Safety, Tolerability, and Immunogenicity of a Recombinant Nonavalent Human Papillomavirus Vaccine (Escherichia coli) in Healthy Chinese Women Aged 18–45 Years: A Phase 1 Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
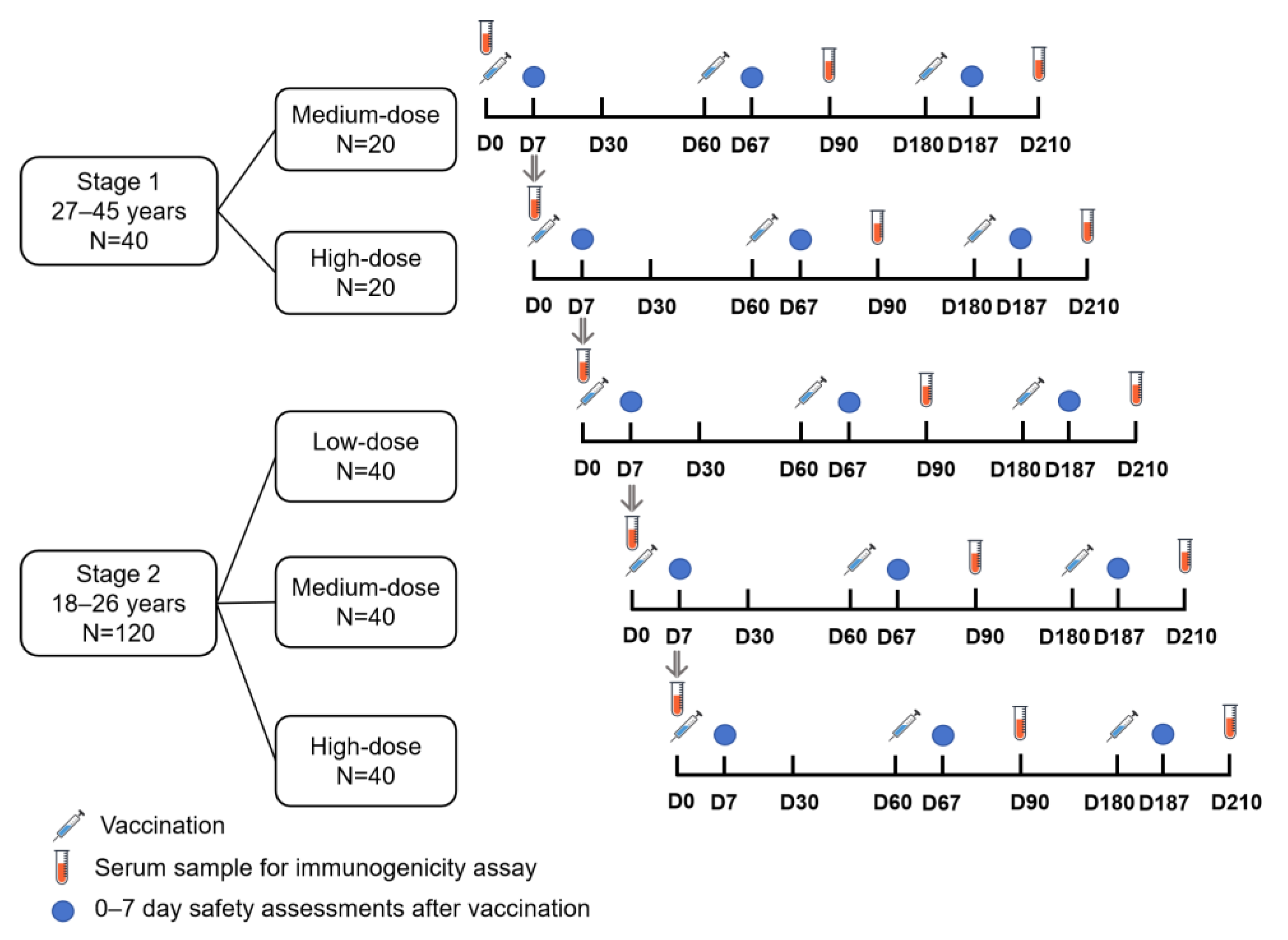
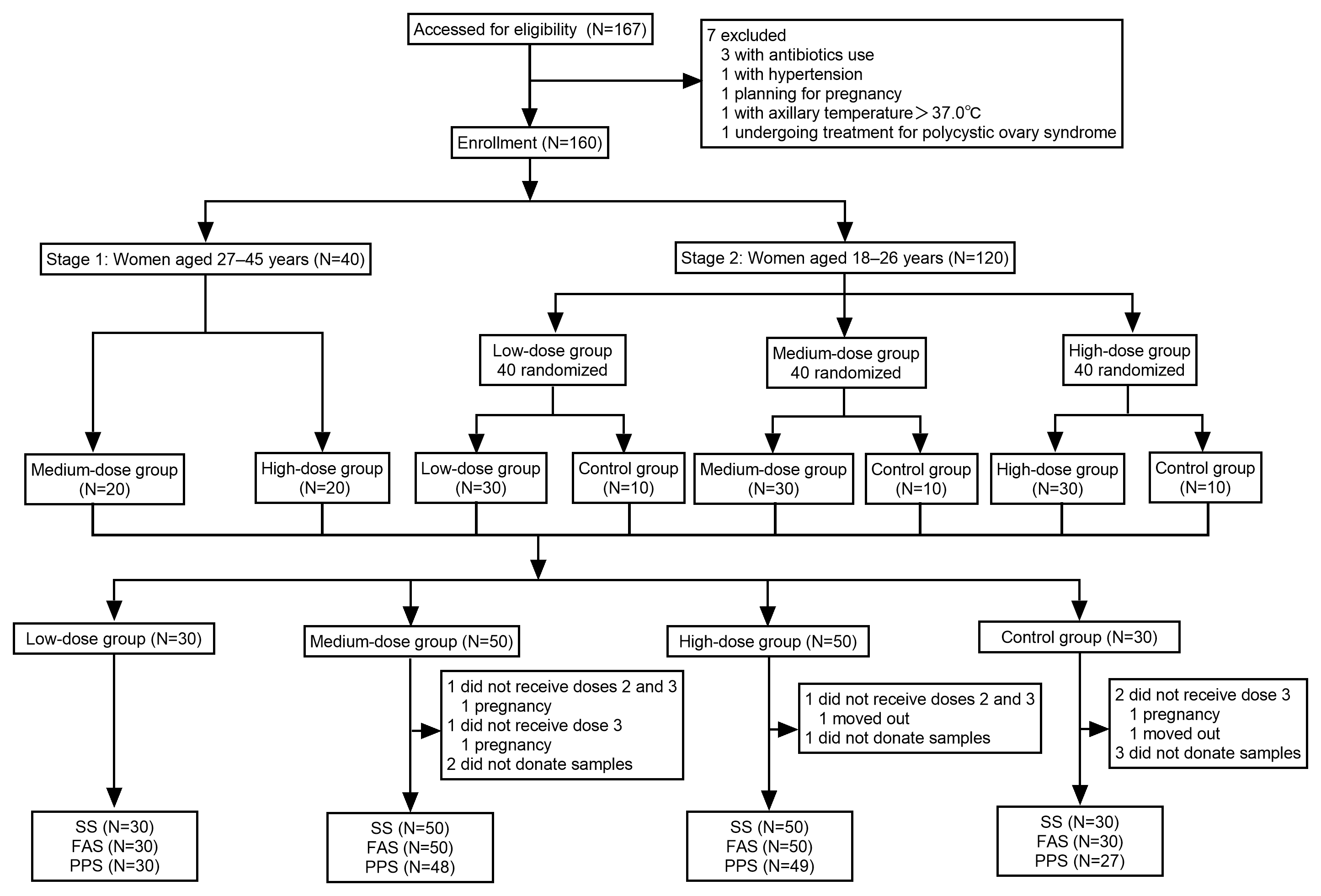
2.1. Study Design and Participants
2.2. Randomization and Masking
2.3. Procedures
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics
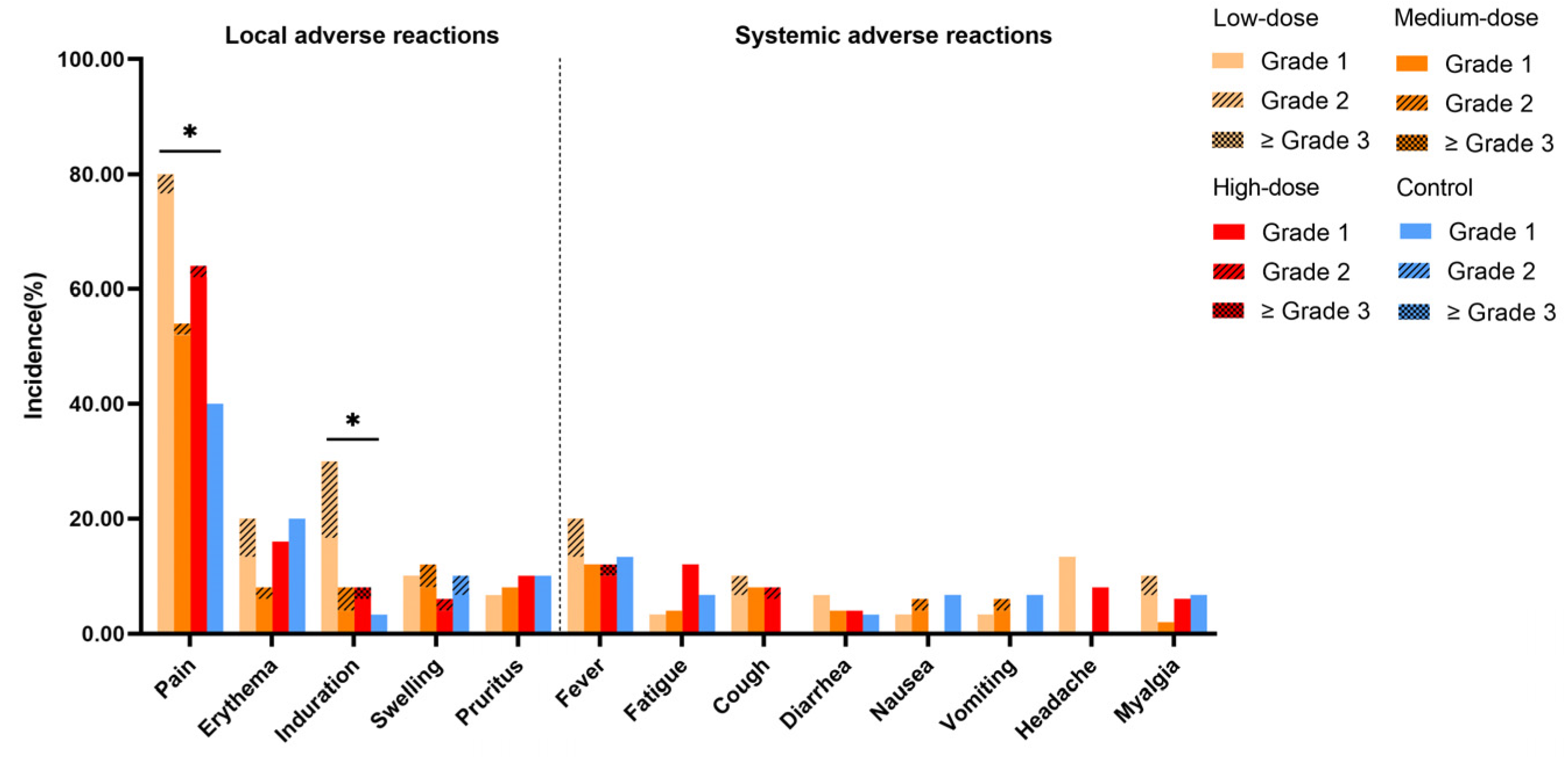
3.2. Safety and Tolerability
3.3. Laboratory Parameters
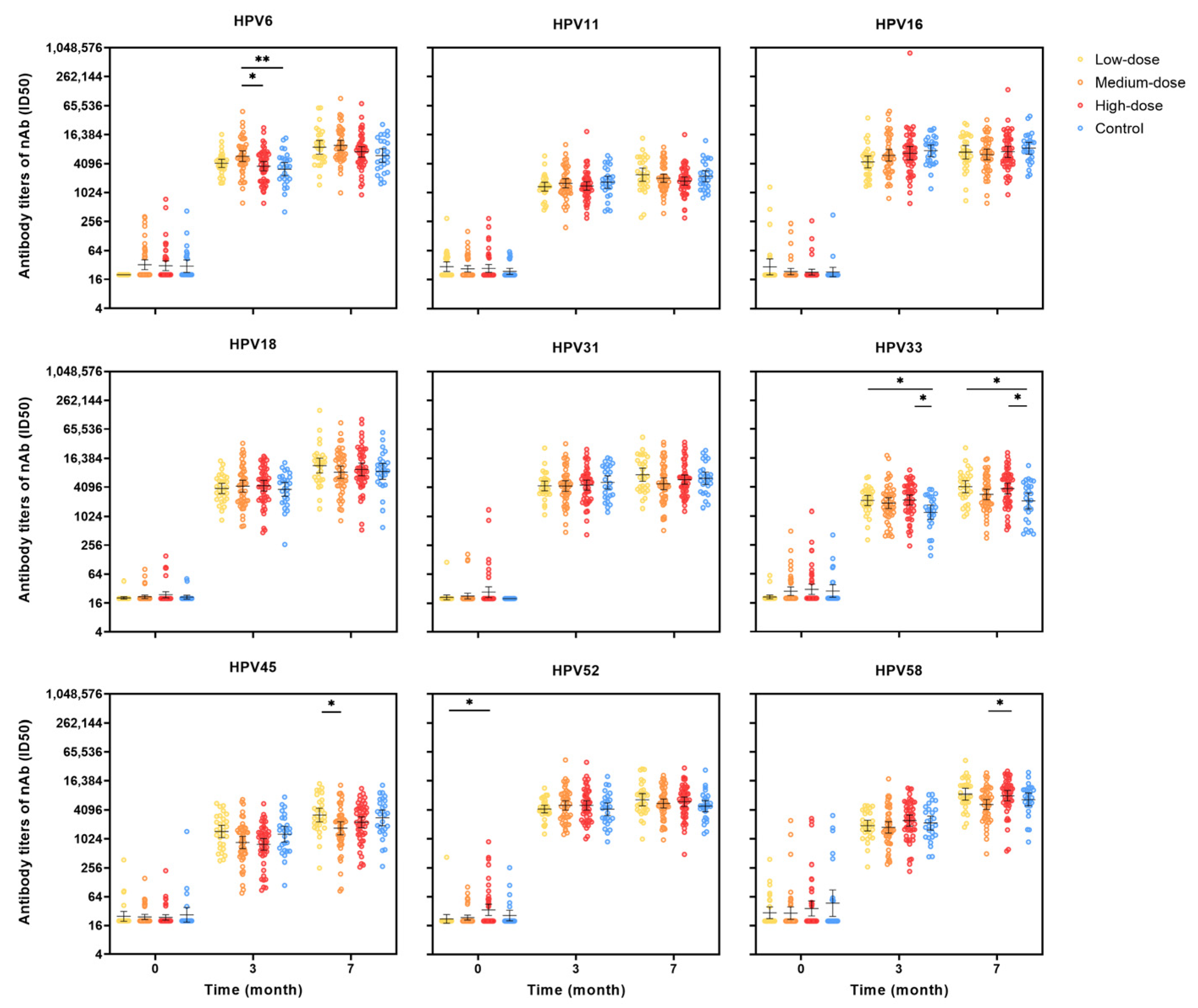
3.4. Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AE | adverse event |
| ALT | alanine aminotransferase |
| AR | adverse reaction |
| APTT | activated partial thromboplastin time |
| AST | aspartate aminotransferase |
| CI | confidential interval |
| CREA | creatinine |
| E. coli | Escherichia coli |
| ELISA | enzyme-linked immunosorbent assay |
| FAS | full analysis set |
| GMI | geometric mean increase |
| GMT | geometric mean titer |
| GST | glutathione-S-transferase |
| HBG | hemoglobin |
| HPV | human papillomavirus |
| IgG | immunoglobulin G |
| nAb | neutralizing antibody |
| NMPA | National Medical Products Administration |
| NIFDC | China National Institute for Food and Drug Control |
| PBNA | pseudovirion-based neutralization assay |
| PPS | per-protocol set |
| PT | prothrombin time |
| SAE | serious adverse event |
| sIgA | secretory immunoglobulin A |
| SS | safety set |
| VLP | virus-like particle |
| WBC | white blood cell count |
| WHO | World Health Organization |
References
- Wu, S.; Jiao, J.; Yue, X.; Wang, Y. Cervical cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with England and India based on the global burden of disease study 2019. Front. Public. Health 2024, 12, 1358433. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Today-World. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 25 February 2025).
- World Health Organization. Cancer Today-China. 2024. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/160-china-fact-sheet.pdf (accessed on 25 February 2025).
- National Cancer Institute. HPV and Cancer. 31 January 2025. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer (accessed on 25 February 2025).
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. 2022. Available online: https://www.who.int/publications/i/item/who-wer9750-645-672 (accessed on 25 February 2025).
- Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. 2020. Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 25 February 2025).
- ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf?t=1693896342213 (accessed on 25 February 2025).
- Hu, Y.M.; Bi, Z.F.; Zheng, Y.; Zhang, L.; Zheng, F.Z.; Chu, K.; Li, Y.F.; Chen, Q.; Quan, J.L.; Hu, X.W.; et al. Immunogenicity and safety of an Escherichia coli-produced human papillomavirus (types 6/11/16/18/31/33/45/52/58) L1 virus-like-particle vaccine: A phase 2 double-blind, randomized, controlled trial. Sci. Bull. 2023, 68, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.H.; Wu, T.; Hu, Y.M.; Wei, L.H.; Li, M.Q.; Huang, W.J.; Chen, W.; Huang, S.J.; Pan, Q.J.; Zhang, X.; et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced Human Papillomavirus (16 and 18) L1 virus-like-particle vaccine: End-of-study analysis of a phase 3, double-blind, randomised, controlled trial. Lancet Infect. Dis. 2022, 22, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.L.; Wu, T.; Li, R.C.; Hu, Y.M.; Wei, L.H.; Li, C.G.; Chen, W.; Huang, S.J.; Zhao, F.H.; Li, M.Q.; et al. Efficacy, Safety, and Immunogenicity of an Escherichia coli-Produced Bivalent Human Papillomavirus Vaccine: An Interim Analysis of a Randomized Clinical Trial. J. Natl. Cancer Inst. 2020, 112, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. NPJ Vaccines 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Bi, Z.F.; Huang, W.J.; Li, Y.F.; Zhang, L.; Yang, C.L.; Jiang, H.M.; Zang, X.; Chen, Q.; Liu, D.L.; et al. Safety and immunogenicity of an Escherichia coli-produced 9-valent human papillomavirus L1 virus-like particle vaccine (types 6/11/16/18/31/33/45/52/58) in healthy adults: An open-label, dose-escalation phase 1 clinical trial. Lancet Reg. Health West. Pac. 2023, 34, 100731. [Google Scholar] [CrossRef]
- Moreira Jr, E.D.; Block, S.L.; Ferris, D.; Giuliano, A.R.; Iversen, O.E.; Joura, E.A.; Kosalaraksa, P.; Schilling, A.; Van Damme, P.; Bornstein, J.; et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics 2016, 138, e20154387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, L.W.; Yu, B.W.; Huang, L.R.; Zhou, L.Y.; Shi, L.; Jiang, Z.W.; Xia, J.L.; Wang, X.Y.; Li, R.C.; et al. Safety and immunogenicity of a pichia pastoris-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine in healthy Chinese women aged 9-45 years: A randomized, double-blind, placebo-controlled phase 1 clinical trial. Vaccine 2023, 41, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Potter, C.S.; Carragher, B.; Lander, G.; Sworen, J.; Towne, V.; Abraham, D.; Duncan, P.; Washabaugh, M.W.; Sitrin, R.D. Characterization of virus-like particles in GARDASIL® by cryo transmission electron microscopy. Hum. Vaccin. Immunother. 2014, 10, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.J.; Bi, Z.F.; Sheng, W.; Chen, Q.; Huang, T.; Li, M.Q.; Cui, X.L.; Wangjiang, Y.H.; Lin, B.Z.; Zheng, F.Z.; et al. Safety and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus type 6/11 vaccine: A dose-escalation, randomized, double-blind, placebo-controlled phase 1 trial. Hum. Vaccin. Immunother. 2022, 18, 2092363. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Tjalma, W.; Matheeussen, V.; Van Damme, P.; Vorsters, A. Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature. Papillomavirus Res. 2019, 8, 100185. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.; Hulskotte, E.; Voeten, T.; Breedveld, B.; Verweij, P.; van Duijnhoven, W.; Rudenko, L.; van Damme, P.; van den Bosch, H. Safety and immunogenicity in man of a cell culture derived trivalent live attenuated seasonal influenza vaccine: A Phase I dose escalating study in healthy volunteers. Vaccine 2014, 32, 5118–5124. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Low-Dose (N = 30) | Medium-Dose (N = 50) | High-Dose (N = 50) | Control (N = 30) | Total (N = 160) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Mean (SD) | 22.60 (2.18) | 28.90 (8.28) | 28.52 (8.10) | 22.73 (2.41) | 26.44 (7.20) | <0.001 |
| Median | 23.00 | 25.50 | 24.50 | 23.00 | 24.00 | |
| Min, Max | 18.00, 26.00 | 18.00, 45.00 | 19.00, 45.00 | 18.00, 26.00 | 18.00, 45.00 | |
| Height (cm) | ||||||
| Mean (SD) | 160.07 (5.12) | 160.90 (5.46) | 161.14 (5.67) | 161.63 (7.65) | 160.96 (5.90) | 0.748 |
| Median | 160.50 | 161.00 | 161.00 | 162.00 | 161.00 | |
| Min, Max | 151.00, 171.00 | 151.00, 172.00 | 146.00, 173.00 | 143.00, 181.00 | 143.00, 181.00 | |
| Weight (kg) | ||||||
| Mean (SD) | 57.97 (9.61) | 59.54 (12.32) | 60.54 (10.39) | 58.57 (11.20) | 59.38 (10.99) | 0.678 |
| Median | 55.50 | 58.50 | 58.50 | 55.00 | 58.00 | |
| Min, Max | 44.00, 80.00 | 41.00, 115.00 | 45.00, 92.00 | 41.00, 97.00 | 41.00, 115.00 | |
| BMI (kg/m2) | ||||||
| Mean (SD) | 22.63 (3.62) | 22.94 (4.23) | 23.34 (3.99) | 22.33 (3.20) | 22.89 (3.85) | 0.693 |
| Median | 21.77 | 22.74 | 22.81 | 21.75 | 22.40 | |
| Min, Max | 16.94, 31.64 | 15.06, 40.75 | 15.57, 35.94 | 16.63, 29.61 | 15.06, 40.75 |
| Low-Dose (N = 30) | Medium-Dose (N = 50) | High-Dose (N = 50) | Control (N = 30) | Total (N = 160) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | ||
| Total adverse reactions a | 92 | 27 (90.00) | 86 | 37 (74.00) | 108 | 37 (74.00) | 52 | 17 (56.67) | 338 | 118 (73.75) | 0.035 b |
| Solicited adverse reactions | 90 | 27 (90.00) | 82 | 35 (70.00) | 104 | 36 (72.00) | 49 | 17 (56.67) | 325 | 115 (71.88) | 0.039 b |
| Local solicited adverse reactions | 65 | 25 (83.33) | 59 | 30 (60.00) | 72 | 34 (68.00) | 36 | 16 (53.33) | 232 | 105 (65.63) | 0.072 |
| Systemic solicited adverse reactions | 25 | 13 (43.33) | 23 | 15 (30.00) | 32 | 16 (32.00) | 13 | 7 (23.33) | 93 | 51 (31.88) | 0.407 |
| Unsolicited adverse reactions | 2 | 2 (6.67) | 4 | 4 (8.00) | 4 | 4 (8.00) | 3 | 2 (6.67) | 13 | 12 (7.50) | >0.999 |
| Adverse reactions ≥ grade 3 | 0 | 0 | 0 | 0 | 2 | 2 (4.00) | 0 | 0 | 2 | 2 (1.25) | 0.332 |
| Low-Dose (N = 30) | Medium-Dose (N = 50) | High-Dose (N = 50) | Control (N = 30) | Total (N = 160) | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | Events | Participants (%) | ||
| Total adverse events | 112 | 27 (90.00) | 106 | 40 (80.00) | 131 | 41 (82.00) | 63 | 20 (66.67) | 412 | 128 (80.00) | 0.149 |
| Adverse reactions a | 93 | 27 (90.00) | 86 | 37 (74.00) | 111 | 37 (74.00) | 52 | 17 (56.67) | 342 | 118 (73.75) | 0.035 b |
| Solicited adverse reactions | 90 | 27 (90.00) | 82 | 35 (70.00) | 104 | 36 (72.00) | 49 | 17 (56.67) | 325 | 115 (71.88) | 0.039 b |
| Local solicited adverse reactions | 65 | 25 (83.33) | 59 | 30 (60.00) | 72 | 34 (68.00) | 36 | 16 (53.33) | 232 | 105 (65.63) | 0.072 |
| Systemic solicited adverse reactions | 25 | 13 (43.33) | 23 | 15 (30.00) | 32 | 16 (32.00) | 13 | 7 (23.33) | 93 | 51 (31.88) | 0.407 |
| Unsolicited adverse reactions | 3 | 3 (10.00) | 4 | 4 (8.00) | 7 | 5 (10.00) | 3 | 2 (6.67) | 17 | 14 (8.75) | 0.981 |
| Solicited adverse events | 90 | 27 (90.00) | 82 | 35 (70.00) | 109 | 36 (72.00) | 49 | 17 (56.67) | 330 | 115 (71.88) | 0.039 b |
| Unsolicited adverse events | 22 | 12 (40.00) | 24 | 14 (28.00) | 22 | 14 (28.00) | 14 | 9 (30.00) | 82 | 49 (30.63) | 0.666 |
| Adverse events ≥ grade 3 | 0 | 0 | 0 | 0 | 2 | 2 (4.00) | 0 | 0 | 2 | 2 (1.25) | 0.332 |
| Serious adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Discontinuation due to adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Indices | Low-Dose (N = 30) | Medium-Dose (N = 50) | High-Dose (N = 50) | Control (N = 30) | Total (N = 160) | p Value | |
|---|---|---|---|---|---|---|---|
| Routine blood indices | WBC | ||||||
| 1st vaccination | 0 | 3 (6.00) | 2 (4.00) | 1 (3.33) | 6 (3.75) | 0.702 | |
| 2nd vaccination | 0 | 0 | 1 (2.04) | 0 | 1 (0.63) | >0.999 | |
| 3rd vaccination | 3 (10.00) | 2 (4.17) | 3 (6.12) | 0 | 8 (5.19) | 0.421 | |
| HGB | |||||||
| 1st vaccination | 0 | 0 | 0 | 0 | 0 | - | |
| 2nd vaccination | 0 | 1 (2.04) | 2 (4.08) | 0 | 3 (1.90) | 0.777 | |
| 3rd vaccination | 0 | 1 (2.08) | 0 | 0 | 1 (0.65) | 0.682 | |
| Serum biochemical indices | ALT | ||||||
| 1st vaccination | 1 (3.33) | 0 | 1 (2.00) | 0 | 2 (1.25) | 0.803 | |
| 2nd vaccination | 1 (3.33) | 1 (2.04) | 1 (2.04) | 0 | 3 (1.90) | >0.999 | |
| 3rd vaccination | 1 (3.33) | 2 (4.17) | 0 | 0 | 3 (1.95) | 0.398 | |
| AST | |||||||
| 1st vaccination | 0 | 0 | 0 | 0 | 0 | - | |
| 2nd vaccination | 0 | 1 (2.04) | 1 (2.04) | 0 | 2 (1.27) | >0.999 | |
| 3rd vaccination | 1 (3.33) | 1 (2.08) | 0 | 0 | 2 (1.30) | 0.676 | |
| CREA | |||||||
| 1st vaccination | 1 (3.33) | 0 | 1 (2.00) | 0 | 2 (1.25) | 0.803 | |
| 2nd vaccination | 0 | 0 | 4 (8.16) | 0 | 4 (2.53) | 0.068 | |
| 3rd vaccination | 2 (6.67) | 3 (6.25) | 1 (2.04) | 2 (7.41) | 8 (5.19) | 0.667 | |
| Blood coagulation function indices | APTT | ||||||
| 1st vaccination | 0 | 1 (2.00) | 1 (2.00) | 0 | 2 (1.25) | >0.999 | |
| 2nd vaccination | 0 | 2 (4.08) | 2 (4.08) | 2 (6.67) | 6 (3.80) | 0.568 | |
| 3rd vaccination | 0 | 2 (4.17) | 0 | 1 (3.70) | 3 (1.95) | 0.288 | |
| PT | |||||||
| 1st vaccination | 1 (3.33) | 0 | 0 | 1 (3.33) | 2 (1.25) | 0.139 | |
| 2nd vaccination | 0 | 2 (4.08) | 0 | 0 | 2 (1.27) | 0.332 | |
| 3rd vaccination | 1 (3.33) | 1 (2.08) | 1 (2.04) | 1 (3.70) | 4 (2.60) | >0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Han, W.; Zhang, J.; Liu, Y.; Yu, H.; Li, J.; Wang, W. Safety, Tolerability, and Immunogenicity of a Recombinant Nonavalent Human Papillomavirus Vaccine (Escherichia coli) in Healthy Chinese Women Aged 18–45 Years: A Phase 1 Clinical Trial. Vaccines 2025, 13, 511. https://doi.org/10.3390/vaccines13050511
Wei M, Han W, Zhang J, Liu Y, Yu H, Li J, Wang W. Safety, Tolerability, and Immunogenicity of a Recombinant Nonavalent Human Papillomavirus Vaccine (Escherichia coli) in Healthy Chinese Women Aged 18–45 Years: A Phase 1 Clinical Trial. Vaccines. 2025; 13(5):511. https://doi.org/10.3390/vaccines13050511
Chicago/Turabian StyleWei, Mingwei, Weiwei Han, Jing Zhang, Yongjiang Liu, Hongyang Yu, Jingxin Li, and Wenjuan Wang. 2025. "Safety, Tolerability, and Immunogenicity of a Recombinant Nonavalent Human Papillomavirus Vaccine (Escherichia coli) in Healthy Chinese Women Aged 18–45 Years: A Phase 1 Clinical Trial" Vaccines 13, no. 5: 511. https://doi.org/10.3390/vaccines13050511
APA StyleWei, M., Han, W., Zhang, J., Liu, Y., Yu, H., Li, J., & Wang, W. (2025). Safety, Tolerability, and Immunogenicity of a Recombinant Nonavalent Human Papillomavirus Vaccine (Escherichia coli) in Healthy Chinese Women Aged 18–45 Years: A Phase 1 Clinical Trial. Vaccines, 13(5), 511. https://doi.org/10.3390/vaccines13050511






