P35: Dominant Interference by N-Terminal Sequences P35(1-76). J. Virol. 1994, 68, 7728. [Google Scholar] [CrossRef]
- Lin, G.; Li, G.; Granados, R.R.; Blissard, G.W. Stable Cell Lines Expressing Baculovirus P35: Resistance to Apoptosis and Nutrient Stress, and Increased Glycoprotein Secretion. In Vitro Cell. Dev. Biol. Anim. 2001, 37, 293–302. [Google Scholar] [CrossRef]
- Fath-Goodin, A.; Kroemer, J.A.; Webb, B.A. The Campoletis Sonorensis Ichnovirus Vankyrin Protein P-Vank-1 Inhibits Apoptosis in Insect Sf9 Cells. Insect Mol. Biol. 2009, 18, 497–506. [Google Scholar] [CrossRef]
- Fath-Goodin, A.; Kroemer, J.; Martin, S.; Reeves, K.; Webb, B.A. Polydnavirus Genes That Enhance the Baculovirus Expression Vector System. Adv. Virus Res. 2006, 68, 75–90. [Google Scholar] [CrossRef]
- Steele, K.H.; Stone, B.J.; Franklin, K.M.; Fath-Goodin, A.; Zhang, X.; Jiang, H.; Webb, B.A.; Geisler, C. Improving the Baculovirus Expression Vector System with Vankyrin-Enhanced Technology. Biotechnol. Prog. 2017, 33, 1496–1507. [Google Scholar] [CrossRef]
- March, J.C.; Bentley, W.E. Engineering Eukaryotic Signal Transduction with RNAi: Enhancing Drosophila S2 Cell Growth and Recombinant Protein Synthesis via Silencing of TSC1. Biotechnol. Bioeng. 2006, 95, 645–652. [Google Scholar] [CrossRef]
- Hebert, C.G.; Valdes, J.J.; Bentley, W.E. In Vitro and in Vivo RNA Interference Mediated Suppression of Tn-Caspase-1 for Improved Recombinant Protein Production in High Five Cell Culture with the Baculovirus Expression Vector System. Biotechnol. Bioeng. 2009, 104, 390–399. [Google Scholar] [CrossRef]
- Lai, Y.K.; Hsu, J.T.A.; Chu, C.C.; Chang, T.Y.; Pan, K.L.; Lin, C.C. Enhanced Recombinant Protein Production and Differential Expression of Molecular Chaperones in Sf-Caspase-1-Repressed Stable Cells after Baculovirus Infection. BMC Biotechnol. 2012, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.; Chen, K.; Ju, X. Suppression of Bm-Caspase-1 Expression in BmN Cells Enhances Recombinant Protein Production in a Baculovirus Expression Vector System. Mol. Biotechnol. 2016, 58, 319–327. [Google Scholar] [CrossRef]
- Naik, N.G.; Lo, Y.W.; Wu, T.Y.; Lin, C.C.; Kuo, S.C.; Chao, Y.C. Baculovirus as an Efficient Vector for Gene Delivery into Mosquitoes. Sci. Rep. 2018, 8, 17778. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Siaterli, E.A.; Nixon, C.P.; King, L.A. Quantitative Real-Time PCR for Rapid and Accurate Titration of Recombinant Baculovirus Particles. Biotechnol. Bioeng. 2007, 96, 810–814. [Google Scholar] [CrossRef]
- Lothert, K.; Bagrin, E.; Wolff, M.W. Evaluating Novel Quantification Methods for Infectious Baculoviruses. Viruses 2023, 15, 998. [Google Scholar] [CrossRef]
- Luo, N.; Chen, X.; Li, J.; Huynh, D.; Li, Y.; Ou, L.; Liu, S. Evaluation of Recombinant Baculovirus Clearance during RAAV Production in Sf9 Cells Using a Newly Developed Fluorescent-TCID50 Assay. Front. Med. 2024, 11, 1302648. [Google Scholar] [CrossRef]
- Hopkins, R.F.; Esposito, D. A Rapid Method for Titrating Baculovirus Stocks Using the Sf-9 Easy Titer Cell Line. Biotechniques 2009, 47, 785–788. [Google Scholar] [CrossRef]
- Lemaitre, R.P.; Bogdanova, A.; Borgonovo, B.; Woodruff, J.B.; Drechsel, D.N. FlexiBAC: A Versatile, Open-Source Baculovirus Vector System for Protein Expression, Secretion, and Proteolytic Processing. BMC Biotechnol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Guardalini, L.G.O.; Cavalcante, P.E.d.S.; Leme, J.; de Mello, R.G.; Bernardino, T.C.; Jared, S.G.S.; Antoniazzi, M.M.; Astray, R.M.; Tonso, A.; Fernández Núñez, E.G.; et al. Performance Comparison of Recombinant Baculovirus and Rabies Virus-like Particles Production Using Two Culture Platforms. Vaccines 2023, 11, 39. [Google Scholar] [CrossRef]
- Kim, K.-S.; Bae, J.-S.; Moon, H.-J.; Kim, D.-Y.; Woo, S.-D. A Novel Transgenic Sf9 Cell Line for Quick and Easy Virus Quantification. Insects 2024, 15, 686. [Google Scholar] [CrossRef]
- Wagner, J.M.; Pajerowski, J.D.; Daniels, C.L.; McHugh, P.M.; Flynn, J.A.; Balliet, J.W.; Casimiro, D.R.; Subramanian, S. Enhanced Production of Chikungunya Virus-Like Particles Using a High-PH Adapted Spodoptera frugiperda Insect Cell Line. PLoS ONE 2014, 9, e94401. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Fernandes, B.; Alves, P.M.; Carrondo, M.J.T.; Roldão, A. Improving Influenza HA-Vlps Production in Insect High Five Cells via Adaptive Laboratory Evolution. Vaccines 2020, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Vidigal, J.; Correia, R.; Carrondo, M.J.T.; Alves, P.M.; Teixeira, A.P.; Roldão, A. Adaptive Laboratory Evolution of Stable Insect Cell Lines for Improved HIV-Gag VLPs Production. J. Biotechnol. 2020, 307, 139–147. [Google Scholar] [CrossRef]
- Fernandes, B.; Correia, R.; Sousa, M.; Carrondo, M.J.T.; Alves, P.M.; Roldão, A. Integrating High Cell Density Cultures with Adapted Laboratory Evolution for Improved Gag-HA Virus-like Particles Production in Stable Insect Cell Lines. Biotechnol. Bioeng. 2021, 118, 2536–2547. [Google Scholar] [CrossRef]
- Fernandes, B.; Correia, R.; Alves, P.M.; Roldão, A. Intensifying Continuous Production of Gag-HA VLPs at High Cell Density Using Stable Insect Cells Adapted to Low Culture Temperature. Front. Bioeng. Biotechnol. 2022, 10, 917746. [Google Scholar] [CrossRef]
- Correia, R.; Fernandes, B.; Alves, P.M.; Roldão, A. Adaptive Laboratory Evolution to Improve Recombinant Protein Production Using Insect Cells. Methods Mol. Biol. 2024, 2829, 79–90. [Google Scholar] [CrossRef]
- Hollister, J.R.; Jarvis, D.L. Engineering Lepidopteran Insect Cells for Sialoglycoprotein Production by Genetic Transformation with Mammalian Beta 1,4-Galactosyltransferase and Alpha 2,6-Sialyltransferase Genes. Glycobiology 2001, 11, 1–9. [Google Scholar] [CrossRef]
- Hollister, J.; Grabenhorst, E.; Nimtz, M.; Conradt, H.; Jarvis, D.L. Engineering the Protein N-Glycosylation Pathway in Insect Cells for Production of Biantennary, Complex N-Glycans. Biochemistry 2002, 41, 15093. [Google Scholar] [CrossRef]
- Aumiller, J.J.; Hollister, J.R.; Jarvis, D.L. A Transgenic Insect Cell Line Engineered to Produce CMP–Sialic Acid and Sialylated Glycoproteins. Glycobiology 2003, 13, 497. [Google Scholar] [CrossRef]
- Aumiller, J.J.; Mabashi-Asazuma, H.; Hillar, A.; Shi, X.; Jarvis, D.L. A New Glycoengineered Insect Cell Line with an Inducibly Mammalianized Protein N-Glycosylation Pathway. Glycobiology 2012, 22, 417–428. [Google Scholar] [CrossRef]
- Mabashi-Asazuma, H.; Shi, X.; Geisler, C.; Kuo, C.W.; Khoo, K.H.; Jarvis, D.L. Impact of a Human CMP-Sialic Acid Transporter on Recombinant Glycoprotein Sialylation in Glycoengineered Insect Cells. Glycobiology 2013, 23, 199. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.M.; Kuo, C.W.; Khoo, K.H.; Jarvis, D.L. A New Insect Cell Glycoengineering Approach Provides Baculovirus-Inducible Glycogene Expression and Increases Human-Type Glycosylation Efficiency. J. Biotechnol. 2014, 182–183, 19–29. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z. CRISPR-Cas Systems: Overview, Innovations and Applications in Human Disease Research and Gene Therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas Systems Target Endogenous Genes to Impact Bacterial Physiology and Alter Mammalian Immune Responses. Mol. Biomed. 2022, 3, 22. [Google Scholar] [CrossRef]
- Schubert, M.S.; Thommandru, B.; Woodley, J.; Turk, R.; Yan, S.; Kurgan, G.; McNeill, M.S.; Rettig, G.R. Optimized Design Parameters for CRISPR Cas9 and Cas12a Homology-Directed Repair. Sci. Rep. 2021, 11, 19482. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome Engineering Using the CRISPR-Cas9 System. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Gratz, S.J.; Cummings, A.M.; Nguyen, J.N.; Hamm, D.C.; Donohue, L.K.; Harrison, M.M.; Wildonger, J.; O’connor-Giles, K.M. Genome Engineering of Drosophila with the CRISPR RNA-Guided Cas9 Nuclease. Genetics 2013, 194, 1029–1035. [Google Scholar] [CrossRef]
- Bassett, A.R.; Tibbit, C.; Ponting, C.P.; Liu, J.L. Highly Efficient Targeted Mutagenesis of Drosophila with the CRISPR/Cas9 System. Cell Rep. 2013, 4, 220. [Google Scholar] [CrossRef]
- Mabashi-Asazuma, H.; Kuo, C.W.; Khoo, K.H.; Jarvis, D.L. Modifying an Insect Cell N-Glycan Processing Pathway Using CRISPR-Cas Technology. ACS Chem. Biol. 2015, 10, 2199–2208. [Google Scholar] [CrossRef]
- Mabashi-Asazuma, H.; Jarvis, D.L. CRISPR-Cas9 Vectors for Genome Editing and Host Engineering in the Baculovirus-Insect Cell System. Proc. Natl. Acad. Sci. USA 2017, 114, 9068–9073. [Google Scholar] [CrossRef]
- Mabashi-Asazuma, H.; Jarvis, D.L. A New Insect Cell Line Engineered to Produce Recombinant Glycoproteins with Cleavable N-Glycans. J. Biol. Chem. 2022, 298, 101454. [Google Scholar] [CrossRef] [PubMed]
- de Malmanche, H.; Marcellin, E.; Reid, S. Knockout of Sf-Caspase-1 Generates Apoptosis-Resistant Sf9 Cell Lines: Implications for Baculovirus Expression. Biotechnol. J. 2022, 17, 2100532. [Google Scholar] [CrossRef]
- Bruder, M.R.; Aucoin, M.G. Evaluation of Virus-Free Manufacture of Recombinant Proteins Using CRISPR-Mediated Gene Disruption in Baculovirus-Infected Insect Cells. Vaccines 2023, 11, 225. [Google Scholar] [CrossRef]
- Pazmiño-Ibarra, V.; Mengual-Martí, A.; Targovnik, A.M.; Herrero, S. Improvement of Baculovirus as Protein Expression Vector and as Biopesticide by CRISPR/Cas9 Editing. Biotechnol. Bioeng. 2019, 116, 2823–2833. [Google Scholar] [CrossRef]
- Fahey, L.M.; Raff, A.B.; Da Silva, D.M.; Kast, W.M. A Major Role for the Minor Capsid Protein of Human Papillomavirus Type 16 in Immune Escape. J. Immunol. 2009, 183, 6151. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA Recommends Nuvaxovid for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-nuvaxovid-authorisation-eu (accessed on 2 March 2025).
- Small, E.J.; Fratesi, P.; Reese, D.M.; Strang, G.; Laus, R.; Peshwa, M.V.; Valone, F.H. Immunotherapy of Hormone-Refractory Prostate Cancer With Antigen-Loaded Dendritic Cells. J. Clin. Oncol. 2000, 18, 3894–3903. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef]
- European Medicines Agency. Provenge: EPAR—Summary for the Public. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/provenge (accessed on 2 March 2025).
- European Commission. Annex I: Summary of Product Characteristics—Glybera. Available online: https://ec.europa.eu/health/documents/community-register/2012/20121025124069/anx_124069_en.pdf (accessed on 2 March 2025).
- Diamyd Medical. Diamyd Medical Receives Second U.S. FDA Fast Track Designation for Diamyd®—For the Prevention of Type 1 Diabetes. Available online: https://www.diamyd.com/docs/pressClips.aspx?ClipID=4870609 (accessed on 2 March 2025).
- Afghah, Z.; Webb, B.; Meng, X.J.; Ramamoorthy, S. Ten Years of PCV2 Vaccines and Vaccination: Is Eradication a Possibility? Vet. Microbiol. 2017, 206, 21–28. [Google Scholar] [CrossRef]
- European Medicines Agency. Porcilis PCV: EPAR—Summary for the Public. Available online: https://www.ema.europa.eu/en/documents/overview/porcilis-pcv-epar-summary-public_en.pdf (accessed on 2 March 2025).
- European Commission. Annex: Summary of Product Characteristics. Available online: https://ec.europa.eu/health/documents/community-register/2013/20130114125035/anx_125035_en.pdf (accessed on 2 March 2025).
- O’Brien, B.; Allen, M.; Roerink, F.; Crowley, A.; Knetter, S.; Morgan, C.; Wei, H.; Segers, R. Circumvent® CML: A Newly Licensed Commercial Vaccine for the Protection against PCV2a, PCV2d, Mycoplasma Hyopneumoniae, and Lawsonia intracellularis. In Proceedings of the AASV Annual Meeting 2023, Aurora, CO, USA, 4–7 March 2023. [Google Scholar] [CrossRef]
- van Aarle, P. Suitability of an E2 Subunit Vaccine of Classical Swine Fever in Combination with the E(Rns)-Marker-Test for Eradication through Vaccination. Dev. Biol. 2003, 114, 193–200. [Google Scholar]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever—An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- Cezar, G.; Leite, F.L.; Fano, E.; Phillips, R.; Waddell, J.; Dion, K.; Magalhães, E.; Trevisan, G.; Silva, G.; Linhares, D.C. Assessing the Detection and Interaction of Lawsonia intracellularis and Porcine Circovirus 2 in Low and High-Performance Wean-to-Finish Pig Groups in Different Porcine Reproductive and Respiratory Syndrome Virus Detection Scenarios. Front. Vet. Sci. 2024, 11, 1535803. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine Circovirus Type 2 and Porcine Circovirus-Associated Disease. J. Vet. Intern. Med. 2009, 23, 1151. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines 2023, 11, 1218. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 Spike Glycoprotein Vaccine Candidate NVX-CoV2373 Immunogenicity in Baboons and Protection in Mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Novavax. NVX-CoV2373 Phase 1, 2, and 3 Clinical Data Appendix; Novavax, Inc.: Gaithersburg, MD, USA, 2022; Available online: https://novavax.investorroom.com/download/NVX-CoV2373_Phase_1_2_and_3_Clinical_Data_Appendix_March_2022.Pdf (accessed on 2 April 2025).
- Senior, M. After Glybera’s Withdrawal, What’s next for Gene Therapy? Nat. Biotechnol. 2017, 35, 491–492. [Google Scholar] [CrossRef]
- Hinke, S. Diamyd, an Alum-Formulated Recombinant Human GAD65 for the Prevention of Autoimmune Diabetes. Curr. Opin. Mol. Ther. 2008, 10, 516–525. [Google Scholar]
- Zarkesh, K.; Akbarian, M.; Tayebi, L.; Uversky, V.N.; Rubio-Casillas, A.; Redwan, E.M. Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control. COVID 2023, 3, 975–986. [Google Scholar] [CrossRef]
- Dayan, G.H.; Rouphael, N.; Walsh, S.R.; Chen, A.; Grunenberg, N.; Allen, M.; Antony, J.; Asante, K.P.; Bhate, A.S.; Beresnev, T.; et al. Efficacy of a Bivalent (D614 + B.1.351) SARS-CoV-2 Recombinant Protein Vaccine with AS03 Adjuvant in Adults: A Phase 3, Parallel, Randomised, Modified Double-Blind, Placebo-Controlled Trial. Lancet Respir. Med. 2023, 11, 975–990. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Smit, M.J.; Sander, A.F.; Ariaans, M.B.P.A.; Fougeroux, C.; Heinzel, C.; Fendel, R.; Esen, M.; Kremsner, P.G.; ter Heine, R.; Wertheim, H.F.; et al. First-in-Human Use of a Modular Capsid Virus-like Vaccine Platform: An Open-Label, Non-Randomised, Phase 1 Clinical Trial of the SARS-CoV-2 Vaccine ABNCoV2. Lancet Microbe 2023, 4, e140–e148. [Google Scholar] [CrossRef]
- López-Macías, C. Virus-like Particle (VLP)-Based Vaccines for Pandemic Influenza: Performance of a VLP Vaccine during the 2009 Influenza Pandemic. Hum. Vaccines Immunother. 2012, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Carascal, M.B.; Pavon, R.D.N.; Rivera, W.L. Recent Progress in Recombinant Influenza Vaccine Development Toward Heterosubtypic Immune Response. Front. Immunol. 2022, 13, 878943. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Xu, W.; Huo, X.; Wang, J.; Xu, Y.; Ding, W.; Guo, Z.; Liu, R. Advances in Protein Subunit Vaccines against H1N1/09 Influenza. Front. Immunol. 2024, 15, 1499754. [Google Scholar] [CrossRef]
- Bernstein, D.I.; El Sahly, H.M.; Keitel, W.A.; Wolff, M.; Simone, G.; Segawa, C.; Wong, S.; Shelly, D.; Young, N.S.; Dempsey, W. Safety and Immunogenicity of a Candidate Parvovirus B19 Vaccine. Vaccine 2011, 29, 7357. [Google Scholar] [CrossRef]
- European Medicines Agency. EudraCT Number: 2016-002302-39. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-002302-39/ES (accessed on 2 March 2025).
- Atmar, R.L.; Bernstein, D.I.; Harro, C.D.; Al-Ibrahim, M.S.; Chen, W.H.; Ferreira, J.; Estes, M.K.; Graham, D.Y.; Opekun, A.R.; Richardson, C.; et al. Norovirus Vaccine against Experimental Human Norwalk Virus Illness. N. Engl. J. Med. 2011, 365, 2178–2187. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649. [Google Scholar] [CrossRef]
- Tiono, A.B.; Nébié, I.; Anagnostou, N.; Coulibaly, A.S.; Bowyer, G.; Lam, E.; Bougouma, E.C.; Ouedraogo, A.; Yaro, J.B.B.; Barry, A.; et al. First Field Efficacy Trial of the ChAd63 MVA ME-TRAP Vectored Malaria Vaccine Candidate in 5-17 Months Old Infants and Children. PLoS ONE 2018, 13, e0208328. [Google Scholar] [CrossRef]
- Suschak, J.J.; Schmaljohn, C.S. Vaccines against Ebola Virus and Marburg Virus: Recent Advances and Promising Candidates. Hum. Vaccines Immunother. 2019, 15, 2359. [Google Scholar] [CrossRef]
- Veeva. A Study to Evaluate the Efficacy, Safety, and Immunogenicity of SCT1000 in Healthy Women Aged 18-45 Years. Available online: https://ctv.veeva.com/study/a-study-to-evaluate-the-efficacy-safety-and-immunogenicity-of-sct1000-in-healthy-women-aged1845 (accessed on 2 March 2025).
- Hu, J.; Zhang, Q.; Peng, P.; Li, R.; Li, J.; Wang, X.; Gu, M.; Hu, Z.; Hu, S.; Liu, X.; et al. Baculovirus-Derived Influenza Virus-like Particle Confers Complete Protection against Lethal H7N9 Avian Influenza Virus Challenge in Chickens and Mice. Vet. Microbiol. 2022, 264, 109306. [Google Scholar] [CrossRef]
- Hevey, M.; Negley, D.; Geisbert, J.; Jahrling, P.; Schmaljohn, A. Antigenicity and Vaccine Potential of Marburg Virus Glycoprotein Expressed by Baculovirus Recombinants. Virology 1997, 239, 206–216. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Lee, H.J.; Lee, R.; Jung, Y.J.; Park, J.; Hahn, T.W. Zika Virus Baculovirus-Expressed Envelope Protein Elicited Humoral and Cellular Immunity in Immunocompetent Mice. Sci. Rep. 2022, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Chun, J.; Park, M.; Kim, S.; Kim, N.; Lee, H.J.; Kim, M.; Shin, H.Y.; Oh, Y.K.; Kim, Y.B. The Safe Baculovirus-Based PrM/E DNA Vaccine Protected Fetuses Against Zika Virus in A129 Mice. Vaccines 2021, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Tomatis, C.; Ferrer, M.F.; Aquila, S.; Thomas, P.D.; Arrías, P.N.; Ferrelli, L.; Pidre, M.; Romanowski, V.; Carrera Silva, E.A.; Gómez, R.M. Baculovirus Surface Display of a Chimeric E-NS1 Protein of YFV Protects against YFV Infection. Vaccine 2024, 42, 126045. [Google Scholar] [CrossRef]
- Zhu, B.; Ye, J.; Lu, P.; Jiang, R.; Yang, X.; Fu, Z.F.; Chen, H.; Cao, S. Induction of Antigen-Specific Immune Responses in Mice by Recombinant Baculovirus Expressing Premembrane and Envelope Proteins of West Nile Virus. Virol. J. 2012, 9, 132. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Wang, Y.; Hao, P.; Jin, X. Elaboration of Tetravalent Antibody Responses against Dengue Viruses Using a Subunit Vaccine Comprised of a Single Consensus Dengue Envelope Sequence. Vaccine 2017, 35, 6308–6320. [Google Scholar] [CrossRef]
- Sung, L.Y.; Chen, C.L.; Lin, S.Y.; Li, K.C.; Yeh, C.L.; Chen, G.Y.; Lin, C.Y.; Hu, Y.C. Efficient Gene Delivery into Cell Lines and Stem Cells Using Baculovirus. Nat. Protoc. 2014, 9, 1882–1899. [Google Scholar] [CrossRef]
- Chen, N.; Kong, X.; Zhao, S.; Xiaofeng, W. Post-Translational Modification of Baculovirus-Encoded Proteins. Virus Res. 2020, 279, 197865. [Google Scholar] [CrossRef]
- Dias, M.M.; Vidigal, J.; Sequeira, D.P.; Alves, P.M.; Teixeira, A.P.; Roldão, A. Insect High FiveTM Cell Line Development Using Site-Specific Flipase Recombination Technology. G3 Genes Genomes Genet. 2021, 11, jkab166. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, J.; Labropoulou, V.; Swevers, L. Beyond Baculoviruses: Additional Biotechnological Platforms Based on Insect RNA Viruses. Adv. Insect Physiol. 2018, 55, 123–162. [Google Scholar] [CrossRef]
- Varikkodan, M.M.; Chen, C.C.; Wu, T.Y. Recombinant Baculovirus: A Flexible Drug Screening Platform for Chikungunya Virus. Int. J. Mol. Sci. 2021, 22, 7891. [Google Scholar] [CrossRef]
- Assenberg, R.; Wan, P.T.; Geisse, S.; Mayr, L.M. Advances in Recombinant Protein Expression for Use in Pharmaceutical Research. Curr. Opin. Struct. Biol. 2013, 23, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, X.; Liu, L. Influenza and Universal Vaccine Research in China. Viruses 2022, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.J.; Hashimoto, Y. A Fast Track Influenza Virus Vaccine Produced in Insect Cells. J. Invertebr. Pathol. 2011, 107, S31–S41. [Google Scholar] [CrossRef]
- Drugmand, J.C.; Schneider, Y.J.; Agathos, S.N. Insect Cells as Factories for Biomanufacturing. Biotechnol. Adv. 2012, 30, 1140–1157. [Google Scholar] [CrossRef]
- Harrison, R.L.; Jarvis, D.L. Protein N-Glycosylation in the Baculovirus-Insect Cell Expression System and Engineering of Insect Cells to Produce “Mammalianized” Recombinant Glycoproteins. Adv. Virus Res. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Shi, X.; Jarvis, D.L. Protein N-Glycosylation in the Baculovirus-Insect Cell System. Curr. Drug Targets 2007, 8, 1116. [Google Scholar] [CrossRef]
- Schaly, S.; Ghebretatios, M.; Prakash, S. Baculoviruses in Gene Therapy and Personalized Medicine. Biologics 2021, 15, 115. [Google Scholar] [CrossRef]
- Wang, F.; Sun, J.; Guo, W.; Wu, Y. Application of the Insect Cell-Baculovirus Expression Vector System in Adeno-Associated Viral Production. Appl. Sci. 2024, 14, 10948. [Google Scholar] [CrossRef]
- Hüser, A.; Rudolph, M.; Hofmann, C. Incorporation of Decay-Accelerating Factor into the Baculovirus Envelope Generates Complement-Resistant Gene Transfer Vectors. Nat. Biotechnol. 2001, 19, 451–455. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Maatta, A.I.; Ylä-Herttuala, S.; Airenne, K.J. Screening of Complement Inhibitors: Shielded Baculoviruses Increase the Safety and Efficacy of Gene Delivery. Mol. Ther. 2010, 18, 987–992. [Google Scholar] [CrossRef]
- Grand View Research. Recombinant Proteins Market Size And Share Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/recombinant-proteins-market-report (accessed on 2 March 2025).
- Research Nester. Recombinant Proteins Market Size & Share, Growth Forecasts 2037. Available online: https://www.researchnester.com/reports/recombinant-proteins-market/4632 (accessed on 2 March 2025).
- Pawlita, M.; Muller, M.; Muller, M.; Oppenlander, M.; Oppenlander, O.; Zentgraf, H.; Herrmann, M. DNA Encapsidation by Viruslike Particles Assembled in Insect Cells from the Major Capsid Protein VP1 of B-Lymphotropic Papovavirus. J. Virol. 1996, 70, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- Puente-Massaguer, E.; González-Domínguez, I.; Strobl, F.; Grabherr, R.; Striedner, G.; Lecina, M.; Gòdia, F. Bioprocess Characterization of Virus-like Particle Production with the Insect Cell Baculovirus Expression System at Nanoparticle Level. J. Chem. Technol. Biotechnol. 2022, 97, 2456–2465. [Google Scholar] [CrossRef]
- van Oers, M.M. Vaccines for Viral and Parasitic Diseases Produced with Baculovirus Vectors. Adv. Virus Res. 2006, 68, 193–253. [Google Scholar] [CrossRef] [PubMed]
- Garretson, T.A.; Shang, H.; Schulz, A.K.; Donohue, B.V.; Cheng, X.W. Expression- and Genomic-Level Changes during Passage of Four Baculoviruses Derived from Bacmids in Permissive Insect Cell Lines. Virus Res. 2018, 256, 117–124. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Grose, C.; Hick, T.A.H.; Breukink, H.E.; van den Braak, R.; Abbo, S.R.; Geertsema, C.; van Oers, M.M.; Martens, D.E.; Esposito, D. Relocation of the AttTn7 Transgene Insertion Site in Bacmid DNA Enhances Baculovirus Genome Stability and Recombinant Protein Expression in Insect Cells. Viruses 2020, 12, 1448. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Possee, R.D.; King, L.A. High-Throughput Baculovirus Expression in Insect Cells. Methods Mol. Biol. 2012, 824, 609–627. [Google Scholar] [CrossRef]
- Nguyen, C.; Ibe-Enwo, A.; Slack, J. A Baculovirus Expression Vector Derived Entirely from Non-Templated, Chemically Synthesized DNA Parts. Viruses 2023, 15, 1981. [Google Scholar] [CrossRef]
- Zitzmann, J.; Sprick, G.; Weidner, T.; Czermak, C.S.P.; Zitzmann, J.; Sprick, G.; Weidner, T.; Czermak, C.S.P. Process Optimization for Recombinant Protein Expression in Insect Cells. In New Insights into Cell Culture Technology; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Food and Drug Administration. Current Good Manufacturing Practice (CGMP) Regulations. Available online: https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations (accessed on 7 May 2025).
- Geisler, C.; Jarvis, D.L. Adventitious Viruses in Insect Cell Lines Used for Recombinant Protein Expression. Protein Expr. Purif. 2018, 144, 25–32. [Google Scholar] [CrossRef]
- Ma, H.; Galvin, T.A.; Glasner, D.R.; Shaheduzzaman, S.; Khan, A.S. Identification of a Novel Rhabdovirus in Spodoptera frugiperda Cell Lines. J. Virol. 2014, 88, 6576–6585. [Google Scholar] [CrossRef]
- Li, T.-C.; Scotti, P.D.; Miyamura, T.; Takeda, N. Latent Infection of a New Alphanodavirus in an Insect Cell Line. J. Virol. 2007, 81, 10890–10896. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q5A(R2) Guideline on Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q5ar2-guideline-viral-safety-evaluation-biotechnology-products-derived-cell-lines-human-or-animal-origin-scientific-guideline (accessed on 8 May 2025).

| Disease | Brand Name | Manufacturer | Antigen/Product Type | References |
|---|---|---|---|---|
| Human Vaccines | ||||
| Cervical cancer | CervarixTM | GSK | L1 capsid protein/VLP | [136] |
| Influenza | FluBlok® | Protein Sciences Corporation | Hemagglutinin/subunit | [48] |
| Influenza | Flublok® Quadrivalent | [48] | ||
| SARS-CoV-2 | Nuvaxovid®/Covovax | Novavax | S protein/subunit | [137] |
| Human therapeutics | ||||
| Prostate cancer | Provenge® | Dendreon | PSA/immunotherapy | [138,139,140] |
| Lipoprotein lipase deficiency | Glybera® | uniQure | rAAV-based gene therapy | [48,141] |
| Type 1 diabetes | Diamyd® | Diamyd Medical | 65 kDa glutamate decarboxylate | [142] |
| Veterinary vaccines | ||||
| Porcine circovirus type 2 | Porcilis® PCV | Schering-Plough/Merck | PCV2 ORF2 protein/VLP | [143,144] |
| Porcine circovirus type 2 | Ingelvac CircoFLEXTM | Boehringer Ingelheim | PCV2 ORF2 protein/VLP | [143,145] |
| Porcine circovirus type 2 | Circumvent® | Intervet/Merck | PCV2 ORF2 protein/VLP | [143] |
| Porcine circovirus type 2 and Mycoplasma hyopneumoniae | Porcilis PCV M Hyo® | Intervet/Merck | PCV2 ORF2 protein/VLP and inactivated Mycoplasma hyopneumoniae | [144] |
| Porcine circovirus type 2, Mycoplasma hyopneumoniae and Lawsonia intracellularis | Circumvent CML® | Merck | PCV2 ORF2 protein/VLP, inactivated M. hyopneumoniae and L. intracellularis | [146] |
| Classical swine fever | Porcilis Pesti® | Boehringer Ingelheim/Merc | E2 protein/subunit | [147] |
| Classical swine fever | Bayovac CSF E2® | Bayer/Pfizer | E2 protein/subunit | [48] |
| Target | Antigen | Manufacturer | Stage/NCT Number | References |
|---|---|---|---|---|
| SARS-CoV-2 | Recombinant RBD monomer | West China Hospital of Sichuan University | Phase III (NCT04904471) | [156] |
| SARS-CoV-2 | CoV-2 preS dTM | Sanofi/GSK | Phase III (NCT04904549) | [157] |
| SARS-CoV-2 | SARS-CoV-2 spike glycoproteins | Novavax | Phase I/II (NCT04368988) | [158] |
| SARS-CoV-2 | SARS-CoV-2 spike glycoproteins | Radboud University Medical Center (Netherlands) | Phase I (NCT04839146) | [159] |
| Influenza A H1N1 | A (H1N1) 2009 Influenza VLP | Novavax | Phase II (NCT01072799) | [160] |
| Seasonal Influenza virus | Hemagglutinin, neuraminidase, and Matrix-M adjuvant | Novavax | Phase III (NCT04120194) | [161] |
| SARS-CoV-2 and Influenza | Quadrivalent Influenza hemagglutinin and CoV-2 rS of SARS-CoV-2 nanoparticles | Novavax | Phase III (NCT06291857) | [162] |
| Human parvovirus B19 | VP1 and VP2 | National Institute of Allergy and Infectious Diseases/Meridian Life Science | Phase I/II (NCT00379938) | [163] |
| Respiratory Syncytial Virus | Fusion glycoprotein | Novavax | Phase III (NCT02624947) | [164] |
| Norwalk virus | Norwalk virus-VLP | Baylor College of Medicine | Phase I/II (NCT00973284) | [165] |
| Norwalk virus | Norwalk virus-VLP | LigoCyte Pharmaceuticals | Phase I/II (NCT00806962) | [166] |
| Malaria | ChAd63-MVA ME-TRAP | Novavax | Phase I/IIb (NCT01635647) | [167] |
| Ebola virus (EBOV) | EBOV surface glycoprotein | Novavax | Phase I (NCT02370589) | [168] |
| Human papillomavirus | HPV (6/11/16/18/31/33/ 35/39/45/51/52/56/ 58/59) L1 protein | SinoCellTech (China) | Phase III (NCT06041061) | [169] |
| Influenza A H7N9 | Hemagglutinin | Animal Infectious Disease Laboratory, School of Veterinary Medicine, Yangzhou University | preclinical | [170] |
| Marburg virus (MARV) | MARV surface glycoprotein | Virology Division, United States Army Medical Research Institute for Infectious Diseases | preclinical | [168,171] |
| Zika virus (ZIKV) | ZIKV E protein | College of Veterinary Medicine and Institute of Veterinary Science, Kangwon National University, Korea | preclinical | [172] |
| Zika virus (ZIKV) | ZIKV prM/E | Department of Bioindustrial Technologies, Konkuk University, Seoul, Korea. | preclinical | [173] |
| Yellow fever virus (YFV) | YFV E-NS1 protein | Laboratorio de Patogénesis Viral, Instituto de Biotecnología y Biología Molecular (IBBM), CONICET-UNLP, La Plata, Buenos Aires, Argentina | preclinical | [174] |
| West Nile virus (WNV) | preM/E proteins | State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University, Wuhan, Hubei, China | preclinical | [175] |
| Dengue virus (DENV) | DENV E protein | Viral Disease and Vaccine Translational Research Unit, Institute Pasteur of Shanghai, China | preclinical | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułek, M.; Szuster-Ciesielska, A. The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review. Vaccines 2025, 13, 556. https://doi.org/10.3390/vaccines13060556
Sułek M, Szuster-Ciesielska A. The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review. Vaccines. 2025; 13(6):556. https://doi.org/10.3390/vaccines13060556
Chicago/Turabian StyleSułek, Michał, and Agnieszka Szuster-Ciesielska. 2025. "The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review" Vaccines 13, no. 6: 556. https://doi.org/10.3390/vaccines13060556
APA StyleSułek, M., & Szuster-Ciesielska, A. (2025). The Bioengineering of Insect Cell Lines for Biotherapeutics and Vaccine Production: An Updated Review. Vaccines, 13(6), 556. https://doi.org/10.3390/vaccines13060556





