Differences in Mpox and Vaccinia Immunity Induced by Non-Replicating and Replicating Vaccinia-Based Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Viruses
2.3. Mpox Focus Reduction Neutralization Test (FRNT)
2.4. Vaccinia Plaque Reduction Neutralization Test (PRNT)
2.5. IFN-γ ELISPOT
2.6. Statistical Analysis
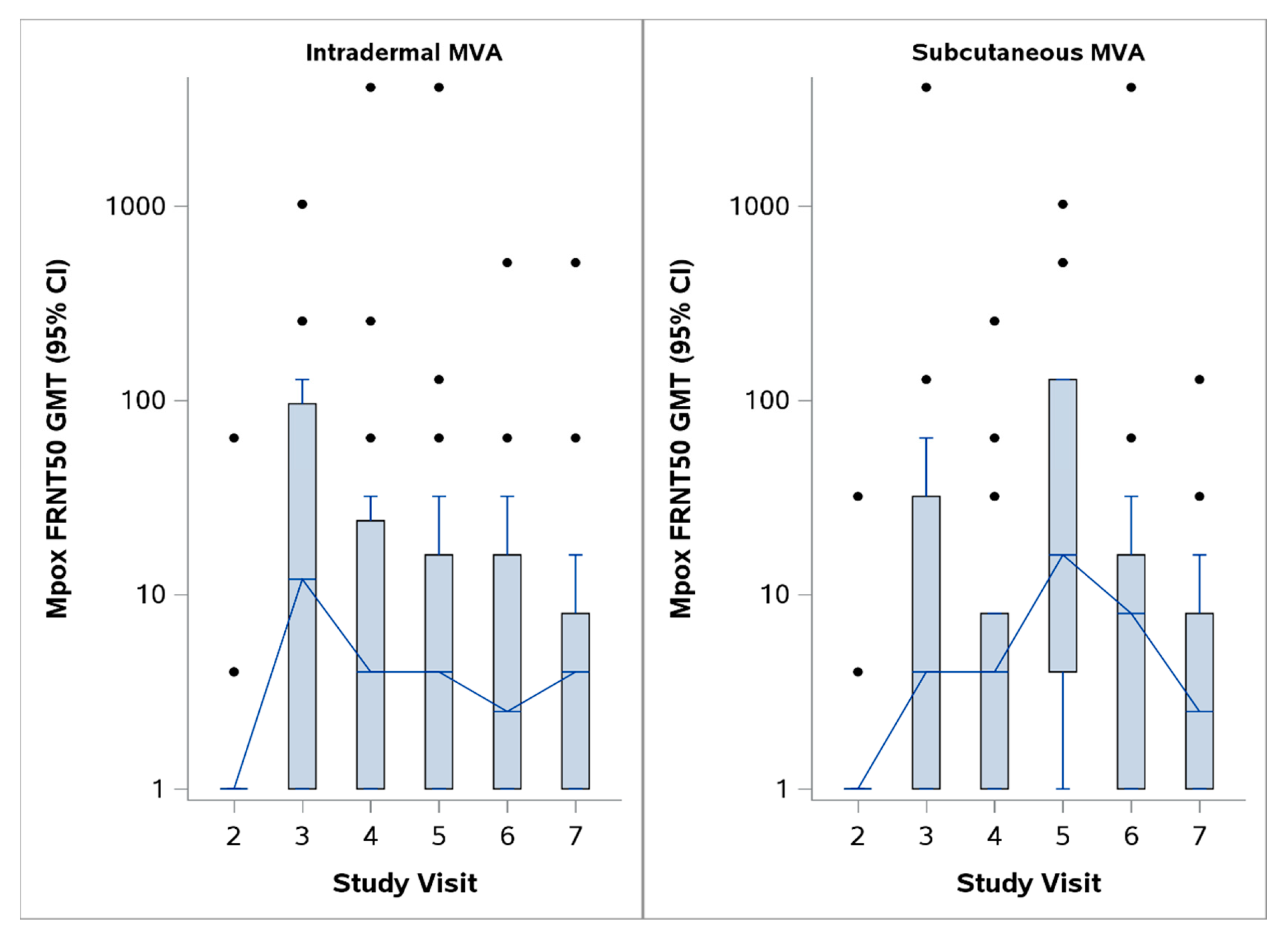
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrino, J.; Graham, B.S. Smallpox vaccines: Past, present, and future. J. Allergy Clin. Immunol. 2006, 118, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A tale of two clades: Monkeypox viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef] [PubMed]
- Okwor, T.; Mbala, P.K.; Evans, D.H.; Kindrachuk, J. A contemporary review of clade-specific virological differences in monkeypox viruses. Clin. Microbiol. Infect. 2023, 29, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of Monkeypox-West and Central Africa, 1970–2017. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 306–310. [Google Scholar] [CrossRef]
- Pan, D.; Nazareth, J.; Sze, S.; Martin, C.A.; Decker, J.; Fletcher, E.; Hollingsworth, T.D.; Barer, M.R.; Pareek, M.; Tang, J.W. Transmission of monkeypox/mpox virus: A narrative review of environmental, viral, host, and population factors in relation to the 2022 international outbreak. J. Med. Virol. 2023, 95. [Google Scholar] [CrossRef]
- CDC. Mpox in the United States and Around the World: Current Situation. Available online: https://www.cdc.gov/mpox/situation-summary/index.html (accessed on 19 March 2025).
- WHO. 2022-24 Mpox (Monkeypox) Outbreak: Global Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 23 March 2023).
- Brand, S.P.C.; Cavallaro, M.; Cumming, F.; Turner, C.; Florence, I.; Blomquist, P.; Hilton, J.; Guzman-Rincon, L.M.; House, T.; Nokes, D.J.; et al. The role of vaccination and public awareness in forecasts of Mpox incidence in the United Kingdom. Nat. Commun. 2023, 14, 4100. [Google Scholar] [CrossRef]
- WHO. WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. 2022. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 23 March 2023).
- Kennedy, R.B.; Ovsyannikova, I.; Poland, G.A. Smallpox vaccines for biodefense. Vaccine 2009, 27, D73–D79. [Google Scholar] [CrossRef]
- Petersen, B.W.; Damon, I.K.; A Pertowski, C.; Meaney-Delman, D.; Guarnizo, J.T.; Beigi, R.H.; Edwards, K.M.; Fisher, M.C.; E Frey, S.; Lynfield, R.; et al. Clinical guidance for smallpox vaccine use in a postevent vaccination program. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1–26. [Google Scholar]
- Damon, I.K.; Davidson, W.B.; Hughes, C.M.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Frey, S.E.; Newman, F.; Belshe, R.B.; Yan, L.; et al. Evaluation of smallpox vaccines using variola neutralization. J. Gen. Virol. 2009, 90, 1962–1966. [Google Scholar] [CrossRef]
- Hughes, C.M.; Newman, F.K.; Davidson, W.B.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Yan, L.; Frey, S.E.; Belshe, R.B.; Karem, K.L.; et al. Analysis of variola and vaccinia virus neutralization assays for smallpox vaccines. Clin. Vaccine Immunol. 2012, 19, 1116–1118. [Google Scholar] [CrossRef]
- Frey, S.E.; Wald, A.; Edupuganti, S.; Jackson, L.A.; Stapleton, J.T.; El Sahly, H.; El-Kamary, S.S.; Edwards, K.; Keyserling, H.; Winokur, P.; et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine 2015, 33, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.E.; Currie, D.W.; Kramarow, E.A.; Siddique, S.; Swanson, M.; Carter, R.J.; Kriss, J.L.; Boersma , P.M.; Lee, F.C.; Spicknall, I.; et al. JYNNEOS Vaccination Coverage Among Persons at Risk for Mpox - United States, May 22, 2022-January 31, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Africa CDC ; Gavi; UNICEF; WHO. Joint Press Release. Vaccine Doses Allocated to 9 African Countries Hardest Hit by Mpox Surge. Available online: https://www.who.int/news/item/06-11-2024-vaccine-doses-allocated-to-9-african-countries-hardest-hit-by-mpox-surge (accessed on 19 March 2025).
- Collier, A.-R.Y.; McMahan, K.; Jacob-Dolan, C.; Liu, J.; Borducchi, E.N.; Moss, B.; Barouch, D.H. Decline of Mpox Antibody Responses After Modified Vaccinia Ankara–Bavarian Nordic Vaccination. JAMA 2024, 332, 1669–1672. [Google Scholar] [CrossRef] [PubMed]
- Moschetta, N.; Raccagni, A.R.; Bianchi, M.; Diotallevi, S.; Lolatto, R.; Candela, C.; Foppa, C.U.; Gismondo, M.R.; Castagna, A.; Nozza, S.; et al. Mpox neutralising antibodies at 6 months from mpox infection or MVA-BN vaccination: A comparative analysis. Lancet Infect. Dis. 2023, 23, e455–e456. [Google Scholar] [CrossRef]
- Kottkamp, A.C.; Samanovic, M.I.; Duerr, R.; Oom, A.L.; Belli, H.M.; Zucker, J.R.; Rosen, J.B.; Mulligan, M.J. Antibody Titers against Mpox Virus after Vaccination. N. Engl. J. Med. 2023, 389, 2299–2301. [Google Scholar] [CrossRef]
- Christodoulidou, M.M.; A Mabbott, N. Efficacy of smallpox vaccines against Mpox infections in humans. Immunother. Adv. 2023, 3, ltad020. [Google Scholar] [CrossRef]
- Frey, S.E.; Winokur, P.L.; Salata, R.A.; El-Kamary, S.S.; Turley, C.B.; Walter, E.B.; Hay, C.M.; Newman, F.K.; Hill, H.R.; Zhang, Y.; et al. Safety and immunogenicity of IMVAMUNE® smallpox vaccine using different strategies for a post event scenario. Vaccine 2013, 31, 3025–3033. [Google Scholar] [CrossRef]
- Yu, Y.; Meza, K.; Colbert, C.; Hoft, D.F.; Jaunarajs, A.; Blazevic, A.; Frey, S.E.; Abate, G. Optimizing Microneutralization and IFN-γ ELISPOT Assays to Evaluate Mpox Immunity. Vaccines 2025, 13, 27. [Google Scholar] [CrossRef]
- Newman, F.K.; Frey, S.E.; Blevins, T.P.; Mandava, M.; Bonifacio, A., Jr.; Yan, L.; Belshe, R.B. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 2003, 41, 3154–3157. [Google Scholar] [CrossRef]
- von Krempelhuber, A.; Vollmar, J.; Pokorny, R.; Rapp, P.; Wulff, N.; Petzold, B.; Handley, A.; Mateo, L.; Siersbol, H.; Kollaritsch, H.; et al. A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE®. Vaccine 2010, 28, 1209–1216. [Google Scholar] [CrossRef]
- Crandell, J.; Monteiro, V.S.; Pischel, L.; Fang, Z.; Zhong, Y.; Lawres, L.; Conde, L.; Meira de Asis, G.; Maciel, G.; Zaleski, A.; et al. The impact of antigenic distance on Orthopoxvirus Vaccination and Mpox Infection for cross-protective immunity. medRxiv 2025, 2024-01. [Google Scholar] [CrossRef]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low levels of monkeypox virus-neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Meisinger-Henschel, C.; Späth, M.; Lukassen, S.; Wolferstätter, M.; Kachelriess, H.; Baur, K.; Dirmeier, U.; Wagner, M.; Chaplin, P.; Suter, M.; et al. Introduction of the six major genomic deletions of modified vaccinia virus Ankara (MVA) into the parental vaccinia virus is not sufficient to reproduce an MVA-like phenotype in cell culture and in mice. J. Virol. 2010, 84, 9907–9919. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Cimini, E.; Mazzotta, V.; Colavita, F.; Maggi, F.; Antinori, A. Mpox Immune response elicited by MVA-BN vaccine over 12 months of follow-up. J. Infect. 2024, 89, 106309. [Google Scholar] [CrossRef]
- Mack, T.M.; Noble, J.; Thomas, D.B. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 1972, 21, 214–218. [Google Scholar] [CrossRef]
- Belshe, R.B.; Newman, F.K.; Frey, S.E.; Couch, R.B.; Treanor, J.J.; Tacket, C.O.; Yan, L. Dose-dependent neutralizing-antibody responses to vaccinia. J. Infect. Dis. 2004, 189, 493–497. [Google Scholar] [CrossRef]
- Marchi, S.; Piccini, G.; Cantaloni, P.; Guerrini, N.; Zannella, R.; Coluccio, R.; Benincasa, L.; Solfanelli, N.; Remarque, E.J.; Viviani, S.; et al. Evaluation of monkeypox- and vaccinia-virus neutralizing antibodies before and after smallpox vaccination: A sero-epidemiological study. J. Med. Virol. 2024, 96, e29728. [Google Scholar] [CrossRef]
- Sarkar, J.; Mitra, A.; Mukherjee, M. The minimum protective level of antibodies in smallpox. Bull World Health Organ. 1975, 52, 307–311. [Google Scholar]
- Ilchmann, H.; Samy, N.; Reichhardt, D.; Schmidt, D.; Powell, J.D.; Meyer, T.P.H.; Silbernagl, G.; Nichols, R.; Weidenthaler, H.; De Moerlooze, L.; et al. One- and Two-Dose Vaccinations with Modified Vaccinia Ankara-Bavarian Nordic Induce Durable B-Cell Memory Responses Comparable to Replicating Smallpox Vaccines. J. Infect. Dis. 2022, 227, 1203–1213. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Bray, M.; Whitehouse, C.A.; Miller, D.; Mucker, E.; Manischewitz, J.; King, L.R.; Robert-Guroff, M.; Hryniewicz, A.; Venzon, D.; et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J. Infect. Dis. 2005, 191, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.M.; Elrosasy, A.; Mahmoud, A.M.; Saed, S.A.A.; Moawad, W.A.E.; Hamouda, E.; Nguyen, D.; Tran, V.P.; Pham, H.T.; Sah, S.; et al. The effect of HIV and mpox co-infection on clinical outcomes: Systematic review and meta-analysis. HIV Med. 2024, 25, 897–909. [Google Scholar] [CrossRef] [PubMed]


| MVA | Dryvax | ||||
|---|---|---|---|---|---|
| Time Point | Statistic | Mpox | Vaccinia | Mpox | Vaccinia |
| Visit 2 (pre-dose 1) | N | 34 | 34 | 6 | 6 |
| GMT (95% CI) a | 1.5 (1.0, 2.1) | 2.0 (NE) | 1.0 (1.0, 1.0) | 2.0 (2.0, 2.0) | |
| Spearman’s correlationcoefficient | -- | NE | -- | NE | |
| Day 14 (13–15) MVA post-dose 2vs. day 14 (13–15) post-Dryvax | N | 34 | 34 | 8 | 8 |
| GMT (95% CI) a | 10.9 (4.9, 24.2) | 179.3 (90.3, 355.9) | 49.4 (8.6, 284.1) | 13.5 (3.1, 59.9) | |
| Spearman’s correlationcoefficient | -- | 0.54 | -- | 0.78 | |
| Day 28 (26–30) MVA post-dose 2vs. day 28 (26–30) post-Dryvax | N | 35 | 35 | 8 | 8 |
| GMT (95% CI) a | 5.7 (2.9, 11.3) | 127.3 (66.0, 245.5) | 608.9 (130.4, 2842.9) | 90.0 (22.9, 353.4) | |
| Spearman’s correlationcoefficient | -- | 0.44 | -- | 0.17 | |
| Day 180 (177–183) MVA post-dose 2vs. day 180 (177–183) post-Dryvax | N | 34 | 34 | 8 | 8 |
| GMT (95% CI) a | 3.7 (2.1, 6.5) | 23.8 (16.1, 35.3) | 76.1 (18.0, 322.7) | 28.4 (11.8, 68.7) | |
| Spearman’s correlationcoefficient | -- | 0.32 | -- | 0.44 | |
| Day 365 (351–379) post-Dryvax | N | -- | -- | 8 | 8 |
| GMT (95% CI) a | -- | -- | 76.1 (18.0, 322.7) | 23.8 (9.0, 62.8) | |
| Spearman’s correlationcoefficient | -- | -- | -- | 0.24 | |
| Peak response b | N | 35 | 35 | 8 | 8 |
| GMT (95% CI) a | 30.8 (14.6, 65.0) | 266.1 (156.0, 453.9) | 789.6 (238.5, 2614.1) | 123.7 (37.6, 406.7) | |
| Spearman’s correlationcoefficient | -- | 0.26 | -- | 0.11 | |
| Time Point | Statistic | All MVA | MVA Subcutaneous | MVA Intradermal | Dryvax |
|---|---|---|---|---|---|
| Visit 2 (pre-dose 1) | N | 34 | 15 | 19 | 17 |
| GMT (95% CI) a | 1.5 (1.0, 2.1) | 1.4 (0.8, 2.3) | 1.5 (0.9, 2.6) | 1.4 (0.9, 2.1) | |
| GMTR c (95% CI) a | 1.5 (1.0, 2.1) | 1.4 (0.8, 2.3) | 1.5 (0.9, 2.6) | -- | |
| Day 14 (13–15) MVA post-dose 2vs. day 14 (13–15) post-Dryvax | N | 34 | 14 | 20 | 8 |
| GMT (95% CI) a | 10.9 (4.9, 24.2) | 21.5 (5.3, 87.5) | 6.7 (2.5, 18.1) | 49.4 (8.6, 284.1) | |
| GMTR c (95% CI) a | 0.2 (0.0, 1.4) | 0.4 (0.1, 3.5) | 0.1 (0.0, 0.9) | -- | |
| GMFR b (95% CI) a | 7.5 (3.9, 14.5) | 15.2 (4.7, 49.0) | 4.5 (2.1, 9.5) | 22.6 (3.6, 140.5) | |
| Day 28 (26–30) MVA post-dose 2vs. day 28 (26–30) post-Dryvax | N | 35 | 15 | 20 | 8 |
| GMT (95% CI) a | 5.7 (2.9, 11.3) | 8.8 (2.6, 30.0) | 4.1 (1.8, 9.6) | 608.9 (130.4, 2842.9) | |
| GMTR c (95% CI) a | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.0) | -- | |
| GMFR b (95% CI) a | 4.1 (2.4, 7.0) | 6.3 (2.6, 15.7) | 2.9 (1.4, 5.8) | 645.1 (68.8, 6048.0) | |
| Day 180 (177–183) MVA post-dose 2vs. day 180 (177–183) post-Dryvax | N | 34 | 14 | 20 | 8 |
| GMT (95% CI) a | 3.7 (2.1, 6.5) | 3.4 (1.4, 8.5) | 3.9 (1.8, 8.5) | 76.1 (18.0, 322.7) | |
| GMTR c (95% CI) a | 0.0 (0.0, 0.2) | 0.0 (0.0, 0.2) | 0.1 (0.0, 0.2) | -- | |
| GMFR b (95% CI) a | 2.6 (1.6, 4.1) | 2.4 (1.1, 5.4) | 2.7 (1.5, 4.9) | 64.0 (7.8, 527.0) | |
| Day 365 (351–379) post-Dryvax | N | -- | -- | -- | 8 |
| GMT (95% CI) a | -- | -- | -- | 76.1 (18.0, 322.7) | |
| GMFR b (95% CI) a | -- | -- | -- | 57.0 (8.4, 388.9) | |
| Peak response d | N | 35 | 15 | 20 | 19 |
| GMT (95% CI) a | 30.8 (14.6, 65.0) | 30.6 (8.6, 108.7) | 30.9 (11.3, 84.6) | 368.7 (188.4, 721.7) | |
| GMTR c (95% CI) a | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.2) | 0.0 (0.0, 0.2) | -- | |
| GMFR b (95% CI) a | 20.0 (10.9, 36.7) | 22.1 (8.3, 59.1) | 18.5 (8.0, 43.1) | 245.8 (100.4, 601.6) |
| Time Point | Statistic | All MVA | MVA Subcutaneous | MVA Intradermal | Dryvax |
|---|---|---|---|---|---|
| Visit 2 (pre-dose 1) | N | 35 | 15 | 20 | 6 |
| Median (min, max) | 4.4 (0, 325.6) | 4.4 (0, 325.6) | 7.2 (0, 34.1) | 9.9 (0, 23.1) | |
| Mean (95% CI) a | 16.8 (5.8, 36.3) | 26.5 (3.5, 70.5) | 9.6 (5.8, 13.8) | 11.6 (5.5, 17.6) | |
| Difference in means (95% CI) a | 5.3 (−8.9, 27.1) | 15 (−11.1, 61.1) | −2 (−9.1, 5.3) | -- | |
| Day 14 (13–15) MVA post-dose 2vs. day 14 (13–15) post-Dryvax | N | 34 | 14 | 20 | 8 |
| Median (min, max) | 13.8 (0, 613.8) | 13.8 (1.1, 613.8) | 14.9 (0, 158.4) | 51.2 (14.3, 90.2) | |
| Mean (95% CI) a | 67.2 (31.1, 115.4) | 106.5 (25.7, 208.4) | 39.8 (20.6, 61.1) | 53.6 (33.9, 72.9) | |
| Mean difference from baseline (95% CI) a | 50 (22.5, 83.6) | 78.2 (19.9, 147.5) | 30.2 (11.2, 50.9) | 38.5 (12.5, 65.7) | |
| Difference in means (95% CI) a | 13.6 (−27.7, 63.4) | 52.8 (−31.9, 158.1) | −13.9 (−41, 15.1) | -- | |
| Day 28 (26–30) MVA post-dose 2vs. day 28 (26–30) post-Dryvax | N | 32 | 12 | 20 | 8 |
| Median (min, max) | 8.3 (0, 511.5) | 23.7 (0, 511.5) | 5 (0, 77) | 17.6 (0, 73.7) | |
| Mean (95% CI) a | 36.4 (14.4, 71.6) | 69.4 (15.8, 155.7) | 16.6 (7.8, 26.9) | 30.9 (14, 49.6) | |
| Mean difference from baseline (95% CI) a | 18.1 (5.7, 33.6) | 36.7 (9.5, 71) | 7 (−2.5, 17.3) | 26 (3.7, 49.9) | |
| Difference in means (95% CI) b | 5.4 (−25.3, 44) | 38.5 (−20, 127.7) | −14.4 (−35.6, 5.6) | -- | |
| Day 180 (177–183) MVA post-dose 2vs. day 180 (177–183) post-Dryvax | N | 34 | 14 | 20 | 8 |
| Median (min, max) | 3.9 (0, 172.7) | 2.2 (0, 172.7) | 4.7 (0, 129.8) | 19.3 (5.5, 25.3) | |
| Mean (95% CI) a | 14.7 (5.1, 28.6) | 17.4 (2.6, 42.5) | 12.9 (4.8, 26.8) | 16.5 (10.7, 22.1) | |
| Mean difference from baseline (95% CI) a | −2.5 (−15.2, 8.7) | −10.8 (−34.4, 3.8) | 3.3 (−6.2, 18.6) | 2.2 (−9, 12.5) | |
| Difference in means (95% CI) b | −1.8 (−13.1, 12.6) | 0.9 (−16.2, 27.7) | −3.6 (−14.8, 11.5) | -- | |
| Day 365 (351–379) post-Dryvax | N | -- | -- | -- | 8 |
| Median (min, max) | -- | -- | -- | 23.7 (0, 79.2) | |
| Mean (95% CI) a | -- | -- | -- | 27.9 (14.1, 45) | |
| Mean difference from baseline (95% CI) a | -- | -- | -- | 19.1 (−3.7, 44.5) | |
| Peak response c | N | 35 | 15 | 20 | 8 |
| Median (min, max) | 38.5 (0, 727.1) | 25.3 (4.4, 727.1) | 39.1 (0, 424.6) | 68.8 (23.1, 90.2) | |
| Mean (95% CI) a | 104.3 (61, 158.7) | 127 (43.1, 238.3) | 87.3 (46, 137) | 61.2 (44.3, 77.7) | |
| Mean difference from baseline (95% CI) a | 87.5 (51.8, 129.1) | 100.5 (38.7, 172.5) | 77.7 (35.9, 127.9) | 48.6 (22.7, 70.8) | |
| Difference in means (95% CI) b | 43.1 (−4.8, 98.6) | 65.8 (−16.8, 178.8) | 26.1 (−18.6, 78.8) | -- |
| Statistic | All MVA | MVA Subcutaneous | MVA Intradermal | Dryvax |
|---|---|---|---|---|
| N | 34 | 14 | 20 | 8 |
| Median (min, max) | 0 (0, 166) | 14 (0, 166) | 0 (0, 166) | 83 (14, 166) |
| Mean (95% CI) a | 20 (7, 37.1) | 29.7 (6, 61.3) | 13.2 (2.8, 30.5) | 88.3 (33, 143.5) |
| Difference in means (95% CI) a | −68.3 (−121.5, −15.4) | −58.5 (−117.3, 0.4) | −75.1 (−126.9, −21.5) | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abate, G.; Meza, K.; Yu, Y.; Colbert, C.; Jaunarajs, A.; Blazevic, A.; Hoft, D.F.; Frey, S.E. Differences in Mpox and Vaccinia Immunity Induced by Non-Replicating and Replicating Vaccinia-Based Vaccines. Vaccines 2025, 13, 520. https://doi.org/10.3390/vaccines13050520
Abate G, Meza K, Yu Y, Colbert C, Jaunarajs A, Blazevic A, Hoft DF, Frey SE. Differences in Mpox and Vaccinia Immunity Induced by Non-Replicating and Replicating Vaccinia-Based Vaccines. Vaccines. 2025; 13(5):520. https://doi.org/10.3390/vaccines13050520
Chicago/Turabian StyleAbate, Getahun, Krystal Meza, Yinyi Yu, Chase Colbert, Anna Jaunarajs, Azra Blazevic, Daniel F. Hoft, and Sharon E. Frey. 2025. "Differences in Mpox and Vaccinia Immunity Induced by Non-Replicating and Replicating Vaccinia-Based Vaccines" Vaccines 13, no. 5: 520. https://doi.org/10.3390/vaccines13050520
APA StyleAbate, G., Meza, K., Yu, Y., Colbert, C., Jaunarajs, A., Blazevic, A., Hoft, D. F., & Frey, S. E. (2025). Differences in Mpox and Vaccinia Immunity Induced by Non-Replicating and Replicating Vaccinia-Based Vaccines. Vaccines, 13(5), 520. https://doi.org/10.3390/vaccines13050520






