A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Preparation
2.2. Animal Studies
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Neutralization Assays
2.5. Analysis of Cytokines Secreted into the Supernatant of Splenocytes
2.6. Enzyme-Linked Immunospot Assay (ELISPOT)
2.7. Flow Cytometry Analysis
2.8. Determination of Viral Loads by Quantitative Polymerase Chain Reaction (qPCR)
2.9. Data Analysis
3. Results
3.1. Efficient mRNA Encapsulation by LNPs with Uniform Particle Size
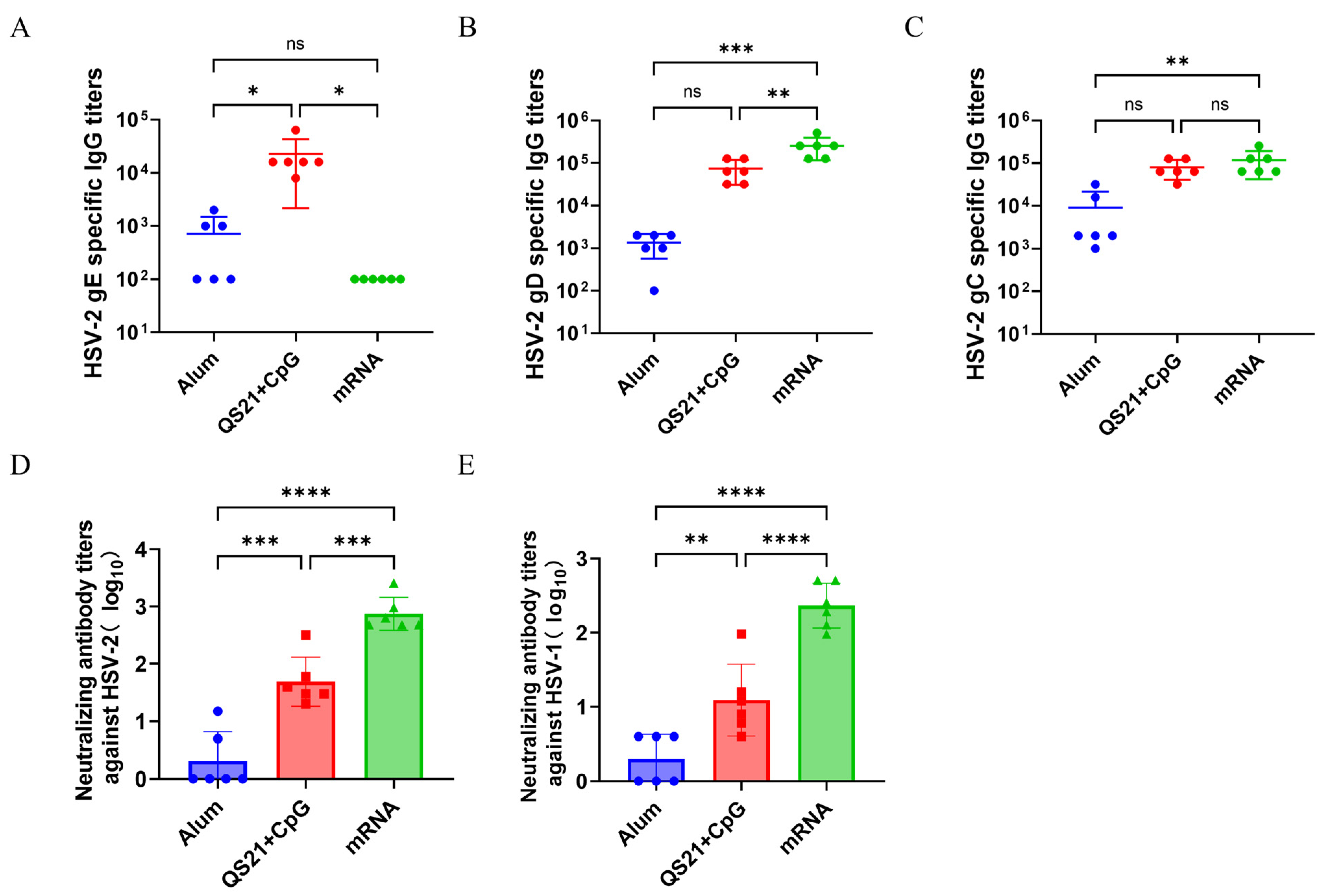
3.2. mRNA Vaccine and QS21 + CpG-Adjuvanted Subunit Vaccine Induced Superior Humoral Immune Response Compared to Aluminum-Adjuvanted Subunit Vaccine
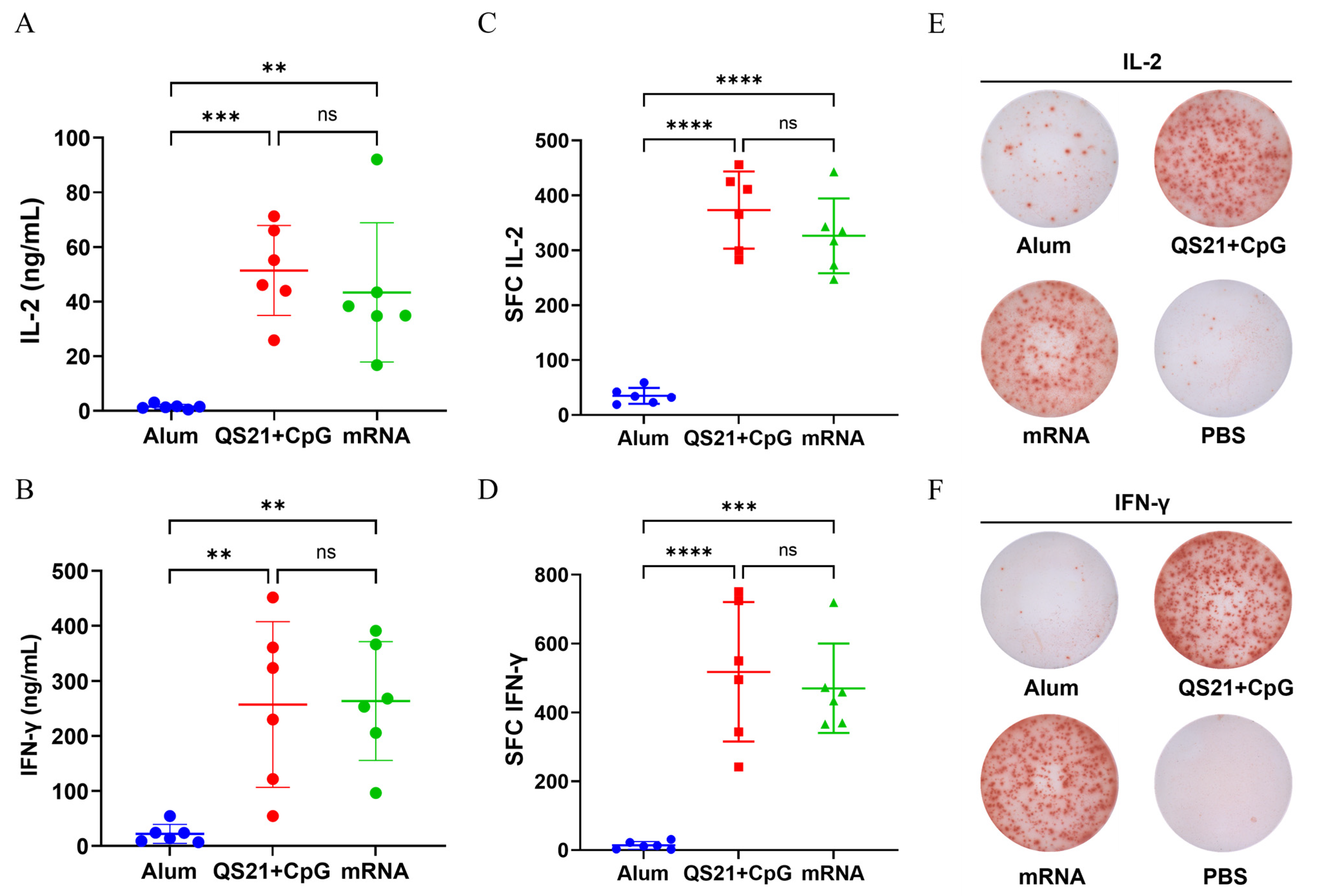
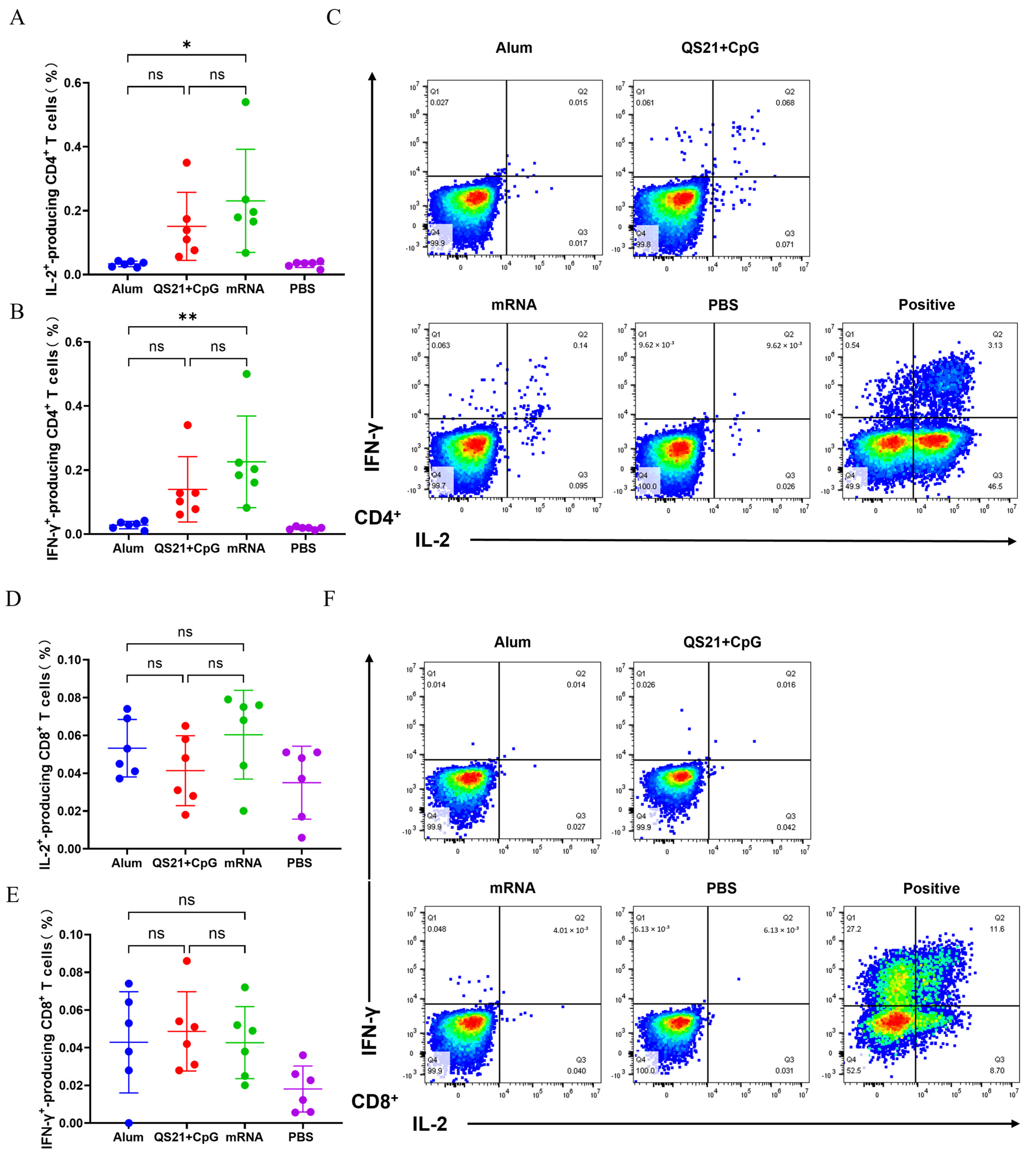
3.3. mRNA Vaccine and QS21 + CpG-Adjuvanted Vaccine Induced Robust Th1 Response
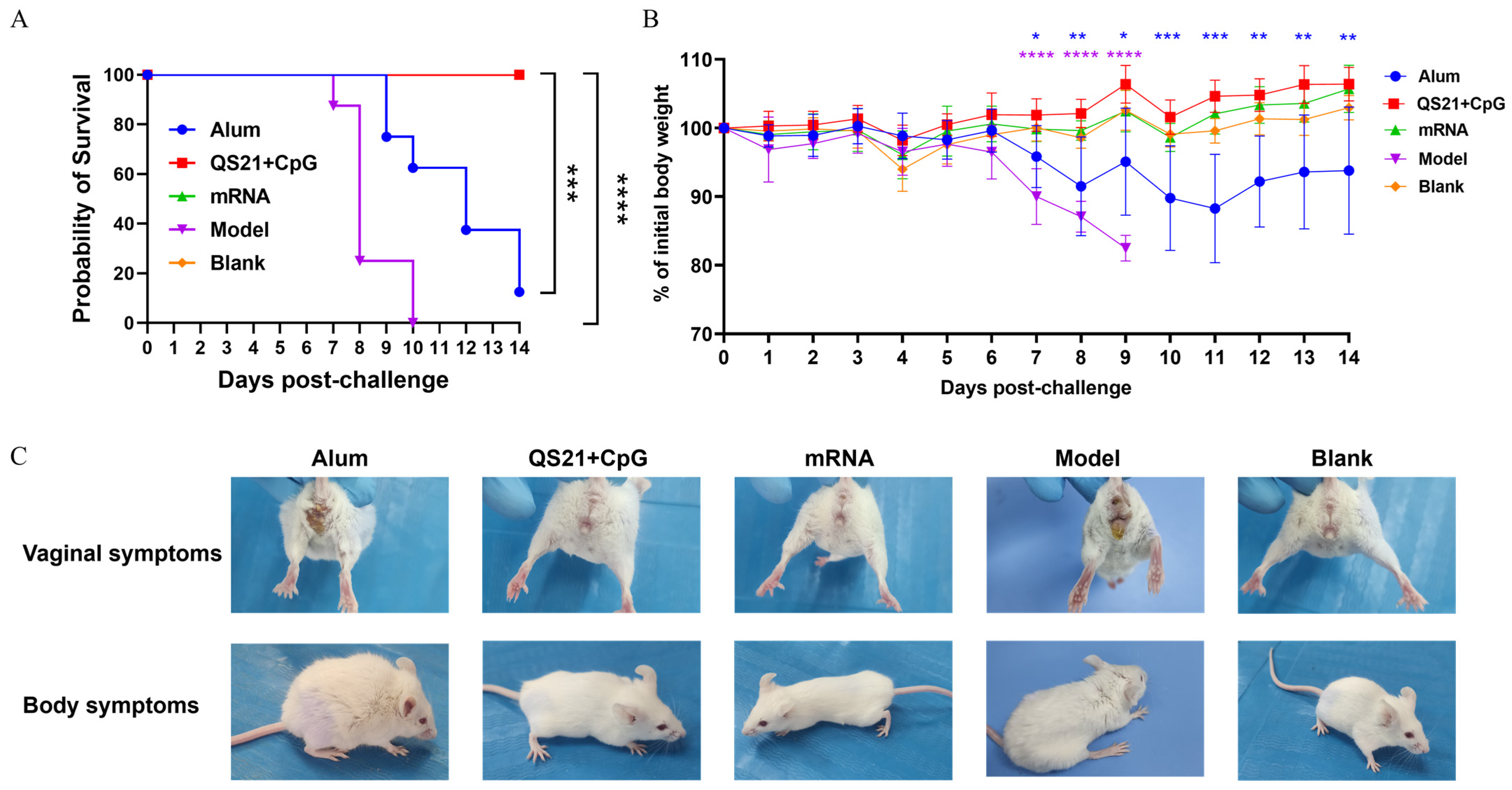
3.4. QS21 + CpG-Adjuvanted Subunit Vaccine and mRNA Vaccine Protected Mice from Lethal HSV-2 Challenge
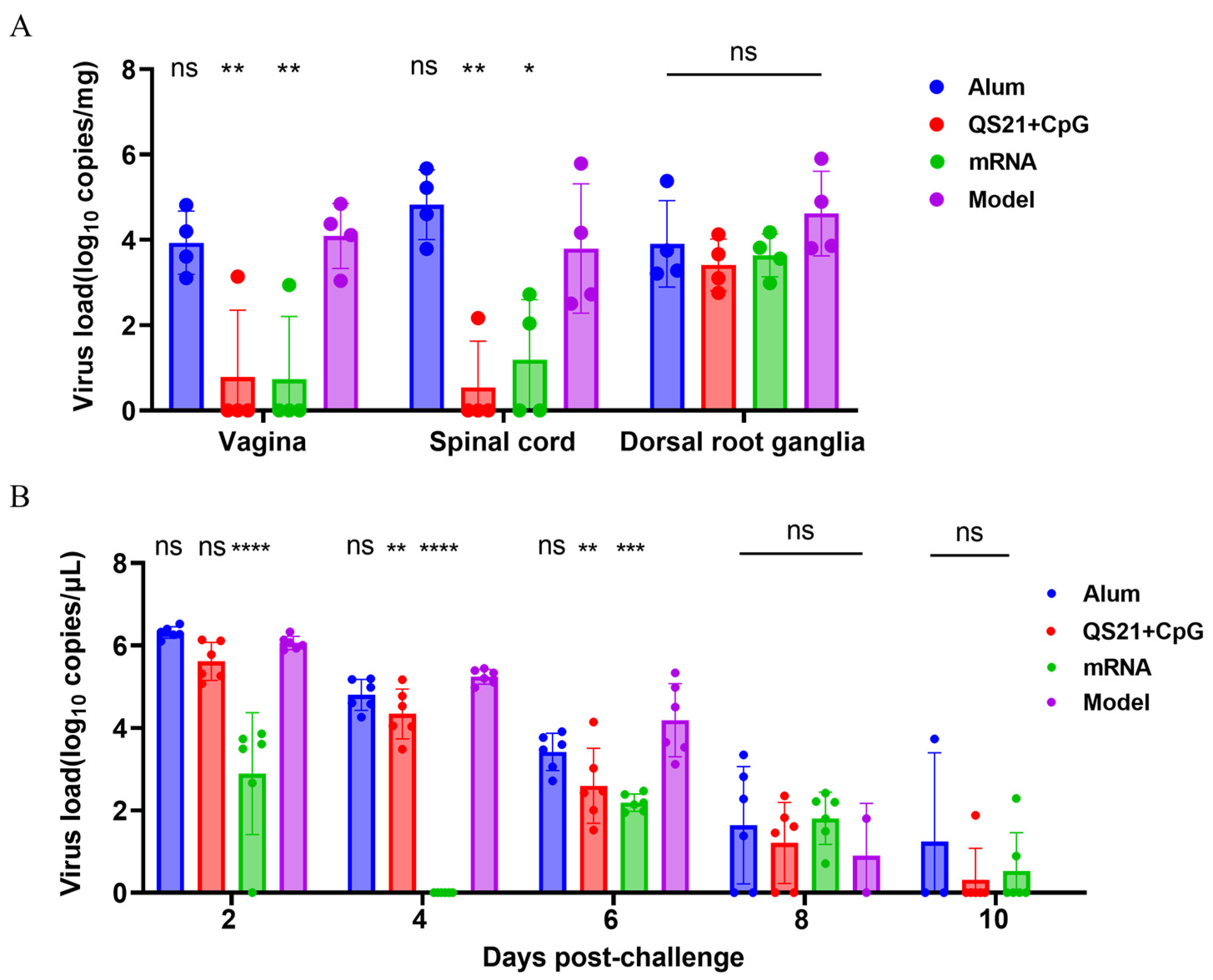
3.5. Viral Load Assessment Demonstrated Robust In Vivo Protection of QS21 + CpG-Adjuvanted and mRNA Vaccines
3.6. The QS21 + CpG-Adjuvanted Subunit Vaccine and mRNA Vaccine Provided Cross-Protection Against HSV-1 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Hayderi, L.; Rübben, A.; Nikkels, A.F. The alpha-herpesviridae in dermatology: Herpes simplex virus types I and II. Der Hautarzt Z. Fur Dermatol. Venerol. Und Verwandte Gebiete 2017, 68 (Suppl. S1), 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Kazanji, N.; Benvenuto, A.; Rizk, D. Understanding Herpes Simplex Virus Type 1 Versus Herpes Simplex Virus Type 2 Encephalitis After Neurosurgery: A Case Series and Literature Review. Surg. Infect. 2023, 24, 583–587. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Tronstein, E.; Johnston, C.; Huang, M.L.; Selke, S.; Magaret, A.; Warren, T.; Corey, L.; Wald, A. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011, 305, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Elmes, J.A.R.; Gottlieb, S.L.; Schiffer, J.T.; Vickerman, P.; Turner, K.M.E.; Boily, M.C. Effect of HSV-2 infection on subsequent HIV acquisition: An updated systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 1303–1316. [Google Scholar] [CrossRef]
- Looker, K.J.; Welton, N.J.; Sabin, K.M.; Dalal, S.; Vickerman, P.; Turner, K.M.E.; Boily, M.C.; Gottlieb, S.L. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: A population attributable fraction analysis using published epidemiological data. Lancet Infect. Dis. 2020, 20, 240–249. [Google Scholar] [CrossRef]
- Cocchi, F.; Fusco, D.; Menotti, L.; Gianni, T.; Eisenberg, R.J.; Cohen, G.H.; Campadelli-Fiume, G. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 2004, 101, 7445–7450. [Google Scholar] [CrossRef]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef]
- Farnsworth, A.; Johnson, D.C. Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread. J. Virol. 2006, 80, 3167–3179. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.D.; Horton, M.; Durr, E.; Heidecker, G.J.; Freed, D.; Fridman, A.; Wang, D.; Zhang, L. Evaluation of HSV-2 gE Binding to IgG-Fc and Application for Vaccine Development. Vaccines 2022, 10, 184. [Google Scholar] [CrossRef]
- Lubinski, J.M.; Lazear, H.M.; Awasthi, S.; Wang, F.; Friedman, H.M. The herpes simplex virus 1 IgG fc receptor blocks antibody-mediated complement activation and antibody-dependent cellular cytotoxicity in vivo. J. Virol. 2011, 85, 3239–3249. [Google Scholar] [CrossRef]
- Kostavasili, I.; Sahu, A.; Friedman, H.M.; Eisenberg, R.J.; Cohen, G.H.; Lambris, J.D. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 1997, 158, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus Evasion of Early Host Antiviral Responses. Front. Cell. Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Hook, L.M.; Lubinski, J.M.; Jiang, M.; Pangburn, M.K.; Friedman, H.M. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J. Virol. 2006, 80, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Ben-Akiva, E.; Chapman, A.; Mao, T.; Irvine, D.J. Linking vaccine adjuvant mechanisms of action to function. Sci. Immunol. 2025, 10, eado5937. [Google Scholar] [CrossRef]
- Kitagawa, S.; Matsuda, T.; Washizaki, A.; Murakami, H.; Yamamoto, T.; Yoshioka, Y. Elucidation of the role of nucleolin as a cell surface receptor for nucleic acid-based adjuvants. NPJ Vaccines 2022, 7, 115. [Google Scholar] [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol. Biol. 2021, 2197, 51–85. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Lee, G.H.; Lim, S.G. CpG-Adjuvanted Hepatitis B Vaccine (HEPLISAV-B®) Update. Expert Rev. Vaccines 2021, 20, 487–495. [Google Scholar] [CrossRef]
- Lv, X.; Martin, J.; Hoover, H.; Joshi, B.; Wilkens, M.; Ullisch, D.A.; Leibold, T.; Juchum, J.S.; Revadkar, S.; Kalinovska, B.; et al. Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture. iScience 2024, 27, 109006. [Google Scholar] [CrossRef] [PubMed]
- Luan, N.; Cao, H.; Wang, Y.; Lin, K.; Liu, C. Ionizable Lipid Nanoparticles Enhanced the Synergistic Adjuvant Effect of CpG ODNs and QS21 in a Varicella Zoster Virus Glycoprotein E Subunit Vaccine. Pharmaceutics 2022, 14, 973. [Google Scholar] [CrossRef]
- Awasthi, S.; Huang, J.; Shaw, C.; Friedman, H.M. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J. Virol. 2014, 88, 8421–8432. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Hook, L.M.; Shaw, C.E.; Pahar, B.; Stagray, J.A.; Liu, D.; Veazey, R.S.; Friedman, H.M. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog. 2017, 13, e1006141. [Google Scholar] [CrossRef]
- Awasthi, S.; Knox, J.J.; Desmond, A.; Alameh, M.G.; Gaudette, B.T.; Lubinski, J.M.; Naughton, A.; Hook, L.M.; Egan, K.P.; Tam, Y.K.; et al. Trivalent nucleoside-modified mRNA vaccine yields durable memory B cell protection against genital herpes in preclinical models. J. Clin. Investig. 2021, 131, e152310. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.; Luan, N.; Lin, K.; Liu, C. Effects of Varicella-Zoster Virus Glycoprotein E Carboxyl-Terminal Mutation on mRNA Vaccine Efficacy. Vaccines 2021, 9, 1440. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.; Cao, H.; Liu, C. Immune Responses to Varicella-Zoster Virus Glycoprotein E Formulated with Poly(Lactic-co-Glycolic Acid) Nanoparticles and Nucleic Acid Adjuvants in Mice. Virol. Sin. 2021, 36, 122–132. [Google Scholar] [CrossRef]
- Wui, S.R.; Kim, K.S.; Ryu, J.I.; Ko, A.; Do, H.T.T.; Lee, Y.J.; Kim, H.J.; Lim, S.J.; Park, S.A.; Cho, Y.J.; et al. Efficient induction of cell-mediated immunity to varicella-zoster virus glycoprotein E co-lyophilized with a cationic liposome-based adjuvant in mice. Vaccine 2019, 37, 2131–2141. [Google Scholar] [CrossRef]
- Laing, K.J.; Russell, R.M.; Dong, L.; Schmid, D.S.; Stern, M.; Magaret, A.; Haas, J.G.; Johnston, C.; Wald, A.; Koelle, D.M. Zoster Vaccination Increases the Breadth of CD4+ T Cells Responsive to Varicella Zoster Virus. J. Infect. Dis. 2015, 212, 1022–1031. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, H.K. Vaccines against Genital Herpes: Where Are We? Vaccines 2020, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Islam, S.; Chakraborty, S.; Mustafa, A.H.; Azim, K.F.; Joy, Z.F.; Hossain, M.N.; Foysal, S.H.; Hasan, M.N. Contriving a chimeric polyvalent vaccine to prevent infections caused by herpes simplex virus (type-1 and type-2): An exploratory immunoinformatic approach. J. Biomol. Struct. Dyn. 2020, 38, 2898–2915. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.H.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing the transitioning epidemiology of herpes simplex virus type 1 in the USA: Model-based predictions. BMC Med. 2019, 17, 57. [Google Scholar] [CrossRef]
- Harfouche, M.; AlMukdad, S.; Alareeki, A.; Osman, A.M.M.; Gottlieb, S.; Rowley, J.; Abu-Raddad, L.J.; Looker, K.J. Estimated global and regional incidence and prevalence of herpes simplex virus infections and genital ulcer disease in 2020: Mathematical modelling analyses. Sex. Transm. Infect. 2024; online first. [Google Scholar] [CrossRef]
- Awasthi, S.; Onishi, M.; Lubinski, J.M.; Fowler, B.T.; Naughton, A.M.; Hook, L.M.; Egan, K.P.; Hagiwara, M.; Shirai, S.; Sakai, A.; et al. Novel Adjuvant S-540956 Targets Lymph Nodes and Reduces Genital Recurrences and Vaginal Shedding of HSV-2 DNA When Administered with HSV-2 Glycoprotein D as a Therapeutic Vaccine in Guinea Pigs. Viruses 2023, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef]


| Vaccine Group | gE + gD + gC | Alum | QS21 | CpG 1018S | mRNA |
|---|---|---|---|---|---|
| 1.Alum | 7.5 μg | 50 μg | - | - | - |
| 2.QS21 + CpG | 7.5 μg | - | 5 μg | 10 μg | - |
| 3.mRNA | - | - | - | - | 15 μg |
| 4.PBS | - | - | - | - | - |
| 5.Model | - | - | - | - | - |
| Name | Sequence |
|---|---|
| HSV-2-gG-F | CGCTCTCGTAAATGCTTCCCT |
| HSV-2-gG-R | TCTACCCACAACAGACCCACG |
| HSV-2-gG-Probe | 6-FAM-CGCGGAGACATTCGAGTACCAGATCG-BHQ1 |
| HSV-1-UL7-F | GGTGCCGGTTGCGGTTCGT |
| HSV-1-UL7-R | GAACCGGTGCAGCAGAACG |
| HSV-1-UL7-Probe | FAM-AGTCCCGAGGACGCCTATGTGACG-TAMRA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Zhang, X.; Cheng, J.; Li, Y.; Luan, N.; Hu, J.; Liang, B.; Zhang, H.; Gao, D.; Lei, Z.; et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines 2025, 13, 497. https://doi.org/10.3390/vaccines13050497
Cao H, Zhang X, Cheng J, Li Y, Luan N, Hu J, Liang B, Zhang H, Gao D, Lei Z, et al. A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines. 2025; 13(5):497. https://doi.org/10.3390/vaccines13050497
Chicago/Turabian StyleCao, Han, Xiaolong Zhang, Jishuai Cheng, Yang Li, Ning Luan, Jingping Hu, Bingyan Liang, Haihao Zhang, Dandan Gao, Zhentao Lei, and et al. 2025. "A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice" Vaccines 13, no. 5: 497. https://doi.org/10.3390/vaccines13050497
APA StyleCao, H., Zhang, X., Cheng, J., Li, Y., Luan, N., Hu, J., Liang, B., Zhang, H., Gao, D., Lei, Z., Yao, Y., & Liu, C. (2025). A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice. Vaccines, 13(5), 497. https://doi.org/10.3390/vaccines13050497






