Indirect Effects of Universal Infant Rotavirus Vaccination: A Narrative Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Analysis
3. Results
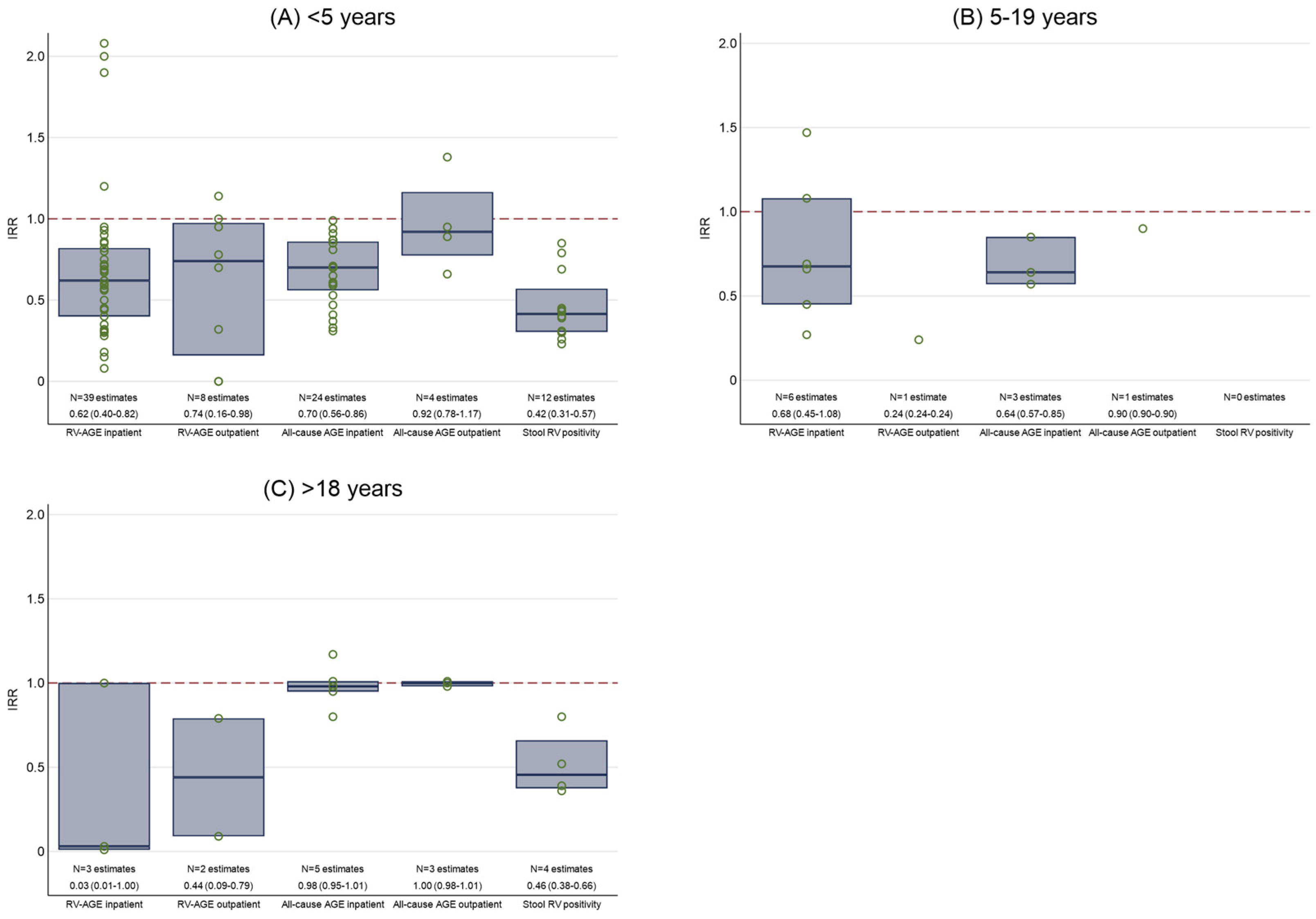
3.1. Inpatient Admissions for Laboratory-Confirmed RV-AGE
3.2. Outpatient Attendances for Laboratory-Confirmed RV-AGE
3.3. Inpatient Admissions for All-Cause AGE
3.4. Outpatient Attendances for All-Cause AGE
3.5. Laboratory-Confirmed RV in Stool Samples
3.6. Mixed Outcomes
3.7. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGE | Acute gastroenteritis |
| CI | Confidence interval |
| IQR | Interquartile range |
| IRR | Incidence rate ratio |
| NIP | National immunisation program |
| ROBINS-E | Risk Of Bias in Non-randomised Studies-of Exposures |
| RV | Rotavirus |
| RV1 | Rotarix™ vaccine |
| RV5 | RotaTeq™ vaccine |
| RV-AGE | Rotavirus-specific acute gastroenteritis |
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Karakusevic, A.; Devaney, P.; Enstone, A.; Kanibir, N.; Hartwig, S.; Carias, C.D.S. The burden of rotavirus-associated acute gastroenteritis in the elderly: Assessment of the epidemiology in the context of universal childhood vaccination programs. Expert. Rev. Vaccines 2022, 21, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; Madhi, S.A.; Cunliffe, N.A.; Vesikari, T.; Phua, K.B.; Lim, F.S.; Nelson, E.A.S.; Lau, Y.-L.; Huang, L.-M.; Karkada, N.; et al. Incidence of rotavirus gastroenteritis by age in African, Asian and European children: Relevance for timing of rotavirus vaccination. Hum. Vaccin. Immunother. 2016, 12, 2406–2412. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Zhang, X.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: An observational trend study. Virol. J. 2022, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-world effectiveness of rotavirus vaccines, 2006–2019: A literature review and meta-analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef]
- Bennett, A.; Pollock, L.; Jere, K.C.; Pitzer, V.E.; Parashar, U.; Tate, J.E.; Heyderman, R.S.; Mwansambo, C.; French, N.; Nakagomi, O.; et al. Direct and possible indirect effects of vaccination on rotavirus hospitalisations among children in Malawi four years after programmatic introduction. Vaccine 2018, 36, 7142–7148. [Google Scholar] [CrossRef]
- Introduction of Rotavirus Vaccine. Available online: https://immunizationdata.who.int/global/wiise-detail-page/introduction-of-rotavirus-vaccine?ISO_3_CODE=&YEAR= (accessed on 19 January 2025).
- World Health Organization (WHO). Rotavirus Vaccines: WHO Position Paper—July 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Soares-Weiser, K.; Maclehose, H.; Bergman, H.; Ben-Aharon, I.; Nagpal, S.; Goldberg, E.; Pitan, F.; Cunliffe, N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2012, 11, CD008521. [Google Scholar] [CrossRef]
- Bennett, A.; Bar-Zeev, N.; Cunliffe, N.A. Measuring indirect effects of rotavirus vaccine in low income countries. Vaccine 2016, 34, 4351–4353. [Google Scholar] [CrossRef]
- Rosettie, K.L.; Vos, T.; Mokdad, A.H.; Flaxman, A.D.; Khalil, I.; Troeger, C.; Weaver, M.R. Indirect rotavirus vaccine effectiveness for the prevention of rotavirus hospitalization: A systematic review and meta-analysis. Am. J. Trop. Med. Hyg. 2018, 98, 1197–1201. [Google Scholar] [CrossRef]
- Chavers, T.; Cates, J.; Burnett, E.; Parashar, U.D.; Tate, J.E. Indirect protection from rotavirus vaccines: A systematic review. Expert. Rev. Vaccines 2024, 23, 789–795. [Google Scholar] [CrossRef]
- Wierzba, T.F. Implications and measurement of herd protection (indirect effects) for enteric vaccine development. Vaccine 2019, 37, 4775–4777. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 19 January 2025).
- Under-Five Mortality. Available online: https://data.unicef.org/topic/child-survival/under-five-mortality/ (accessed on 19 January 2025).
- Sharrow, D.; Hug, L.; You, D.; Alkema, L.; Black, R.; Cousens, S.; Croft, T.; Gaigbe-Togbe, V.; Gerland, P.; Guillot, M.; et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health 2022, 10, e195–e206. [Google Scholar] [CrossRef] [PubMed]
- Rotavirus Vaccination Coverage. Available online: https://immunizationdata.who.int/global/wiise-detail-page/rotavirus-vaccination-coverage?ANTIGEN=ROTAC&YEAR=&CODE= (accessed on 3 May 2025).
- Risk of Bias in Non-Randomized Studies—Of Exposure (ROBINS-E). Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 19 January 2025).
- Sahakyan, G.; Grigoryan, S.; Wasley, A.; Mosina, L.; Sargsyan, S.; Asoyan, A.; Gevorgyan, Z.; Kocharyan, K.; Avagyan, T.; Lopman, B.; et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S147–S154. [Google Scholar] [CrossRef]
- David, R.L.; Kirk, M.D. Rotavirus gastroenteritis hospitalisations following introduction of vaccination, Canberra. Commun. Dis. Intell. Q. Rep. 2014, 38, E3–E8. [Google Scholar]
- Paulke-Korinek, M.; Kollaritsch, H.; Aberle, S.W.; Zwazl, I.; Schmidle-Loss, B.; Vécsei, A.; Kundi, M. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine 2013, 31, 2686–2691. [Google Scholar] [CrossRef]
- Sáfadi, M.A.P.; Berezin, E.N.; Munford, V.; Almeida, F.J.; de Moraes, J.C.; Pinheiro, C.F.; Racz, M.L. Hospital-based surveillance to evaluate the impact of rotavirus vaccination in Sao Paulo, Brazil. Pediatr. Infect. Dis. J. 2010, 29, 1019–1022. [Google Scholar] [CrossRef]
- Wilson, S.E.; Rosella, L.C.; Wang, J.; Renaud, A.; Le Saux, N.; Crowcroft, N.S.; Desai, S.; Harris, T.; Bolotin, S.; Gubbay, J.; et al. Equity and impact: Ontario’s infant rotavirus immunization program five years following implementation. A population-based cohort study. Vaccine 2019, 37, 2408–2414. [Google Scholar] [CrossRef]
- Kõivumägi, K.; Toompere, K.; Soeorg, H.; Kallas, E.; Jõgeda, E.-L.; Huik, K.; Lutsar, I. Acute gastroenteritis hospitalizations after implementation of universal mass vaccination against rotavirus. Vaccine 2020, 38, 2879–2886. [Google Scholar] [CrossRef]
- Solastie, A.; Leino, T.; Ollgren, J. Success of rotavirus vaccination in Finland, a register based study measuring impact beyond overall effectiveness. Vaccine 2020, 38, 3766–3772. [Google Scholar] [CrossRef]
- Pietsch, C.; Liebert, U.G. Rotavirus vaccine effectiveness in preventing hospitalizations due to gastroenteritis: A descriptive epidemiological study from Germany. Clin. Microbiol. Infect. 2019, 25, 102–106. [Google Scholar] [CrossRef]
- Armah, G.; Pringle, K.; Enweronu-Laryea, C.C.; Ansong, D.; Mwenda, J.M.; Diamenu, S.K.; Narh, C.; Lartey, B.; Binka, F.; Grytdal, S.; et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S200–S207. [Google Scholar] [CrossRef] [PubMed]
- Enweronu-Laryea, C.C.; Armah, G.; Sagoe, K.W.; Ansong, D.; Addo-Yobo, E.; Diamenu, S.K.; Mwenda, J.M.; Parashar, U.D.; Tate, J.E. Sustained impact of rotavirus vaccine introduction on rotavirus gastroenteritis hospitalizations in children <5 years of age, Ghana, 2009–2016. Vaccine 2018, 36, 7131–7134. [Google Scholar] [CrossRef] [PubMed]
- Wandera, E.A.; Mohammad, S.; Bundi, M.; Komoto, S.; Nyangao, J.; Kathiiko, C.; Odoyo, E.; Miring’U, G.; Taniguchi, K.; Ichinose, Y. Impact of rotavirus vaccination on rotavirus and all-cause gastroenteritis in peri-urban Kenyan children. Vaccine 2017, 35, 5217–5223. [Google Scholar] [CrossRef]
- Gheorghita, S.; Birca, L.; Donos, A.; Wasley, A.; Birca, I.; Cojocaru, R.; Melnick, A.; Ciobanu, S.; Mosina, L.; Cortese, M.M.; et al. Impact of rotavirus vaccine introduction and vaccine effectiveness in the Republic of Moldova. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S140–S146. [Google Scholar] [CrossRef]
- Abeid, K.A.; Jani, B.; Cortese, M.M.; Kamugisha, C.; Mwenda, J.M.; Pandu, A.S.; Msaada, K.A.; Mohamed, A.S.; Khamis, A.U.; Parashar, U.D.; et al. Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar, Tanzania: Data from the first 3 years after introduction. J. Infect. Dis. 2017, 215, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jani, B.; Hokororo, A.; Mchomvu, J.; Cortese, M.M.; Kamugisha, C.; Mujuni, D.; Kallovya, D.; Parashar, U.D.; Mwenda, J.M.; Lyimo, D.; et al. Detection of rotavirus before and after monovalent rotavirus vaccine introduction and vaccine effectiveness among children in mainland Tanzania. Vaccine 2018, 36, 7149–7156. [Google Scholar] [CrossRef]
- Tharmaphornpilas, P.; Jiamsiri, S.; Boonchaiya, S.; Rochanathimoke, O.; Thinyounyong, W.; Tuntiwitayapun, S.; Guntapong, R.; Riewpaiboon, A.; Rasdjarmrearnsook, A.-O.; Glass, R.I. Evaluating the first introduction of rotavirus vaccine in Thailand: Moving from evidence to policy. Vaccine 2017, 35, 796–801. [Google Scholar] [CrossRef]
- Forrest, R.; Jones, L.; Willocks, L.; Hardie, A.; Templeton, K. Impact of the introduction of rotavirus vaccination on paediatric hospital admissions, Lothian, Scotland: A retrospective observational study. Arch. Dis. Child. 2017, 102, 323–327. [Google Scholar] [CrossRef]
- Hungerford, D.; Read, J.; Cooke, R.; Vivancos, R.; Iturriza-Gómara, M.; Allen, D.; French, N.; Cunliffe, N. Early impact of rotavirus vaccination in a large paediatric hospital in the UK. J. Hosp. Infect. 2016, 93, 117–120. [Google Scholar] [CrossRef]
- Panozzo, C.A.; Becker-Dreps, S.; Pate, V.; Weber, D.J.; Funk, M.J.; Stürmer, T.; Brookhart, M.A. Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007–2010. Am. J. Epidemiol. 2014, 179, 895–909. [Google Scholar] [CrossRef]
- Payne, D.C.; Staat, M.A.; Edwards, K.M.; Szilagyi, P.G.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Curns, A.T.; Wikswo, M.; Tate, J.E.; et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006–2009. Clin. Infect. Dis. 2011, 53, 245–253. [Google Scholar] [CrossRef]
- Dahl, R.M.; Curns, A.T.; Tate, J.E.; Parashar, U.D. Effect of rotavirus vaccination on acute diarrheal hospitalizations among low and very low birth weight US infants, 2001–2015. Pediatr. Infect. Dis. J. 2018, 37, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Eberly, M.D.; Gorman, G.H.; Eide, M.B.; Olsen, C.H.; Rajnik, M. The effect of rotavirus immunization on rotavirus gastroenteritis hospitalization rates in military dependents. Vaccine 2011, 29, 650–659. [Google Scholar] [CrossRef]
- Leshem, E.; Moritz, R.E.; Curns, A.T.; Zhou, F.; Tate, J.E.; Lopman, B.A.; Parashar, U.D. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics 2014, 134, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Shippee, D.B.; Weinrobe, M.H.; Davila, M.D.; Katz, B.Z.; Reddy, S.; Cuyugan, M.G.K.P.; Lee, S.Y.; Simons, Y.M.; Yogev, R.; et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin. Infect. Dis. 2013, 56, 755–760. [Google Scholar] [CrossRef]
- Mpabalwani, E.M.; Simwaka, C.J.; Mwenda, J.M.; Mubanga, C.P.; Monze, M.; Matapo, B.; Parashar, U.D.; Tate, J.E. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged <5 years in Lusaka, Zambia. Clin. Infect. Dis. 2016, 62 (Suppl. 2), S183–S187. [Google Scholar] [CrossRef]
- Mpabalwani, E.M.; Simwaka, J.C.; Mwenda, J.M.; Matapo, B.; Parashar, U.D.; Tate, J.E. Sustained impact of rotavirus vaccine on rotavirus hospitalisations in Lusaka, Zambia, 2009–2016. Vaccine 2018, 36, 7165–7169. [Google Scholar] [CrossRef] [PubMed]
- Marlow, R.; Muir, P.; Vipond, B.; Lyttle, M.; Trotter, C.; Finn, A. Assessing the impacts of the first year of rotavirus vaccination in the United Kingdom. Euro Surveill. 2015, 20, 30077. [Google Scholar] [CrossRef]
- Mujuru, H.A.M.C.; Yen, C.; Nathoo, K.J.M.C.; Gonah, N.A.M.C.M.; Ticklay, I.M.C.M.; Mukaratirwa, A.M.; Berejena, C.H.; Tapfumanei, O.B.; Chindedza, K.B.; Rupfutse, M.M.C.; et al. Reduction in diarrhea- and rotavirus-related healthcare visits among children <5 years of age after national rotavirus vaccine introduction in Zimbabwe. Pediatr. Infect. Dis. J. 2017, 36, 995–999. [Google Scholar] [CrossRef]
- Jenney, A.W.; Reyburn, R.; Ratu, F.T.; Tuivaga, E.; Nguyen, C.; Covea, S.; Thomas, S.; Rafai, E.; Devi, R.; Bright, K.; et al. The impact of the rotavirus vaccine on diarrhoea, five years following national introduction in Fiji. Lancet Reg. Health West. Pac. 2021, 6, 100053. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Nguyen, C.; Tabwaia, B.; Nikuata, A.; Baueri, N.; Timeon, E.; Diaaldeen, M.; Iuta, T.; Ozturk, M.H.; Moore, A.; et al. Temporal decline in diarrhea episodes and mortality in Kiribati children two years following rotavirus vaccine introduction, despite high malnutrition rates: A retrospective review. BMC Infect. Dis. 2020, 20, 207. [Google Scholar] [CrossRef] [PubMed]
- Bruun, T.; Salamanca, B.V.; Bekkevold, T.; Døllner, H.; Gibory, M.; Gilje, A.M.; Haarr, E.; Kran, A.-M.B.; Leegaard, T.M.; Nakstad, B.; et al. Impact of the rotavirus vaccination program in Norway after four years with high coverage. Pediatr. Infect. Dis. J. 2021, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Diop, A.; Thiongane, A.; Mwenda, J.M.; Aliabadi, N.; Sonko, M.A.; Diallo, A.; Ndoye, B.; Faye, P.M.; Ba, I.D.; Parashar, U.D.; et al. Impact of rotavirus vaccine on acute gastroenteritis in children under 5 years in Senegal: Experience of sentinel site of the Albert Royer Children’s Hospital in Dakar. Vaccine 2018, 36, 7192–7197. [Google Scholar] [CrossRef]
- Tsolenyanu, E.; Djadou, K.E.; Fiawoo, M.; Akolly, D.A.; Mwenda, J.M.; Leshem, E.; Tate, J.E.; Aliabadi, N.; Koudema, W.; Guedenon, K.M.; et al. Evidence of the impact of monovalent rotavirus vaccine on childhood acute gastroenteritis hospitalization in Togo. Vaccine 2018, 36, 7185–7191. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Ionides, E.L.; Lee, G.O.; Trujillo, W.F.C.; Eisenberg, J.N.S. Effect of childhood rotavirus vaccination on community rotavirus prevalence in rural Ecuador, 2008–2013. Int. J. Epidemiol. 2020, 49, 1691–1701. [Google Scholar] [CrossRef]
- Maphalala, G.; Phungwayo, N.; Masona, G.; Lukhele, N.; Tsegaye, G.; Dube, N.; Sindisiwe, D.; Khumalo, L.; Daniel, F.; Katsande, R.; et al. Early impact of rotavirus vaccine in under 5 year old children hospitalized due to diarrhea, Swaziland. Vaccine 2018, 36, 7210–7214. [Google Scholar] [CrossRef]
- Yandle, Z.; Coughlan, S.; Dean, J.; Hare, D.; De Gascun, C.F. Indirect impact of rotavirus vaccination on viral causes of acute gastroenteritis in the elderly. J. Clin. Virol. 2021, 137, 104780. [Google Scholar] [CrossRef]
- de Deus, N.; Chilaule, J.J.; Cassocera, M.; Bambo, M.; Langa, J.S.; Sitoe, E.; Chissaque, A.; Anapakala, E.; Sambo, J.; Guimarãesetal, E.L.; et al. Early impact of rotavirus vaccination in children less than five years of age in Mozambique. Vaccine 2018, 36, 7205–7209. [Google Scholar] [CrossRef]
- Chissaque, A.; Bauhofer, A.F.L.; Cossa-Moiane, I.; Sitoe, E.; Munlela, B.; João, E.D.; Langa, J.S.; Chilaúle, J.J.; Boene, S.S.; Cassocera, M.; et al. Rotavirus A infection in pre- and post-vaccine period: Risk factors, genotypes distribution by vaccination status and age of children in Nampula Province, Northern Mozambique (2015–2019). PLoS ONE 2021, 16, e0255720. [Google Scholar] [CrossRef]
- Andersson, M.; Kabayiza, J.C.; Lindh, M. Comparison of rotavirus frequency and genotype distribution in Rwanda before and after vaccine introduction. J. Clin. Virol. 2016, 82 (Suppl. 1), S63. [Google Scholar] [CrossRef]
- Nazurdinov, A.; Azizov, Z.; Mullojonova, M.; Sadykova, U.; Mosina, L.; Singh, S.; Suleymonova, S.; Tishkova, F.; Videbaek, D.; Cortese, M.M.; et al. Impact and effectiveness of monovalent rotavirus vaccine in Tajik children. Vaccine 2022, 40, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.; Rasanen, S.; Huhti, L.; Paloniemi, M.; Salminen, M.; Vesikari, T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013, 172, 739–746. [Google Scholar] [CrossRef]
- Hemming-Harlo, M.; Markkula, J.; Huhti, L.; Salminen, M.; Vesikari, T. Decrease of rotavirus gastroenteritis to a low level without resurgence for five years after universal RotaTeq vaccination in Finland. Pediatr. Infect. Dis. J. 2016, 35, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Givon-Lavi, N.; Ben-Shimol, S.; Cohen, R.; Greenberg, D.; Dagan, R. Rapid impact of rotavirus vaccine introduction to the National Immunization plan in southern Israel: Comparison between 2 distinct populations. Vaccine 2015, 33, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Mandolo, J.J.; Henrion, M.Y.R.; Mhango, C.; Chinyama, E.; Wachepa, R.; Kanjerwa, O.; Malamba-Banda, C.; Shawa, I.T.; Hungerford, D.; Kamng’ona, A.W.; et al. Reduction in severity of all-cause gastroenteritis requiring hospitalisation in children vaccinated against rotavirus in Malawi. Viruses 2021, 13, 2491. [Google Scholar] [CrossRef]
- Zaman, K.; Sack, D.A.; Neuzil, K.M.; Yunus, M.; Moulton, L.H.; Sugimoto, J.D.; Fleming, J.A.; Hossain, I.; El Arifeen, S.; Azim, T.; et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLoS Med. 2021, 14, e1002282. [Google Scholar] [CrossRef]
- Baker, J.M.; Dahl, R.M.; Cubilo, J.; Parashar, U.D.; Lopman, B.A. Effects of the rotavirus vaccine program across age groups in the United States: Analysis of national claims data, 2001–2016. BMC Infect. Dis. 2019, 19, 186. [Google Scholar] [CrossRef]
- Hasso-Agopsowicz, M.; Ladva, C.N.; Lopman, B.; Sanderson, C.; Cohen, A.L.; E Tate, J.; Riveros, X.; Henao-Restrepo, A.M.; Clark, A. Global review of the age distribution of rotavirus disease in children aged <5 years before the introduction of rotavirus vaccination. Clin. Infect. Dis. 2019, 69, 1071–1078. [Google Scholar] [CrossRef]
- Payne, D.C.; McNeal, M.; Staat, M.A.; Piasecki, A.M.; Cline, A.; DeFranco, E.; Goveia, M.G.; Parashar, U.D.; Burke, R.M.; Morrow, A.L. Persistence of maternal anti-rotavirus immunoglobulin G in the post-rotavirus era. J. Infect. Dis. 2021, 224, 133–136. [Google Scholar] [CrossRef]
- Varghese, T.; Kang, G.; Steele, A.D. Understanding rotavirus vaccine efficacy and effectiveness in countries with high child mortality. Vaccines 2022, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.; Victor, J.; Carey, M.; Tate, J.; Atherly, D.; Pecenka, C.; Diaz, Z.; Parashar, U.; Kirkwood, C. Experiences with rotavirus vaccines: Can we improve rotavirus vaccine impact in developing countries? Hum. Vaccin. Immunother. 2019, 15, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; van Zandvoort, K.; Flasche, S.; Sanderson, C.; Bines, J.; Tate, J.; Parashar, U.; Jit, M. Efficacy of live oral rotavirus vaccines by duration of follow-up: A meta-regression of randomised controlled trials. Lancet Infect. Dis. 2019, 19, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-W.; Fu, Y.; Lu, H.-L.; Yang, R.-X.; Goyal, H.; Jiang, Y.; Xu, H.-G. Association of rotavirus vaccines with reduction in rotavirus gastroenteritis in children younger than 5 years: A systematic review and meta-analysis of randomized clinical trials and observational studies. JAMA Pediatr. 2021, 175, e210347. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Chaney, D.M.; Deshpande, A.; Pitzer, V.E.; Lopman, B.A. Predicting indirect effects of rotavirus vaccination programs on rotavirus mortality among children in 112 countries. NPJ Vaccines 2023, 8, 32. [Google Scholar] [CrossRef]
- Hierink, F.; Okiro, E.A.; Flahault, A.; Ray, N. The winding road to health: A systematic scoping review on the effect of geographical accessibility to health care on infectious diseases in low- and middle-income countries. PLoS ONE 2021, 16, e0244921. [Google Scholar] [CrossRef]
- Adegbija, O.; Walker, J.; Smoll, N.; Khan, A.; Graham, J.; Khandaker, G. Notifiable diseases after implementation of COVID-19 public health prevention measures in Central Queensland, Australia. Commun. Dis. Intell. 2021, 45. [Google Scholar] [CrossRef]
- Munos, M.K.; Walker, C.L.; Black, R.E. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int. J. Epidemiol. 2010, 39 (Suppl. 1), i75–i87. [Google Scholar] [CrossRef]
- Parashar, U.D.; Nelson, E.A.; Kang, G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ 2013, 347, f7204. [Google Scholar] [CrossRef]

| Study Characteristics | N = 44 |
|---|---|
| Article type | |
| Peer-reviewed | 43 (98%) |
| Conference abstract | 1 (2%) |
| Study design | |
| Hospital-based surveillance or observational study | 25 (57%) |
| Register-based study | 5 (11%) |
| Population-based surveillance or observational study | 4 (9%) |
| Laboratory-based study | 4 (9%) |
| Observational study (unspecified) | 4 (9%) |
| Clinical audit | 1 (2%) |
| Population and hospital-based observational study | 1 (2%) |
| Country income level | |
| High | 20 (45%) |
| Upper-middle | 6 (14%) |
| Lower-middle | 12 (27%) |
| Low | 6 (14%) |
| National under-five mortality level (deaths per 1000 live births) | |
| ≤10 | 21 (48%) |
| >10–25 | 4 (9%) |
| >25–50 | 12 (27%) |
| >50–75 | 7 (16%) |
| Vaccine formulation | |
| RV1 Rotarix™ | 29 (66%) |
| RV5 RotaTeq™ | 5 (11%) |
| RV1/RV5 combination | 10 (23%) |
| Age group | |
| Children <5 years | 32 (73%) |
| Children <18 years | 7 (16%) |
| All people ≥5 years | 2 (5%) |
| All people ≥18 years | 1 (2%) |
| Older adults ≥65 years | 1 (2%) |
| All age groups | 1 (2%) |
| Outcome measure 1 | |
| Inpatient admissions for laboratory-confirmed RV-AGE | 23 (52%) |
| Outpatient attendances for laboratory-confirmed RV-AGE | 5 (11%) |
| Inpatient admissions for all-cause AGE | 12 (27%) |
| Outpatient attendances for all-cause AGE | 3 (7%) |
| Laboratory-confirmed RV in stool samples | 9 (20%) |
| Indirect vaccine effectiveness against the above outcomes | 3 (7%) |
| Mixed outcomes | 5 (11%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, D.S.; Harris, M.; Hart, J.D.; Russell, F.M. Indirect Effects of Universal Infant Rotavirus Vaccination: A Narrative Systematic Review. Vaccines 2025, 13, 503. https://doi.org/10.3390/vaccines13050503
Ong DS, Harris M, Hart JD, Russell FM. Indirect Effects of Universal Infant Rotavirus Vaccination: A Narrative Systematic Review. Vaccines. 2025; 13(5):503. https://doi.org/10.3390/vaccines13050503
Chicago/Turabian StyleOng, Darren Suryawijaya, Matthew Harris, John D. Hart, and Fiona M. Russell. 2025. "Indirect Effects of Universal Infant Rotavirus Vaccination: A Narrative Systematic Review" Vaccines 13, no. 5: 503. https://doi.org/10.3390/vaccines13050503
APA StyleOng, D. S., Harris, M., Hart, J. D., & Russell, F. M. (2025). Indirect Effects of Universal Infant Rotavirus Vaccination: A Narrative Systematic Review. Vaccines, 13(5), 503. https://doi.org/10.3390/vaccines13050503





