SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposures: HIV Serostatus and COVID-19 Vaccination Status
2.3. Outcomes: SARS-CoV-2 Antibodies
2.4. Covariates: Sociodemographic Characteristics, Substance Use, and Comorbidities
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Li, Z.; Ding, S.; Liu, S.; Tang, Z.; Jia, L.; Liu, J.; Liu, Y. HIV infection and risk of COVID-19 mortality: A meta-analysis. Medicine 2021, 100, e26573. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Casado, J.L.; Härter, G.; Vizcarra, P.; Moreno, A.; Cattaneo, D.; Meraviglia, P.; Spinner, C.D.; Schabaz, F.; Grunwald, S.; et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ming, F.; Dong, Y.; Zhang, Q.; Liu, L.; Gao, M.; Zhang, X.; Mo, P.; Feng, Y.; Tang, W.; et al. Driving Force of COVID-19 Among People Living with HIV/AIDS in Wuhan, China. Res. Sq. 2020, 34, 1364–1371. [Google Scholar] [CrossRef]
- Goodnow, C.C.; Vinuesa, C.G.; Randall, K.L.; Mackay, F.; Brink, R. Control systems and decision making for antibody production. Nat. Immunol. 2010, 11, 681–688. [Google Scholar] [CrossRef]
- Uhl, L.F.K.; Gérard, A. Modes of Communication Between T Cells and Relevance for Immune Responses. Int. J. Mol. Sci. 2020, 21, 2674. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Le Hingrat, Q.; Sereti, I.; Landay, A.L.; Pandrea, I.; Apetrei, C. The Hitchhiker Guide to CD4+ T-Cell Depletion in Lentiviral Infection. A Critical Review of the Dynamics of the CD4+ T Cells in SIV and HIV Infection. Front. Immunol. 2021, 12, 695674. [Google Scholar] [CrossRef]
- Cai, C.W.; Sereti, I. Residual immune dysfunction under antiretroviral therapy. Semin. Immunol. 2021, 51, 101471. [Google Scholar] [CrossRef]
- El Chaer, F.; El Sahly, H.M. Vaccination in the Adult Patient Infected with HIV: A Review of Vaccine Efficacy and Immunogenicity. Am. J. Med. 2019, 132, 437–446. [Google Scholar] [CrossRef]
- Nunes, M.C.; Madhi, S.A. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum. Vaccin. Immunother. 2012, 8, 161–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitaker, J.A.; Rouphael, N.G.; Edupuganti, S.; Lai, L.; Mulligan, M.J. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect. Dis. 2012, 12, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Belaunzarán-Zamudio, P.F.; García-León, M.L.; Wong-Chew, R.M.; Villasís-Keever, A.; Cuellar-Rodríguez, J.; Mosqueda-Gómez, J.L.; Muñoz-Trejo, T.; Escobedo, K.; Santos, J.I.; Ruiz-Palacios, G.M.; et al. Early loss of measles antibodies after MMR vaccine among HIV-infected adults receiving HAART. Vaccine 2009, 27, 7059–7064. [Google Scholar] [CrossRef] [PubMed]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.-Y. Long-Term Immune Responses to Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Veit, O.; Niedrig, M.; Chapuis-Taillard, C.; Cavassini, M.; Mossdorf, E.; Schmid, P.; Bae, H.G.; Litzba, N.; Staub, T.; Hatz, C.; et al. Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin. Infect. Dis. 2009, 48, 659–666. [Google Scholar] [CrossRef]
- Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations#:~:text=For%20people%20with%20HIV%20receiving,years%20throughout%20life%20(BIII) (accessed on 29 January 2023).
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2023, 42, 393–414. [Google Scholar] [CrossRef]
- Bian, L.; Gao, F.; Zhang, J.; He, Q.; Mao, Q.; Xu, M.; Liang, Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 365–373. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Kandikattu, H.K.; Yadavalli, C.S.; Venkateshaiah, S.U.; Mishra, A. Vaccine efficacy in mutant SARS-CoV-2 variants. Int. J. Cell Biol. Physiol. 2021, 4, 1–12. [Google Scholar]
- Bian, L.; Liu, J.; Gao, F.; Gao, Q.; He, Q.; Mao, Q.; Wu, X.; Xu, M.; Liang, Z. Research progress on vaccine efficacy against SARS-CoV-2 variants of concern. Human. Vaccines Immunother. 2022, 18, 2057161. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Benefits of Getting A COVID-19 Vaccine. Available online: https://www.cdc.gov/covid/vaccines/benefits.html?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html (accessed on 29 January 2023).
- Levy, I.; Wieder-Finesod, A.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Nault, L.; Marchitto, L.; Goyette, G.; Tremblay-Sher, D.; Fortin, C.; Martel-Laferrière, V.; Trottier, B.; Richard, J.; Durand, M.; Kaufmann, D.; et al. COVID-19 vaccine immunogenicity in people living with HIV-1. Vaccine 2022, 40, 3633–3637. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, H.R.; Mwimanzi, F.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Speckmaier, S.; Moran-Garcia, N.; Datwani, S.; et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses. medRxiv 2022. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2022, 74, 1268–1270. [Google Scholar] [CrossRef]
- Hassold, N.; Brichler, S.; Ouedraogo, E.; Leclerc, D.; Carroue, S.; Gater, Y.; Alloui, C.; Carbonnelle, E.; Bouchaud, O.; Mechai, F.; et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. Aids 2022, 36, F1–F5. [Google Scholar] [CrossRef]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef]
- Lugoboni, F.; Quaglio, G.; Pajusco, B.; Civitelli, P.; Romanò, L.; Bossi, C.; Spilimbergo, I.; Mezzelani, P. Immunogenicity, reactogenicity and adherence to a combined hepatitis A and B vaccine in illicit drug users. Addiction 2004, 99, 1560–1564. [Google Scholar] [CrossRef]
- Kuronuma, K.; Takahashi, H. Immunogenicity of pneumococcal vaccines in comorbid autoimmune and chronic respiratory diseases. Human. Vaccines Immunother. 2019, 15, 859–862. [Google Scholar] [CrossRef]
- Dietz, L.L.; Juhl, A.K.; Søgaard, O.S.; Reekie, J.; Nielsen, H.; Johansen, I.S.; Benfield, T.; Wiese, L.; Stærke, N.B.; Jensen, T.Ø.; et al. Impact of age and comorbidities on SARS-CoV-2 vaccine-induced T cell immunity. Commun. Med. 2023, 3, 58. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol. Res. 2015, 37, 185–197. [Google Scholar]
- Shaikh, S.R.; Beck, M.A.; Alwarawrah, Y.; MacIver, N.J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 2023, 20, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef] [PubMed]
- Honardoost, M.; Janani, L.; Aghili, R.; Emami, Z.; Khamseh, M.E. The Association between Presence of Comorbidities and COVID-19 Severity: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2021, 50, 132–140. [Google Scholar] [CrossRef]
- Faizo, A.A.; Qashqari, F.S.; El-Kafrawy, S.A.; Barasheed, O.; Almashjary, M.N.; Alfelali, M.; Bawazir, A.A.; Albarakati, B.M.; Khayyat, S.A.; Hassan, A.M.; et al. A potential association between obesity and reduced effectiveness of COVID-19 vaccine-induced neutralizing humoral immunity. J. Med. Virol. 2023, 95, e28130. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH) Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP). About RADx-UP. Available online: https://radx-up.org/about/ (accessed on 8 March 2023).
- Degarege, A.; Krupp, K.; Tamargo, J.; Martinez, S.S.; Campa, A.; Baum, M. Polysubstance use and adherence to antiretroviral treatment in the Miami Adult Studies on HIV (MASH) cohort. AIDS Care 2022, 34, 639–646. [Google Scholar] [CrossRef]
- Tamargo, J.A.; Martin, H.R.; Diaz-Martinez, J.; Delgado-Enciso, I.; Johnson, A.; Bastida Rodriguez, J.A.; Trepka, M.J.; Brown, D.R.; Garba, N.A.; Roldan, E.O.; et al. Drug use and COVID-19 testing, vaccination, and infection among underserved, minority communities in Miami, Florida. PLoS ONE 2024, 19, e0297327. [Google Scholar] [CrossRef]
- Adjobimey, T.; Meyer, J.; Sollberg, L.; Bawolt, M.; Berens, C.; Kovacevic, P.; Trudic, A.; Parcina, M.; Hoerauf, A. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization with Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm's COVID-19 Vaccines. Front. Immunol. 2022, 13, 917905. [Google Scholar] [CrossRef]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.E.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliveira, M.H.; Catahay, J.A.; Khan, M.S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Kelliher, M.T.; Levy, J.J.; Nerenz, R.D.; Poore, B.; Johnston, A.A.; Rogers, A.R.; Stella, M.E.O.; Snow, S.E.; Cervinski, M.A.; Hubbard, J.A. Comparison of Symptoms and Antibody Response Following Administration of Moderna or Pfizer SARS-CoV-2 Vaccines. Arch. Pathol. Lab. Med. 2022, 146, 677–685. [Google Scholar] [CrossRef] [PubMed]
- RADx® Underserved Populations (RADx-UP). NIH RADx-UP Common Data Elements. Available online: https://radx-up.org/about/ (accessed on 13 March 2023).
- National Institute on Alcohol Abuse and Alcoholism. The Physicians' Guide to Helping Patients with Alcohol Problems; Government Printing Office: Washington, DC, USA, 1995. [Google Scholar]
- Johns Hopkins University. Community Collaboration to Combat Coronavirus (C4-Ward) Module Five: Comorbidities and Care Engagement. Available online: https://www.phenxtoolkit.org/toolkit_content/PDF/JHU_C4WARD_Health.pdf (accessed on 13 March 2023).
- Little, R.J.; D’Agostino, R.; Cohen, M.L.; Dickersin, K.; Emerson, S.S.; Farrar, J.T.; Frangakis, C.; Hogan, J.W.; Molenberghs, G.; Murphy, S.A.; et al. The prevention and treatment of missing data in clinical trials. N. Engl. J. Med. 2012, 367, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.E.; Frontera, W.R.; Andrasik, M.P.; del Rio, C.; Mondríguez-González, A.; Price, S.A.; Krantz, E.M.; Pergam, S.A.; Silver, J.K. Assessment of the inclusion of racial/ethnic minority, female, and older individuals in vaccine clinical trials. JAMA Netw. Open 2021, 4, e2037640. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 15 January 2025).
- Tamargo, J.A.; Martin, H.R.; Diaz-Martinez, J.; Trepka, M.J.; Delgado-Enciso, I.; Johnson, A.; Mandler, R.N.; Siminski, S.; Gorbach, P.M.; Baum, M.K. COVID-19 Testing and the Impact of the Pandemic on the Miami Adult Studies on HIV Cohort. J. Acquir. Immune Defic. Syndr. 2021, 87, 1016–1023. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y. Racial and ethnic and income disparities in COVID-19 vaccination among Medicare beneficiaries. J. Am. Geriatr. Soc. 2022, 70, 2638–2645. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, C.; Li, Q.; Wang, Z.; Jiang, Y. COVID-19 vaccine hesitancy cannot fully explain disparities in vaccination coverage across the contiguous United States. Vaccine 2022, 40, 5471–5482. [Google Scholar] [CrossRef]
- Martin, H.R.; Brown, D.R.; Fluney, E.; Trepka, M.J.; Marty, A.M.; Roldan, E.O.; Liu, Q.; Barbieri, M.A.; Baum, M.K. Community-Engaged Research: COVID-19 Testing, Infection, and Vaccination among Underserved Minority Communities in Miami, Florida. Vaccines 2024, 12, 117. [Google Scholar] [CrossRef]
- Smith, B.A.; Ricotta, E.E.; Kwan, J.L.; Evans, N.G. COVID-19 risk perception and vaccine acceptance in individuals with self-reported chronic respiratory or autoimmune conditions. Allergy Asthma Clin. Immunol. 2023, 19, 37. [Google Scholar] [CrossRef]
- Molina, J.M.; Grund, B.; Gordin, F.; Williams, I.; Schechter, M.; Losso, M.; Law, M.; Ekong, E.; Mwelase, N.; Skoutelis, A.; et al. Which HIV-infected adults with high CD4 T-cell counts benefit most from immediate initiation of antiretroviral therapy? A post-hoc subgroup analysis of the START trial. Lancet HIV 2018, 5, e172–e180. [Google Scholar] [CrossRef]
- Sharma, S.; Schlusser, K.E.; de la Torre, P.; Tambussi, G.; Draenert, R.; Pinto, A.N.; Metcalf, J.A.; Neaton, J.D.; Laeyendecker, O. The benefit of immediate compared with deferred antiretroviral therapy on CD4+ cell count recovery in early HIV infection. Aids 2019, 33, 1335–1344. [Google Scholar] [CrossRef]
- Tchakoute, C.T.; Rhee, S.-Y.; Hare, C.B.; Shafer, R.W.; Sainani, K. Adherence to contemporary antiretroviral treatment regimens and impact on immunological and virologic outcomes in a US healthcare system. PLoS ONE 2022, 17, e0263742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, Y.; Zeng, F.; Meng, Y.; Liu, H.; Deng, G. Correlation between CD4 T-Cell Counts and Seroconversion Among COVID-19 Vaccinated Patients with HIV: A Meta-Analysis. Vaccines 2023, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Koblischke, M.; Traugott, M.T.; Medits, I.; Spitzer, F.S.; Zoufaly, A.; Weseslindtner, L.; Simonitsch, C.; Seitz, T.; Hoepler, W.; Puchhammer-Stöckl, E.; et al. Dynamics of CD4 T Cell and Antibody Responses in COVID-19 Patients with Different Disease Severity. Front. Med. 2020, 7, 592629. [Google Scholar] [CrossRef]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Surgo Ventures. Surgo Precision for COVID: The U.S. COVID Community Vulnerability Index (CCVI). Available online: https://precisionforcovid.org/ccvi (accessed on 23 January 2023).
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
| People Without HIV (n = 927) | PLWH (n = 390) | p k | |||||
|---|---|---|---|---|---|---|---|
| Variable a | Non-Vaccinated (n = 352) | Vaccinated (n = 575, 62.0%) | p | Non-Vaccinated (n = 93) | Vaccinated (n = 297, 76.2%) | p | |
| Sex, male | 169 (48.0) | 265 (46.0) | 0.569 | 54 (58.1) | 168 (56.6) | 0.893 | <0.001 |
| Age, years | 53.6 (41.5–60.5) | 59.5 (51.1–64.8) | <0.001 | 56.2 (52.5–63.0) | 58.4 (54.8–63.7) | 0.102 | 0.075 |
| Race/Ethnicity | |||||||
| Black, non-Hispanic | 183 (52.0) | 218 (37.9) | <0.001 | 75 (80.6) | 183 (61.6) | 0.004 | <0.001 |
| White, non-Hispanic | 22 (6.3) | 25 (4.3) | 3 (3.2) | 19 (6.4) | |||
| White, Hispanic | 120 (34.1) | 291 (50.6) | 11 (11.8) | 82 (27.6) | |||
| Other b | 27 (7.7) | 41 (7.1) | 4 (4.3) | 13 (4.4) | |||
| CD4+ T, (cells/µL) | N/A | N/A | N/A | 517.5 (244.5–722.0) | 577.5 (416.8–876.8) | 0.011 | N/A |
| <200 | 17 (18.2) | 23 (7.7) | 0.010 | N/A | |||
| ≥200–<500 | 21 (22.6) | 81 (27.2) | |||||
| ≥500 | 40 (43.0) | 160 (53.9) | |||||
| Unknown/missing | 15 (16.1) | 33 (11.1) | |||||
| On ART | N/A | N/A | N/A | 89 (95.7) | 275 (92.6) | 0.770 | N/A |
| HIV viral load, (copies/mL) | N/A | N/A | N/A | ||||
| <200 | 51 (54.8) | 227 (76.4) | <0.001 | N/A | |||
| ≥200–<5000 | 10 (10.8) | 17 (5.7) | |||||
| >5000 | 13 (14.0) | 19 (6.4) | |||||
| Unknown/missing | 19 (20.4) | 34 (11.4) | |||||
| Log(10) HIV viral load (copies/mL) | 1.24 (0.70–3.27) | 1.17 (0.70–1.76) | 0.004 | N/A | |||
| BMI, kg/m2 | 27.7 (24.4–33.0) | 28.3 (25.0–32.9) | 0.928 | 25.8 (23.1–30.2) | 27.4 (24.0–31.7) | 0.054 | 0.040 |
| <18.5 | 2 (0.6) | 5 (0.9) | 0.610 | 3 (3.2) | 8 (2.7) | 0.163 | 0.046 |
| ≥18.5–<25 | 103 (29.3) | 138 (24.0) | 37 (39.8) | 84 (28.2) | |||
| ≥25–<30 | 119 (33.8) | 211 (36.7) | 29 (31.2) | 102 (34.2) | |||
| ≥30 | 128 (36.4) | 221 (38.4) | 24 (25.8) | 103 (34.6) | |||
| Interval between vaccination dose 2 and serology collection, days | N/A | 100.0 (65.0–133.3) | N/A | N/A | 101.4 (56.0–127.0) | N/A | 0.051 |
| <14 | 27 (4.7) | 17 (5.7) | <0.001 | ||||
| ≥14–<180 | 415 (72.2) | 243 (81.8) | |||||
| ≥180 | 133 (23.1) | 37 (12.5) | |||||
| Substance use | 174 (49.4) | 225 (39.1) | 0.002 | 70 (75.3) | 232 (78.1) | 0.567 | <0.001 |
| Hazardous drinking c | 31 (8.8) | 28 (4.9) | 0.972 | 25 (26.7) | 43 (14.5) | 0.006 | <0.001 |
| Drug use, any d | 118 (33.5) | 101 (17.5) | <0.001 | 35 (37.6) | 94 (31.6) | 0.345 | <0.001 |
| Marijuana | 105 (29.8) | 88 (15.3) | <0.001 | 29 (31.2) | 79 (26.6) | 0.004 | <0.001 |
| Cocaine | 36 (10.2) | 26 (4.5) | 15 (16.1) | 30 (10.1) | |||
| Other drugs e | 4 (1.1) | 4 (0.7) | 3 (3.3) | 6 (2.4) | |||
| Cigarette smoking | 149 (42.3) | 162 (28.1) | <0.001 | 41 (44.1) | 103 (34.7) | 0.101 | 0.047 |
| Substance use disorder | 33 (9.4) | 27 (4.7) | 0.005 | 11 (11.8) | 28 (9.4) | 0.501 | 0.006 |
| Comorbidities | |||||||
| Hypertension | 118 (33.5) | 251 (43.7) | 0.002 | 45 (48.4) | 162 (54.5) | 0.358 | 0.002 |
| Diabetes | 45 (12.8) | 123 (21.4) | 0.001 | 13 (14.0) | 73 (24.6) | 0.045 | 0.286 |
| Autoimmune disease | 7 (2.0) | 21 (3.7) | 0.159 | 4 (4.3) | 16 (5.4) | 0.794 | 0.231 |
| Obesity | 128 (36.4) | 221 (38.4) | 0.248 | 24 (25.8) | 103 (34.7) | 0.142 | <0.001 |
| Chronic kidney disease | 3 (0.9) | 13 (2.3) | 0.124 | 1 (1.1) | 21 (7.1) | 0.036 | <0.001 |
| ≥Comorbidity f | 199 (56.5) | 378 (65.7) | 0.015 | 59 (63.4) | 225 (75.8) | 0.028 | 0.002 |
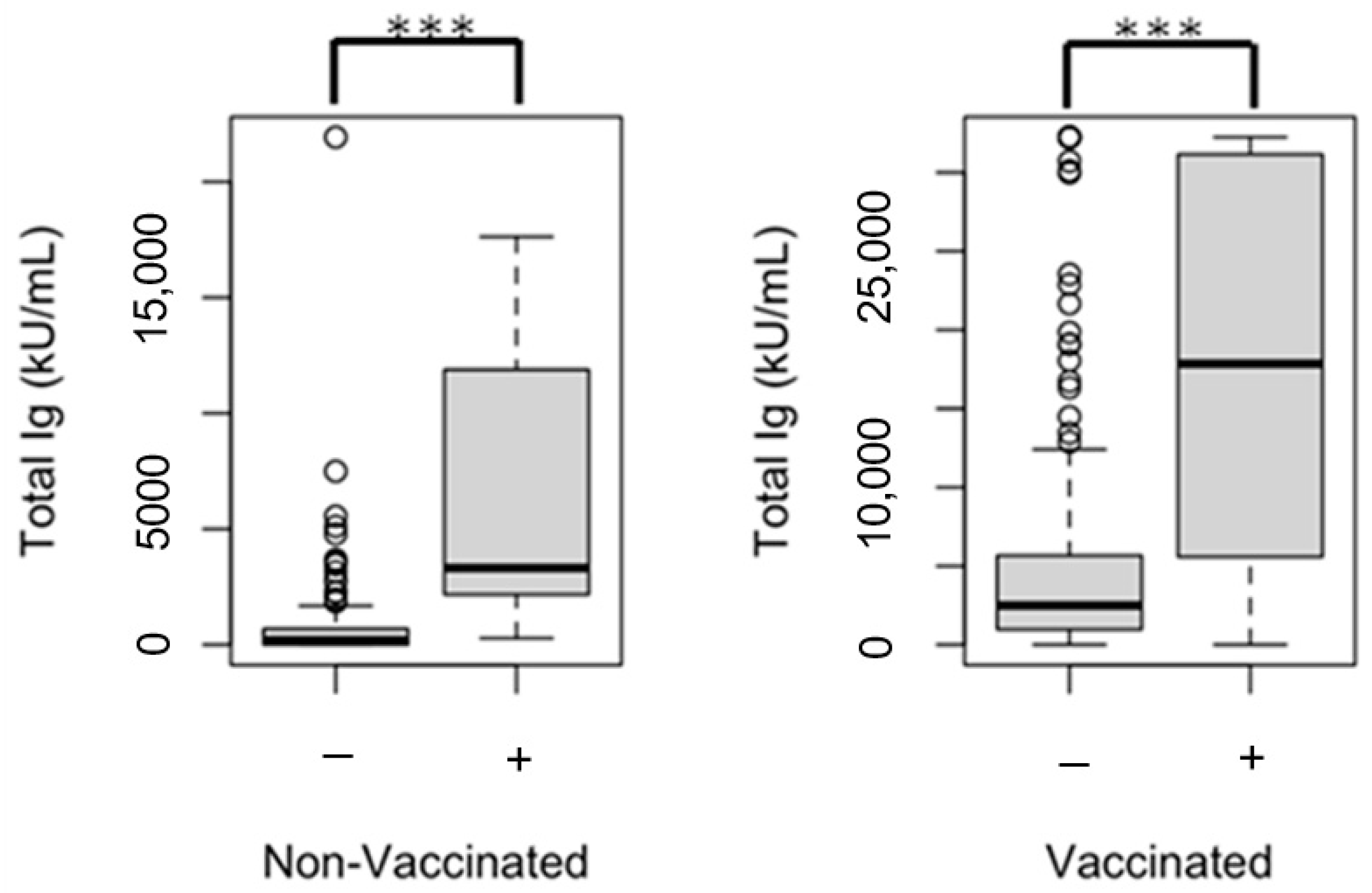
| SARS-CoV-2 spike (trimer) total Ig, (kU/mL) g | 473 (0–2251) | 2927 (1114–6411) | <0.001 | 209 (0–1353) | 2786 (1049–6415) | <0.001 | 0.650 |
| ≥1000 h | 125 (35.5) | 446 (77.6) | <0.001 | 25 (26.9) | 207 (74.2) | <0.001 | 0.453 |
| SARS-CoV-2 nucleocapsid IgG, (U/mL) i | 2.26 (1.34–4.36) | 2.16 (1.19–4.16) | 0.001 | 2.12 (1.38–4.75) | 2.40 (1.20–6.72) | 0.698 | 0.044 |
| ≥20 j | 33 (9.4) | 22 (3.8) | 0.001 | 5 (5.4) | 24 (8.1) | 0.522 | 0.008 |
| Variable a | 1 Dose (n = 33) | 2 Doses (n = 264, 88.9%) | p |
|---|---|---|---|
| Sex, male | 22 (66.7) | 146 (55.3) | 0.214 |
| Age, years | 56.2 (52.8–60.1) | 58.9 (55.2–63.9) | 0.647 |
| Race/Ethnicity | 0.843 | ||
| Black, non-Hispanic | 18 (54.6) | 165 (62.5) | |
| White, non-Hispanic | 3 (9.1) | 16 (6.1) | |
| White, Hispanic | 12 (36.4) | 70 (26.5) | |
| Other b | 0 (0) | 13 (4.9) | |
| Vaccine brand | N/A k | ||
| Ad26.COV2.S COVID-19 vaccine | 22 (66.7) | 3 (1.1) | |
| mRNA-1273 or BNT162b2 mRNA COVID-19 vaccine | 11 (33.3) | 253 (95.8) | |
| Other | 0 (0) | 8 (3.0) | |
| CD4+ T, (cells/µL) | 554.0 (385.0–743.5) | 581.0 (419.0–886.0) | 0.488 |
| <200 | 5 (15.2) | 18 (6.0) | 0.523 |
| ≥200–<500 | 8 (24.2) | 73 (24.5) | |
| ≥500 | 18 (54.5) | 142 (47.7) | |
| Unknown/missing | 2 (6.1) | 32 (10.7) | |
| On ART | 30 (90.9) | 245 (92.8) | |
| HIV viral load, (copies/mL) | |||
| <200 | 27 (81.8) | 200 (75.8) | 0.900 |
| ≥200–<5000 | 1 (3.0) | 16 (6.1) | |
| >5000 | 3 (9.1) | 16 (6.1) | |
| Unknown/missing | 2 (6.1) | 33 (12.5) | |
| Log(10) HIV viral load (copies/mL) | 1.18 (0.7–1.98) | 1.18 (0.69–1.71) | 0.805 |
| BMI, kg/m2 | 27.0 (23.1–31.6) | 27.5 (24.2–31.8) | 0.584 |
| <18.5 | 1 (3.0) | 7 (2.7) | 0.991 |
| ≥18.5–<25 | 11 (33.3) | 73 (27.7) | |
| ≥25–<30 | 10 (30.3) | 92 (34.8) | |
| ≥30 | 11 (33.3) | 92 (34.8) | |
| Interval between vaccination dose 2 and serology collection, days | 81.0 (50.0–104.0) | 88.5 (56.0–136.0) | 0.887 |
| <14 | 6 (18.2) | 11 (4.2) | 0.711 |
| ≥14–<180 | 26 (78.8) | 217 (82.2) | |
| ≥180 | 1 (3.0) | 36 (13.6) | |
| Substance use | 26 (78.8) | 206 (78.0) | 0.860 |
| Hazardous drinking c | 4 (12.1) | 41 (15.5) | 0.206 |
| Drug use, any d | 15 (45.5) | 79 (29.9) | 0.059 |
| Marijuana | 14 (42.4) | 65 (24.6) | 0.086 |
| Cocaine | 5 (15.2) | 25 (9.5) | 0.188 |
| Other drugs e | 2 (6.1) | 5 (1.9) | 0.980 |
| Cigarette smoking | 17 (51.5) | 86 (32.6) | 0.275 |
| Substance use disorder | 3 (9.1) | 25 (9.5) | 0.540 |
| Comorbidities | |||
| Hypertension | 16 (48.5) | 146 (55.3) | 0.970 |
| Diabetes | 7 (21.2) | 66 (25.0) | 0.456 |
| Autoimmune disease | 1 (3.0) | 15 (5.7) | 0.630 |
| Obesity | 11 (33.3) | 92 (34.8) | 0.532 |
| Chronic kidney disease | 1 (3.0) | 20 (7.6) | 0.266 |
| ≥comorbidity f | 23 (69.7) | 202 (76.5) | 0.577 |
| SARS-CoV-2 spike (trimer) total Ig, (kU/mL) g | 610 (72–2553) | 2949 (1404–6718) | 0.048 |
| ≥1000 h | 11 (33.3) | 214 (81.1) | <0.001 |
| SARS-CoV-2 nucleocapsid IgG, (U/mL) i | 1.73 (0.87–3.54) | 2.44 (1.24–7.24) | 0.700 |
| ≥20 U/mL j | 2 (6.1) | 22 (8.3) | 0.524 |
| Variable | Adjusted Model | Unadjusted Model | ||||
|---|---|---|---|---|---|---|
| β [95% CI] | t | p | β | t | p | |
| Sex | ||||||
| Female | −0.072 [−0.232, 0.088] | −1.162 | 0.247 | 0.038 | 0.655 | 0.513 |
| Male | Reference | Reference | ||||
| Age, years | −0.044 [−0.214, 0.126] | −0.351 | 0.726 | 0.031 | 0.338 | 0.736 |
| <50 | −0.044 [−0.164, 0.076] | −0.351 | 0.726 | 0.031 | 0.338 | 0.736 |
| ≥50–<55 | −0.083 [−0.193, 0.027] | −0.775 | 0.440 | 0.052 | 0.612 | 0.541 |
| ≥55–<60 | −0.124 [−0.274, 0.026] | −1.422 | 0.157 | 0.016 | 0.15 | 0.881 |
| ≥60–<65 | −0.026 [−0.126, 0.074] | −0.275 | 0.783 | 0.117 | 0.948 | 0.344 |
| ≥65 | Reference | Reference | ||||
| Race/Ethnicity | ||||||
| White, non-Hispanic | −0.123 [−0.237, −0.101] | −1.041 | 0.300 | −0.062 | −0.517 | 0.606 |
| White, Hispanic | 0.064 [0.058, 0.069] | 0.877 | 0.382 | −0.015 | −0.203 | 0.839 |
| Other a | −0.163 [−0.343, 0.017] | −1.186 | 0.238 | 0.011 | 0.079 | 0.937 |
| Black, non-Hispanic | Reference | Reference | ||||
| CD4+ T, (cells/µL) | ||||||
| <200 | −0.279 [−0.439, −0.119] | −2.394 | 0.018 | −0.304 | −2.692 | 0.008 |
| ≥200–<500 | 0.004 [0.003, 0.006] | 0.053 | 0.958 | −0.059 | −0.938 | 0.35 |
| ≥500 | Reference | Reference | ||||
| HIV viral load, (copies/mL) | ||||||
| ≥200–<5000 | −0.35 [−0.45, −0.25] | −3.1 | 0.002 | −0.271 | −2.52 | 0.01 |
| ≥5000 | −0.009 [−0.099, 0.081] | −0.079 | 0.937 | −0.024 | −0.232 | 0.817 |
| <200 | Reference | Reference | ||||
| BMI, kg/m2 | ||||||
| ≥27 | 0.151 [0.144, 0.158] | 1.907 | 0.058 | 0.151 | 2.635 | 0.009 |
| <27 | Reference | Reference | ||||
| Interval between vaccination dose 2 and serology collection, days | −0.003 [−0.153, 0.147] | −4.228 | <0.001 | −0.003 | −3.488 | <0.001 |
| Substance use | ||||||
| Hazardous drinking b | 0.060 [−0.05, 0.17] | 0.816 | 0.416 | 0.033 | 0.432 | 0.666 |
| Marijuana | −0.023 [−0.223, 0.177] | −0.321 | 0.215 | −0.077 | −1.172 | 0.243 |
| Cocaine | −0.132 [−0.272, 0.008] | −1.245 | 0.544 | −0.195 | −1.933 | 0.055 |
| Other drugs c | −0.166 [−0.356, 0.024] | −0.609 | 0.469 | −0.051 | −0.192 | 0.848 |
| Cigarette smoking | 0.046 [−0.124, 0.216] | 0.726 | 0.397 | −0.01 | −0.161 | 0.872 |
| Substance use disorder | −0.09 [−0.25, 0.07] | −0.85 | 0.400 | −0.085 | −0.857 | 0.393 |
| Comorbidities | ||||||
| Hypertension | −0.121 [−0.301, 0.059] | −1.906 | 0.059 | −0.092 | −1.591 | 0.113 |
| Diabetes | 0.129 [−0.081, 0.339] | 1.937 | 0.055 | 0.101 | 1.52 | 0.130 |
| Autoimmune disease | 0.013 [−0.097, 0.123] | 0.11 | 0.912 | 0.072 | 0.616 | 0.539 |
| Obesity | 0.011 [−0.099, 0.121] | 0.13 | 0.896 | 0.106 | 1.792 | 0.075 |
| Chronic kidney disease | 0.029 [−0.141, 0.199] | 0.279 | 0.781 | −0.091 | −0.89 | 0.375 |
| ≥Comorbidity d | −0.06 [−0.28, 0.16] | −0.819 | 0.414 | −0.042 | −0.602 | 0.548 |
| Variables | Adjusted Model | ||
|---|---|---|---|
| β [95%CI] | t | p | |
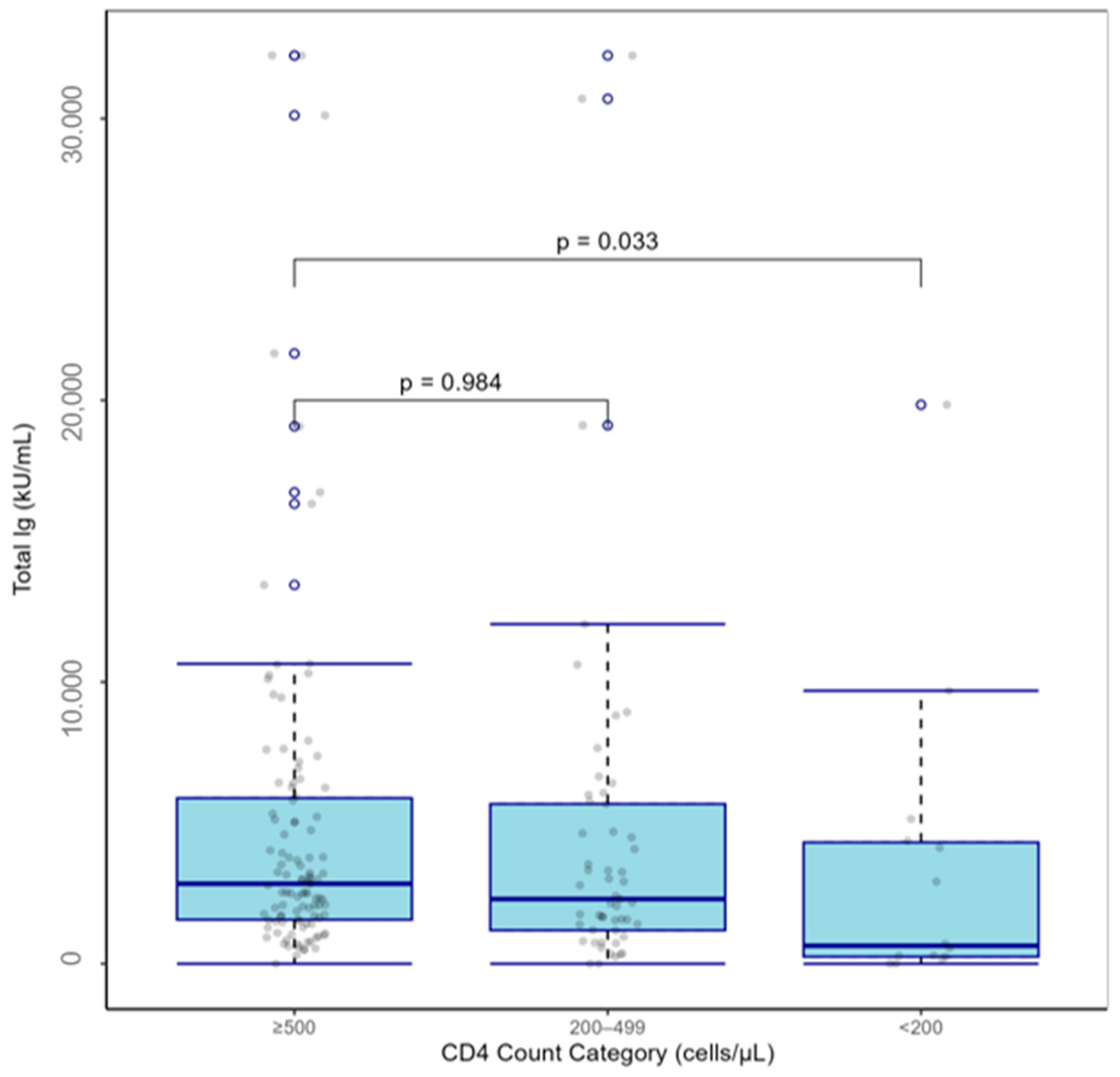
| CD4+ T, (cells/µL) | |||
| <200 | −0.400 [−0.59, −0.21] | −2.151 | 0.033 |
| ≥200–<500 | 0.001 [−0.109, 0.111] | 0.02 | 0.984 |
| ≥500 | Reference | ||
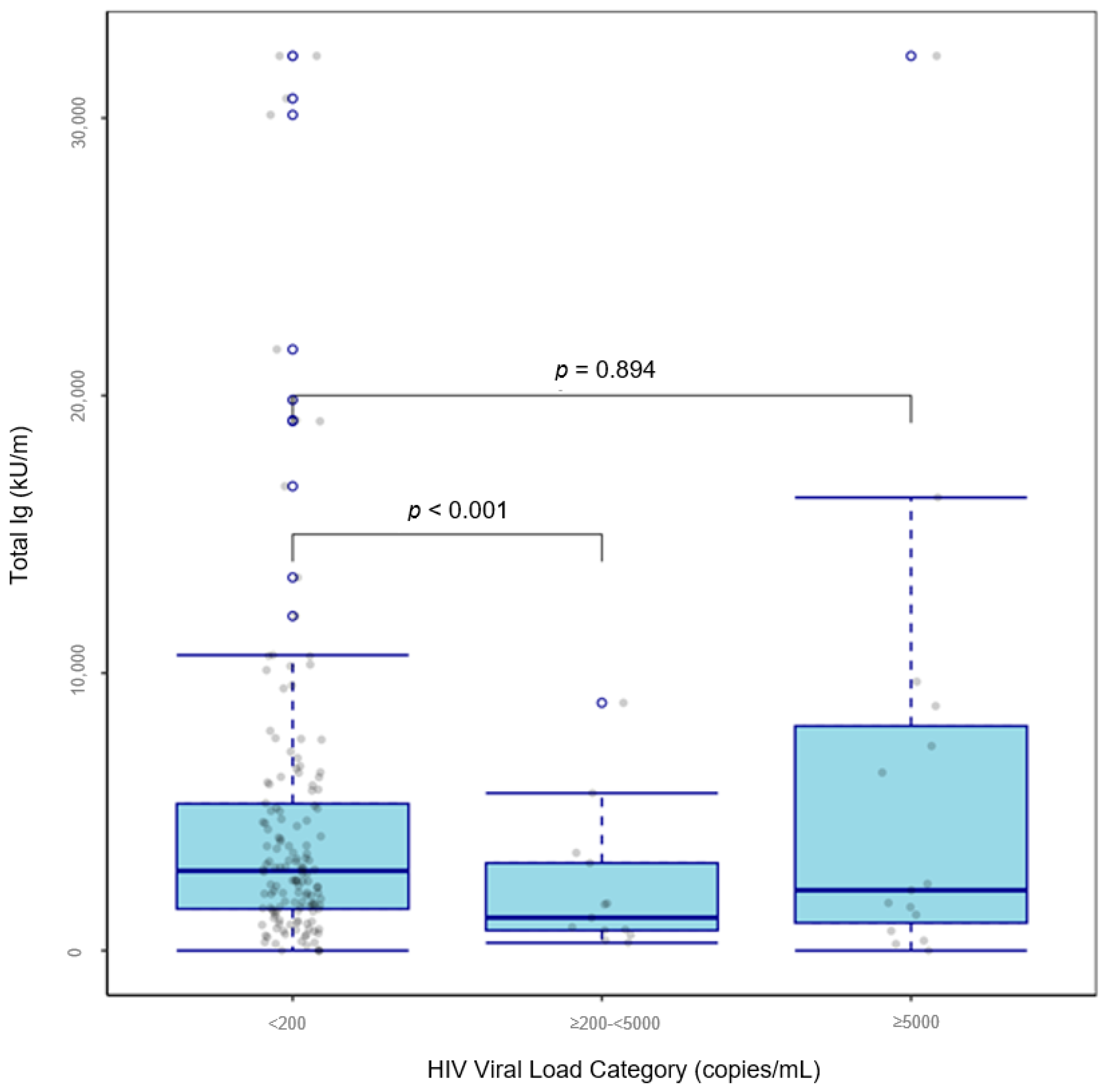
| HIV viral load, (copies/mL) | |||
| ≥200–<5000 | −0.275 [−0.485, −0.065] | −2.654 | <0.001 |
| ≥5000 | 0.0135 [−0.117, 0.144] | 0.133 | 0.894 |
| <200 | Reference | ||
| BMI, (kg/m2) | |||
| ≥27 | 0.107 [−0.043, 0.257] | 1.74 | 0.050 |
| <27 | Reference | ||
| Interval between vaccination dose 2 and serology collection, days | −0.003 [−0.247, 0.253] | 3.764 | <0.001 |
| Substance use | |||
| Cocaine | −0.15 [−0.34, 0.04] | 0.545 | 0.124 |
| Comorbidities | |||
| Hypertension | −0.08 [−0.22, 0.06] | −1.427 | 0.156 |
| Diabetes | 0.117 [0.07, 0.227] | 1.868 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Fonseca, H.R.; Acuna, L.; Wu, W.; Wang, X.; Gonzales, S.; Barbieri, M.; Brown, D.R.; Baum, M.K. SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV. Vaccines 2025, 13, 517. https://doi.org/10.3390/vaccines13050517
Huang Y, Fonseca HR, Acuna L, Wu W, Wang X, Gonzales S, Barbieri M, Brown DR, Baum MK. SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV. Vaccines. 2025; 13(5):517. https://doi.org/10.3390/vaccines13050517
Chicago/Turabian StyleHuang, Yongjun, Haley R. Fonseca, Leonardo Acuna, Wensong Wu, Xuexia Wang, Samantha Gonzales, Manuel Barbieri, David R. Brown, and Marianna K. Baum. 2025. "SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV" Vaccines 13, no. 5: 517. https://doi.org/10.3390/vaccines13050517
APA StyleHuang, Y., Fonseca, H. R., Acuna, L., Wu, W., Wang, X., Gonzales, S., Barbieri, M., Brown, D. R., & Baum, M. K. (2025). SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV. Vaccines, 13(5), 517. https://doi.org/10.3390/vaccines13050517







