Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cells and Viruses
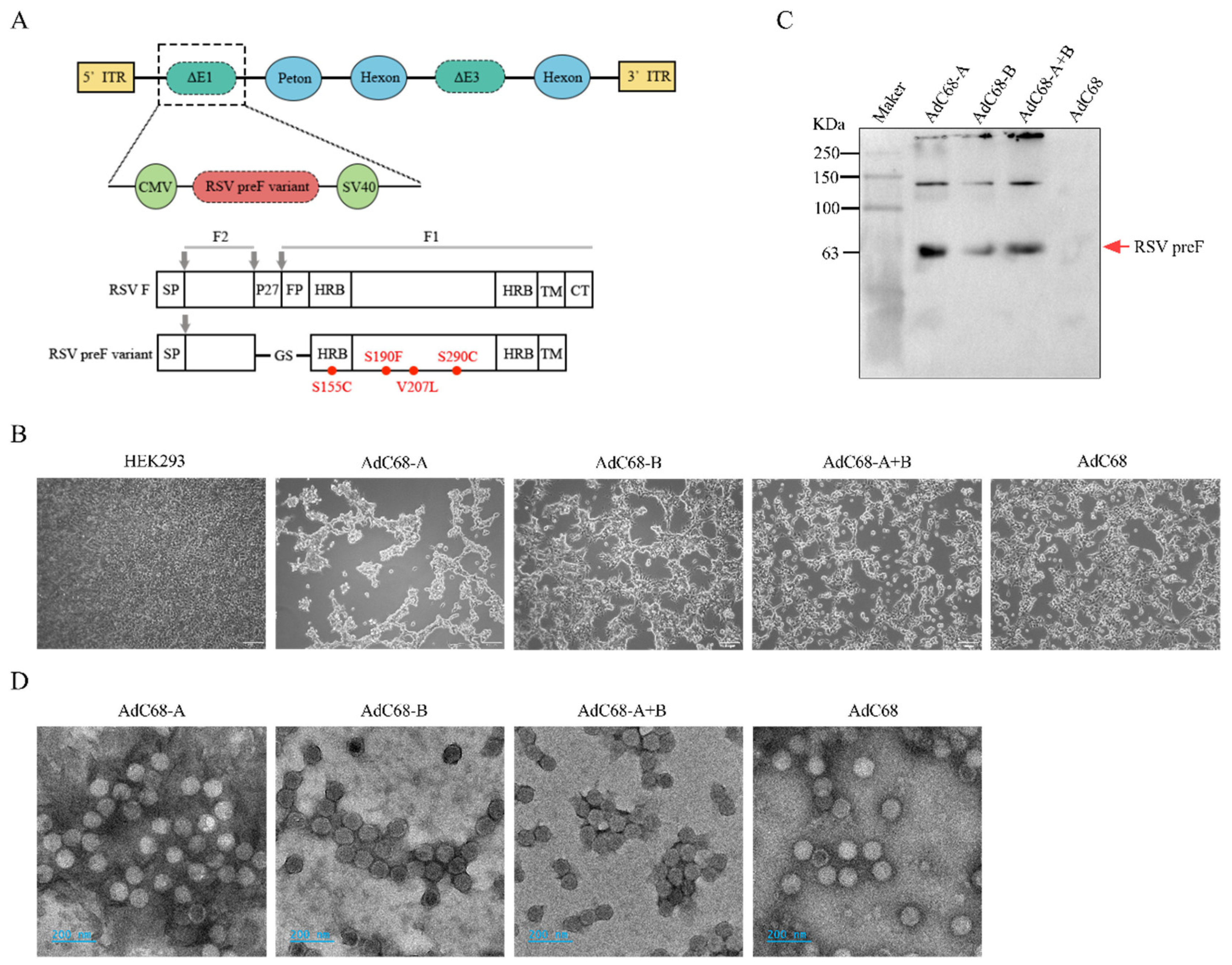
2.3. Production of AdC68-Based RSV Vaccines
2.4. The Cytopathic Effect (CPE) Observation and Western Blot Analysis
2.5. Visualization of Viral Particles by Negative Staining Electron Microscopy (EM)
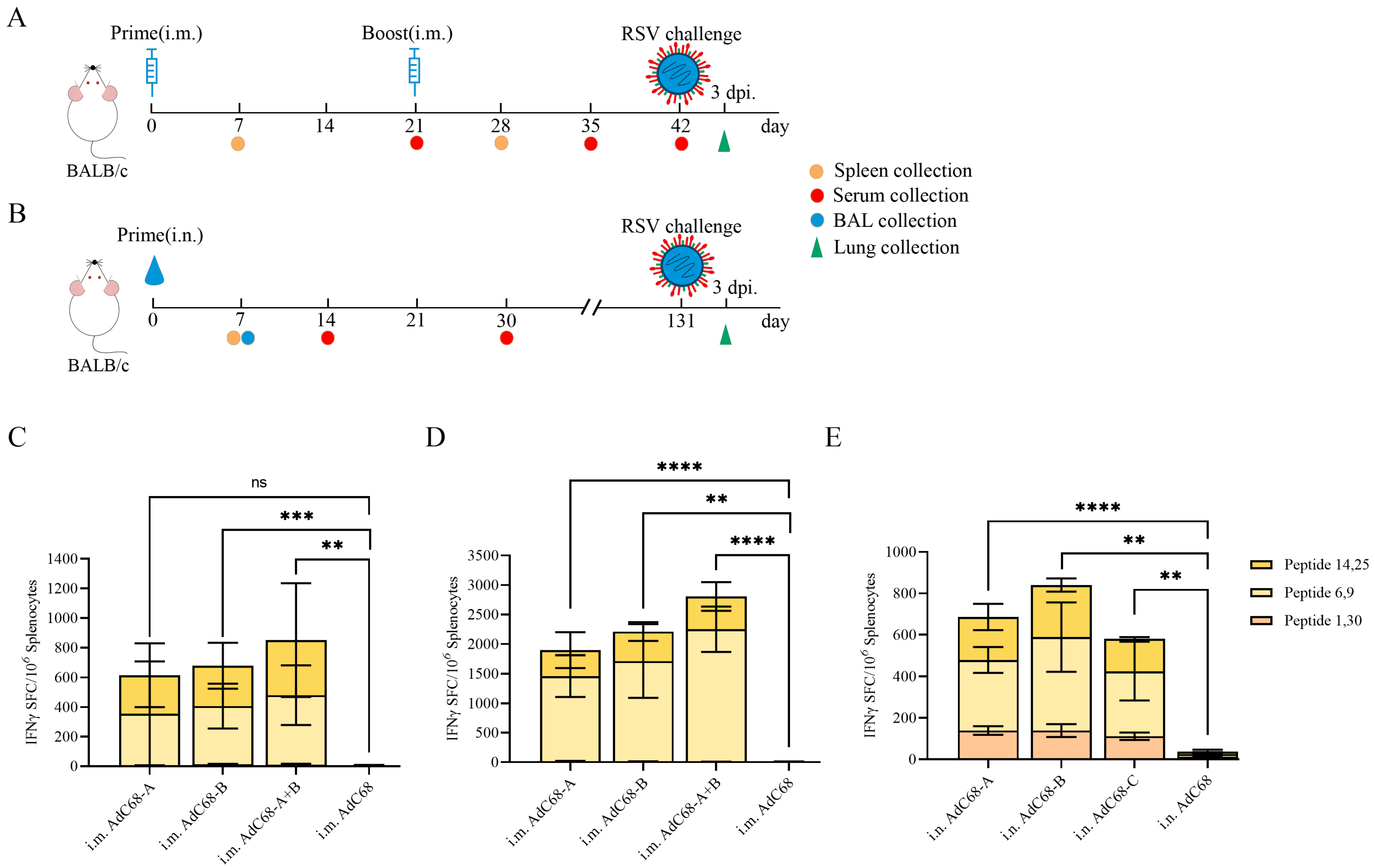
2.6. Animals, Immunization, Challenge, and Sample Collection
2.7. Antibody Detection by Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Evaluation of Antigen-Specific T-Cell Responses by Enzyme-Linked Immunospot (ELISpot) Assay
2.9. Neutralization Assay
2.10. Viral Load Detection by Real-Time Quantitative Reverse Transcriptase PCR (qRT-PCR)
2.11. Statistical Analysis
3. Results
3.1. Successful Rescue of Recombinant Chimpanzee Adenovirus Expressing RSV-A and/or B pre-F Protein
3.2. Single Intranasal Administration of Recombinant AdC68-RSV Vaccines Elicits Robust Cellular Immunity in Mice
3.3. Single Intranasal Administration of Recombinant AdC68-RSV Vaccines Induces Antigen-Specific Humoral Responses in Mice
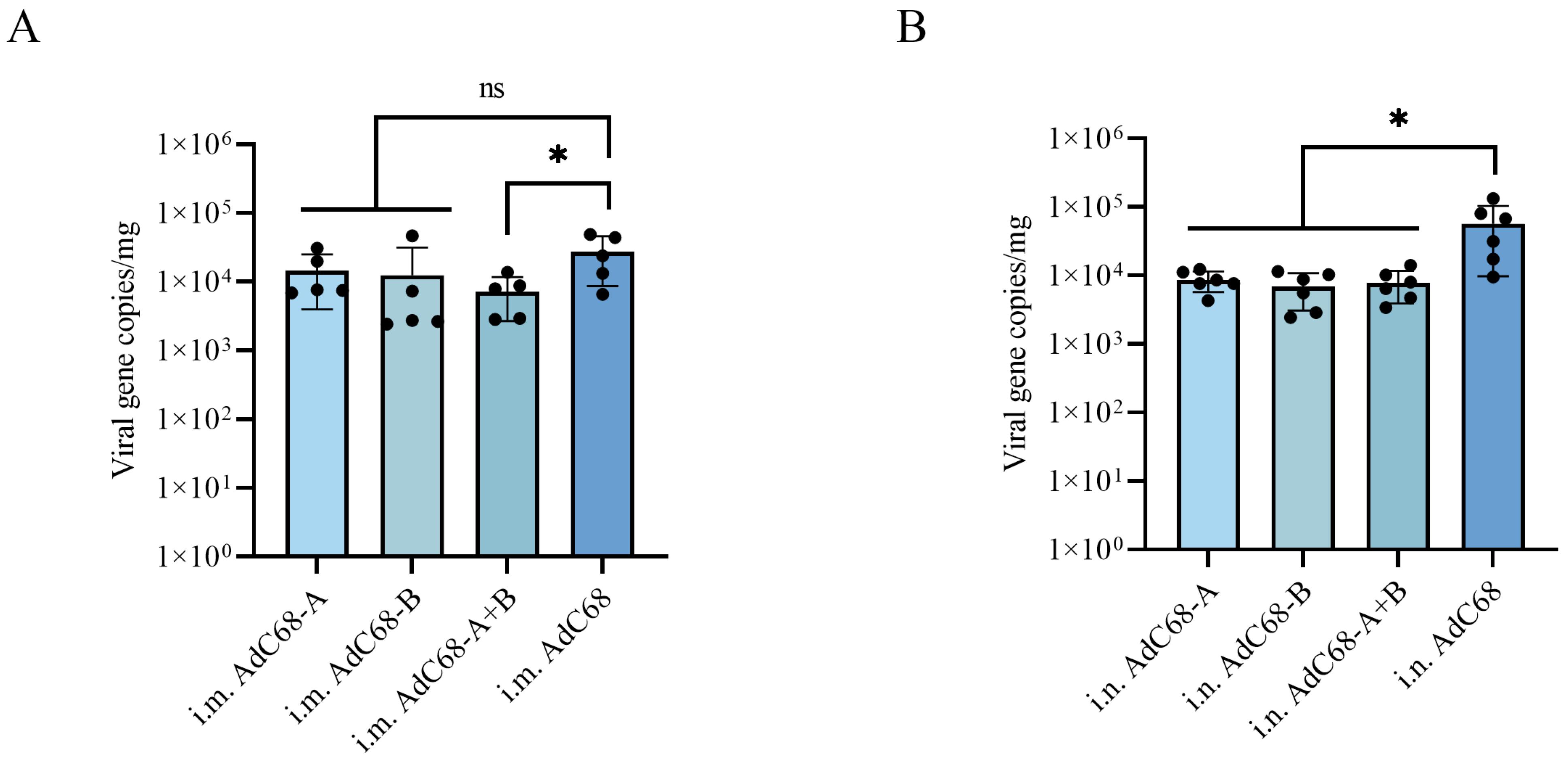
3.4. Single Intranasal Administration of AdC68-RSV Vaccines Confers Prolonged Protection Against Pulmonary RSV Replication in BALB/c Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O′Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Coultas, J.A.; Smyth, R.; Openshaw, P.J. Respiratory syncytial virus (RSV): A scourge from infancy to old age. Thorax 2019, 74, 986–993. [Google Scholar] [CrossRef]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef]
- Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019, 394, 757–779. [Google Scholar] [CrossRef]
- Langley, J.M.; Bianco, V.; Domachowske, J.B.; Madhi, S.A.; Stoszek, S.K.; Zaman, K.; Bueso, A.; Ceballos, A.; Cousin, L.; D’Andrea, U.; et al. Incidence of Respiratory Syncytial Virus Lower Respiratory Tract Infections During the First 2 Years of Life: A Prospective Study Across Diverse Global Settings. J. Infect. Dis. 2022, 226, 374–385. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Martinón-Torres, F.; Carmo, M.; Platero, L.; Drago, G.; López-Belmonte, J.L.; Bangert, M.; Díez-Domingo, J.; Garcés-Sánchez, M. Clinical and economic burden of respiratory syncytial virus in Spanish children: The BARI study. BMC Infect. Dis. 2022, 22, 759. [Google Scholar] [CrossRef]
- Wildenbeest, J.G.; Billard, M.N.; Zuurbier, R.P.; Korsten, K.; Langedijk, A.C.; van de Ven, P.M.; Snape, M.D.; Drysdale, S.B.; Pollard, A.J.; Robinson, H.; et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: A prospective birth cohort study. Lancet. Respir. Med. 2023, 11, 341–353. [Google Scholar] [CrossRef]
- See, K.C. Vaccination for Respiratory Syncytial Virus: A Narrative Review and Primer for Clinicians. Vaccines 2023, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines 2023, 8, 138. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef]
- Ruiz-Galiana, J.; Cantón, R.; De Lucas Ramos, P.; García-Botella, A.; García-Lledó, A.; Hernández-Sampelayo, T.; Gómez-Pavón, J.; González Del Castillo, J.; Martín-Delgado, M.C.; Martín Sánchez, F.J.; et al. Respiratory syncytial virus: A new era. Rev. Esp. Quimioter. 2024, 37, 134–148. [Google Scholar] [CrossRef]
- Stephens, L.M.; Varga, S.M. Nanoparticle vaccines against respiratory syncytial virus. Future Virol. 2020, 15, 763–778. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Kerstetter, L.J.; Buckley, S.; Bliss, C.M.; Coughlan, L. Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses. Front. Immunol. 2020, 11, 607333. [Google Scholar] [CrossRef]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef]
- Roberts, S.R.; Compans, R.W.; Wertz, G.W. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J. Virol. 1995, 69, 2667–2673. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus--a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- de Bree, G.J.; Heidema, J.; van Leeuwen, E.M.; van Bleek, G.M.; Jonkers, R.E.; Jansen, H.M.; van Lier, R.A.; Out, T.A. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J. Infect. Dis. 2005, 191, 1710–1718. [Google Scholar] [CrossRef]
- Rock, M.T.; Crowe, J.E., Jr. Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology 2003, 108, 474–480. [Google Scholar] [CrossRef]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.Y.; et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015, 7, 309ra162. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Mas, V.; Chappell, K.; Vázquez, M.; Cano, O.; Luque, D.; Terrón, M.C.; Melero, J.A.; Palomo, C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl. Acad. Sci. USA 2012, 109, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Center, K.J.; Tita, A.T.N.; Swanson, K.A.; Radley, D.; Houghton, J.; McGrory, S.B.; Gomme, E.; Anderson, M.; Roberts, J.P.; et al. Prefusion F Protein-Based Respiratory Syncytial Virus Immunization in Pregnancy. N. Engl. J. Med. 2022, 386, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Crank, M.C.; Ruckwardt, T.J.; Chen, M.; Morabito, K.M.; Phung, E.; Costner, P.J.; Holman, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J.; et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019, 365, 505–509. [Google Scholar] [CrossRef]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- Ledgerwood, J.E.; DeZure, A.D.; Stanley, D.A.; Coates, E.E.; Novik, L.; Enama, M.E.; Berkowitz, N.M.; Hu, Z.; Joshi, G.; Ploquin, A.; et al. Chimpanzee Adenovirus Vector Ebola Vaccine. N. Engl. J. Med. 2017, 376, 928–938. [Google Scholar] [CrossRef]
- Tapia, M.D.; Sow, S.O.; Mbaye, K.D.; Thiongane, A.; Ndiaye, B.P.; Ndour, C.T.; Mboup, S.; Keshinro, B.; Kinge, T.N.; Vernet, G.; et al. Safety, reactogenicity, and immunogenicity of a chimpanzee adenovirus vectored Ebola vaccine in children in Africa: A randomised, observer-blind, placebo-controlled, phase 2 trial. Lancet. Infect. Dis. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef]
- Widjojoatmodjo, M.N.; Bogaert, L.; Meek, B.; Zahn, R.; Vellinga, J.; Custers, J.; Serroyen, J.; Radošević, K.; Schuitemaker, H. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine 2015, 33, 5406–5414. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Bolder, R.; Heemskerk-van der Meer, M.; Drijver, J.; van Polanen, Y.; Serroyen, J.; Langedijk, J.P.M.; Schuitemaker, H.; Saeland, E.; Zahn, R. Adenovector 26 encoded prefusion conformation stabilized RSV-F protein induces long-lasting Th1-biased immunity in neonatal mice. NPJ Vaccines 2020, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine With an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged ≥60 Years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [CrossRef]
- Sadoff, J.; De Paepe, E.; DeVincenzo, J.; Gymnopoulou, E.; Menten, J.; Murray, B.; Rosemary Bastian, A.; Vandebosch, A.; Haazen, W.; Noulin, N.; et al. Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.preF in a Human Challenge Study. J. Infect. Dis. 2022, 226, 396–406. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Xing, M.; Zhuo, Y.; Guo, J.; Du, M.; Wang, Q.; An, Y.; Li, J.; Gao, P.; et al. Heterologous prime-boost immunizations with chimpanzee adenoviral vectors elicit potent and protective immunity against SARS-CoV-2 infection. Cell Discov. 2021, 7, 123. [Google Scholar] [CrossRef]
- Lu, M.; Yao, Y.; Zhang, X.; Liu, H.; Gao, G.; Peng, Y.; Chen, M.; Zhao, J.; Zhang, X.; Yin, C.; et al. Both chimpanzee adenovirus-vectored and DNA vaccines induced long-term immunity against Nipah virus infection. NPJ Vaccines 2023, 8, 170. [Google Scholar] [CrossRef]
- Liu, C.-F.; Yin, G.-R.; Zhao, Y.-H. Effect of intranasal immunization with soluble tachyzoites antigen in combination with propolis and IFN-γ on antibody levels in mice. Chin. J. Public Health 2011, 27, 1582–1583. [Google Scholar] [CrossRef]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Raju, S.; et al. Mucosal vaccine-induced cross-reactive CD8(+) T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 2024, 25, 537–551. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Xing, M.; Hu, G.; Wang, X.; Wang, Y.; He, F.; Dai, W.; Wang, X.; Niu, Y.; Liu, J.; Liu, H.; et al. An intranasal combination vaccine induces systemic and mucosal immunity against COVID-19 and influenza. NPJ Vaccines 2024, 9, 64. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, J.; Li, X.; Li, Y.; Mao, L.; Suo, W.; Lan, J. Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models. Vaccines 2025, 13, 528. https://doi.org/10.3390/vaccines13050528
Miao J, Li X, Li Y, Mao L, Suo W, Lan J. Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models. Vaccines. 2025; 13(5):528. https://doi.org/10.3390/vaccines13050528
Chicago/Turabian StyleMiao, Jing, Xuejie Li, Yingwen Li, Lingjing Mao, Wenkai Suo, and Jiaming Lan. 2025. "Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models" Vaccines 13, no. 5: 528. https://doi.org/10.3390/vaccines13050528
APA StyleMiao, J., Li, X., Li, Y., Mao, L., Suo, W., & Lan, J. (2025). Single-Dose Intranasal Immunization with ChAd68-Vectored Prefusion F Vaccines Confers Sustained Protection Against Respiratory Syncytial Virus in Murine Models. Vaccines, 13(5), 528. https://doi.org/10.3390/vaccines13050528





