Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Thyroglobulin Assay
2.3. Thyroglobulin Antibody Assay
2.4. Thyroid-Stimulating Hormone Assay
2.5. Free T4 Assay
2.6. Human Papillomavirus DNA Testing
2.7. Variables
2.8. Statistical Analysis
2.9. Ethics Approval and Consent to Participate
3. Results
3.1. Characteristics of Participants
3.2. The Relationship Between HPV Vaccination and the Risk of HT Development
3.3. The Relationship Between HPV Vaccination and TPOAb/TGAb
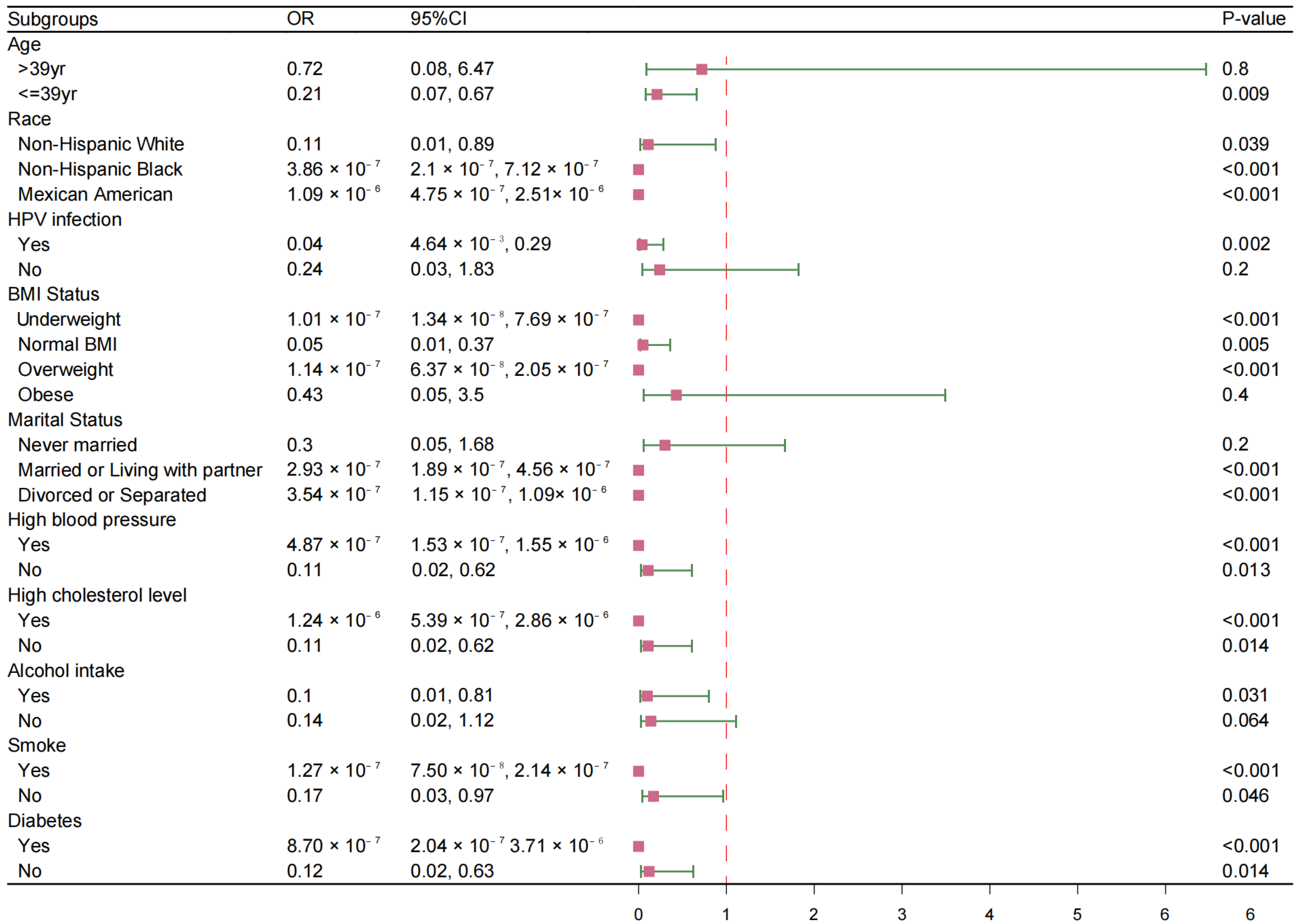
3.4. Subgroups Analysis Between HPV Vaccination and the Risk of HT Development
3.5. Subgroups Analysis Between HPV Vaccination and TPOAb/TGAb
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| TGAb | thyroglobulin antibodies |
| TPOAb | thyroid peroxidase antibodies |
| TSH | thyroid-stimulating hormone |
| FT4 | free thyroxine |
| OR | odds ratio |
| CI | confidence interval |
| 95% CI | 95% confidence interval |
Appendix A
| Exposure | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV Infection | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| No | - | - | - | - | - | - | |||
| Yes | 0.78 | 0.58, 1.05 | 0.1 | 0.92 | 0.67, 1.26 | 0.6 | 1.03 | 0.72, 1.48 | 0.9 |
References
- Saslow, D.; Andrews, K.S.; Manassaram-Baptiste, D.; Loomer, L.; Lam, K.E.; Fisher-Borne, M.; Smith, R.A.; Fontham, E.T.H. Human papillomavirus vaccination guideline update: American Cancer Society guideline endorsement. CA Cancer J. Clin. 2016, 66, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev. Vaccines 2018, 17, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; Singleton, J.A.; Stokley, S. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.M.; Benard, V.; Roland, K.B.; Watson, M.; Liddon, N.; Stokley, S. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA Pediatr. 2014, 168, 76–82. [Google Scholar] [CrossRef]
- Liddon, N.C.; Hood, J.E.; Leichliter, J.S. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: Findings from the 2006–2008 National Survey of Family Growth. Vaccine 2012, 30, 2676–2682. [Google Scholar] [CrossRef]
- Wraith, D.C.; Goldman, M.; Lambert, P.H. Vaccination and autoimmune disease: What is the evidence? Lancet 2003, 362, 1659–1666. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Slade, B.A.; Leidel, L.; Vellozzi, C.; Woo, E.J.; Hua, W.; Sutherland, A.; Izurieta, H.S.; Ball, R.; Miller, N.; Braun, M.M.; et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009, 302, 750–757. [Google Scholar] [CrossRef]
- Genovese, C.; Laf, V.; Squeri, A.; Trimarchi, G.; Squeri, R. HPV vaccine and autoimmune diseases: Systematic review and meta-analysis of the literature. J. Prev. Med. Hyg. 2018, 59, e194–e199. [Google Scholar] [CrossRef]
- Liu, E.Y.; Smith, L.M.; Ellis, A.K.; Whitaker, H.; Law, B.; Kwong, J.C.; Farrington, P.; Lévesque, L.E. Quadrivalent human papillomavirus vaccination in girls and the risk of autoimmune disorders: The Ontario Grade 8 HPV Vaccine Cohort Study. Can. Med. Assoc. J. 2018, 190, e648–e655. [Google Scholar] [CrossRef]
- Grimaldi-Bensouda, L.; Guillemot, D.; Godeau, B.; Bénichou, J.; Lebrun-Frenay, C.; Papeix, C.; Labauge, P.; Berquin, P.; Penfornis, A.; Benhamou, P.; et al. Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J. Intern. Med. 2014, 275, 398–408. [Google Scholar] [CrossRef] [PubMed]
- De Leo, S.; Lee, S.Y.; Braverman, L.E. Hyperthyroidism. Lancet 2016, 388, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Uricoechea, H.; Nogueira, J.P.; Pinzón-Fernández, M.V.; Schwarzstein, D. The Usefulness of Thyroid Antibodies in the Diagnostic Approach to Autoimmune Thyroid Disease. Antibodies 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhane, T.; Krishna, K.; Ranganathan, V.; Jayaraman, V.; Wang, T.; Bei, K.; Ashman, S.; Rajasekaran, K.; Rajasekaran, J.J.; Krishnamurthy, H. Significance of Anti-TPO as an Early Predictive Marker in Thyroid Disease. Autoimmune Dis. 2019, 2019, 1684074. [Google Scholar] [CrossRef]
- Rizzo, M.; Rossi, R.; Bonaffini, O.; Scisca, C.; Altavilla, G.; Calbo, L.; Rosanò, A.; Sindoni, A.; Trimarchi, F.; Benvenga, S. Increased annual frequency of Hashimoto’s thyroiditis between years 1988 and 2007 at a cytological unit of Sicily. Ann. Endocrinol. 2010, 71, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ying, Y.-X.; Liang, J.; Geng, H.-F.; Zhang, Q.-Y.; Zhang, C.-R.; Chen, F.-X.; Li, Y.; Feng, Y.; Wang, Y.; et al. Urinary Iodine and Genetic Predisposition to Hashimoto’s Thyroiditis in a Chinese Han Population: A Case-Control Study. Thyroid 2020, 30, 1820–1830. [Google Scholar] [CrossRef]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef]
- Siolos, A.; Gartzonika, K.; Tigas, S. Thyroiditis following vaccination against COVID-19: Report of two cases and review of the literature. Metab. Open 2021, 12, 100136. [Google Scholar] [CrossRef]
- Şendur, S.N.; Oğuz, H.; Ünlütürk, U. COVID-19 vaccination and thyroiditis. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101759. [Google Scholar] [CrossRef]
- Altay, F.A.; Güz, G.; Altay, M. Subacute thyroiditis following seasonal influenza vaccination. Hum. Vaccines Immunother. 2016, 12, 1033–1034. [Google Scholar] [CrossRef]
- Meng, R.; Ma, R.; Wang, J.; Liu, P.; Liu, Z.; He, B.; Liu, Z.; Yang, Y.; Zhan, S. Post-marketing surveillance for the safety of the 9-valent human papillomavirus vaccine: A retrospective real-world study in China. Expert Rev. Vaccines 2023, 22, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Lee, J.H.; Lee, H.; Shin, J.Y. Association between human papillomavirus vaccination and serious adverse events in South Korean adolescent girls: Nationwide cohort study. BMJ 2021, 372, m4931. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Chaignot, C.; Collin, C.; Dray-Spira, R.; Weill, A.; Zureik, M. Human papillomavirus vaccination and risk of autoimmune diseases: A large cohort study of over 2million young girls in France. Vaccine 2017, 35, 4761–4768. [Google Scholar] [CrossRef]
- Pagliusi, S.R.; Garland, S.M. International standard reagents for HPV detection. Dis. Markers 2007, 23, 283–296. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Perry, C.M. Human papillomavirus quadrivalent (types 6, 11, 16, 18) recombinant vaccine (Gardasil). Drugs 2006, 66, 1263–1271; discussion 1272–1263. [Google Scholar] [CrossRef]
- Eileen, F.D.; Lauri, E.M.; Harrell, C.; Robinette, C.C.; Mona, S.; Julianne, G.; Elizabeth, R.U. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1705–1708. [Google Scholar]
- Graham, D.M.; Isaranuwatchai, W.; Habbous, S.; de Oliveira, C.; Liu, G.; Siu, L.L.; Hoch, J.S. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer 2015, 121, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Trim, K.; Nagji, N.; Elit, L.; Roy, K. Parental Knowledge, Attitudes, and Behaviours towards Human Papillomavirus Vaccination for Their Children: A Systematic Review from 2001 to 2011. Obstet. Gynecol. Int. 2012, 2012, 921236. [Google Scholar] [CrossRef]
- Soldevilla, H.F.; Briones, S.F.; Navarra, S.V. Systemic lupus erythematosus following HPV immunization or infection? Lupus 2012, 21, 158–161. [Google Scholar] [CrossRef]
- Arnheim-Dahlström, L.; Pasternak, B.; Svanström, H.; Sparén, P.; Hviid, A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: Cohort study. BMJ 2013, 347, f5906. [Google Scholar] [CrossRef]
- Naleway, A.L.; Gold, R.; Drew, L.; Riedlinger, K.; Henninger, M.L.; Gee, J. Reported adverse events in young women following quadrivalent human papillomavirus vaccination. J. Women’s Health 2012, 21, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Lawson, H.W.; Chesson, H.; Unger, E.R. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–24. [Google Scholar] [PubMed]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar]
- Effraimidis, G.; Tijssen, J.G.; Wiersinga, W.M. Alcohol consumption as a risk factor for autoimmune thyroid disease: A prospective study. Eur. Thyroid. J. 2012, 1, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P. Cellular immune responses in autoimmune thyroid disease. Clin. Endocrinol. 2004, 61, 405–413. [Google Scholar] [CrossRef]
- Walsh, J.P.; Bremner, A.P.; Feddema, P.; Leedman, P.J.; Brown, S.J.; O’Leary, P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: A 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J. Clin. Endocrinol. Metab. 2010, 95, 1095–1104. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Li, B.; Liu, D.; Liu, Y.-H.; Mo, R.; Lai, F.; Liu, R.; Peng, S.; Li, Y.; et al. Effect of Inactivated SARS-CoV-2 Vaccine on Thyroid Function and Autoimmunity Within 28 Days After the Second Dose. Thyroid 2022, 32, 1051–1058. [Google Scholar] [CrossRef]


| Characteristic | n 1 | Total, n = 39,622,694 2 | Unvaccinated n = 37,196,826 2 | Vaccinated n = 2,425,868 2 | p Value 3 |
|---|---|---|---|---|---|
| Age, years | 2717 | 39 [29, 49] | 41 [30, 50] | 22 [20, 27] | <0.001 |
| Race | 2717 | 0.027 | |||
| Non-Hispanic White | 1125 (67%) | 1053 (67%) | 72 (70%) | ||
| Non-Hispanic Black | 552 (12%) | 518 (12%) | 34 (11%) | ||
| Mexican American | 511 (8.7%) | 496 (9.0%) | 15 (3.1%) | ||
| Other Hispanic | 341 (6.0%) | 313 (5.8%) | 28 (9.3%) | ||
| Other | 188 (6.5%) | 173 (6.5%) | 15 (7.2%) | ||
| Education | 2561 | 0.002 | |||
| Less Than High School | 596 (15%) | 585 (16%) | 11 (4.8%) | ||
| High School | 543 (20%) | 527 (21%) | 16 (12%) | ||
| Some College | 829 (34%) | 771 (33%) | 58 (51%) | ||
| College or above | 593 (31%) | 564 (31%) | 29 (32%) | ||
| BMI status | 2693 | 0.079 | |||
| underweight | 77 (2.8%) | 73 (2.9%) | 4 (2.3%) | ||
| normal BMI | 846 (36%) | 775 (36%) | 71 (49%) | ||
| overweight | 749 (27%) | 709 (27%) | 40 (24%) | ||
| obese | 1021 (34%) | 976 (35%) | 45 (25%) | ||
| Marital Status | 2561 | <0.001 | |||
| Never married | 567 (20%) | 501 (18%) | 66 (53%) | ||
| Married or Living with partner | 1518 (64%) | 1477 (66%) | 41 (40%) | ||
| Divorced or Separated | 476 (16%) | 469 (17%) | 7 (6.8%) | ||
| Alcohol intake | 2717 | 0.9 | |||
| No | 1115 (34%) | 1044 (34%) | 71 (35%) | ||
| Yes | 1602 (66%) | 1509 (66%) | 93 (65%) | ||
| Smoke | 2717 | 0.013 | |||
| No | 1744 (62%) | 1619 (62%) | 125 (73%) | ||
| Yes | 973 (38%) | 934 (38%) | 39 (27%) | ||
| High blood pressure | 2717 | 0.005 | |||
| No | 2162 (80%) | 2014 (79%) | 148 (92%) | ||
| Yes | 555 (20%) | 539 (21%) | 16 (8.2%) | ||
| High cholesterol level | 2717 | <0.001 | |||
| No | 2147 (77%) | 1993 (75%) | 154 (96%) | ||
| Yes | 570 (23%) | 560 (25%) | 10 (4.2%) | ||
| Diabetes | 2717 | 0.04 | |||
| No | 2509 (93%) | 2350 (93%) | 159 (98%) | ||
| Borderline | 32 (1.3%) | 31 (1.4%) | 1 (0.4%) | ||
| Yes | 176 (5.5%) | 172 (5.7%) | 4 (1.9%) | ||
| Ratio of family income to poverty | 2516 | 0.023 | |||
| high income | 910 (51%) | 864 (51%) | 46 (43%) | ||
| middle income | 960 (32%) | 908 (32%) | 52 (30%) | ||
| low income | 646 (17%) | 591 (17%) | 55 (27%) | ||
| HPV infection | 2717 | <0.001 | |||
| No | 1492 (59%) | 1432 (60%) | 60 (37%) | ||
| Yes | 1225 (41%) | 1121 (40%) | 104 (63%) | ||
| TPOAb | 2717 | 0.002 | |||
| Negative | 2374 (86%) | 2216 (86%) | 158 (97%) | ||
| Positive | 343 (14%) | 337 (14%) | 6 (3.4%) | ||
| TGAb | 2717 | 0.3 | |||
| Negative | 2689 (99%) | 2525 (99%) | 164 (100%) | ||
| Positive | 28 (1.0%) | 28 (1.1%) | 0 (0%) | ||
| TSH | 2716 | 0.4 | |||
| 0.34–5.6 mIU/mL | 2588 (95%) | 2430 (95%) | 158 (97%) | ||
| <0.34 mIU/mL | 71 (2.1%) | 66 (2.1%) | 5 (2.2%) | ||
| >5.60 mIU/mL | 57 (2.6%) | 56 (2.7%) | 1 (0.8%) | ||
| FT4 | 2717 | 0.8 | |||
| 0.60–1.60 ng/dL | 2617 (97%) | 2458 (97%) | 159 (97%) | ||
| <0.60 ng/dL | 87 (3.0%) | 82 (3.0%) | 5 (3.1%) | ||
| >1.60 ng/dL | 13 (0.3%) | 13 (0.3%) | 0 (0%) |
| Girls Vaccinated | 164 | |
|---|---|---|
| Age at vaccination | ||
| 18~26 | 125 | 76.22% |
| 27~45 | 34 | 20.73% |
| >45 | 5 | 3.05% |
| Number of doses per girl vaccinated * | ||
| 1 dose | 37 | 23.87% |
| 2 doses | 45 | 29.03% |
| 3 doses | 73 | 47.10% |
| Type of vaccine | ||
| 2007–2010 | Gardasil | |
| 2011–2012 | Cervarix/Gardasil |
| Exposure | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV Vaccination | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| No | - | - | - | - | - | - | |||
| Yes | 0.21 | 0.07, 0.58 | 0.004 | 0.28 | 0.10, 0.78 | 0.016 | 0.13 | 0.02, 0.76 | 0.025 |
| Exposure | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV Vaccination | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| No | - | - | - | - | - | - | |||
| 1 dose | 1.05 × 10−6 | 6.56 × 10−7, 1.69 × 10−6 | <0.001 | 1.63 × 10−6 | 9.87 × 10−7, 2.69 × 10−6 | <0.001 | 1.72 × 10−6 | 2.02 × 10−7, 1.47 × 10−5 | <0.001 |
| 2 doses | 0.518 | 0.129, 2.088 | 0.3 | 0.66 | 0.168, 2.585 | 0.5 | 0.247 | 0.034, 1.796 | 0.2 |
| 3 doses | 0.099 | 0.018, 0.535 | 0.008 | 0.136 | 0.026, 0.725 | 0.021 | 9.93 × 10−7 | 1.19 × 10−7, 8.32 × 10−6 | <0.001 |
| Exposure | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TPOAb: | |||||||||
| HPV vaccination | Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value |
| No | - | - | - | - | - | - | |||
| Yes | −20.34 | −32.11, −8.57 | 0.001 | −14.1 | −26.45, −1.74 | 0.026 | −22.27 | −34.86, −9.68 | 0.001 |
| TGAb: | |||||||||
| HPV vaccination | Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value |
| No | - | - | - | - | - | - | |||
| Yes | −6.12 | −9.41, −2.84 | <0.001 | −4.89 | −9.63, −0.14 | 0.044 | −7.53 | −14.88, −0.18 | 0.045 |
| Exposure | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TPOAb: | |||||||||
| HPV vaccination | Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value |
| No | - | - | - | - | - | - | |||
| 1 dose | −28.73 | −34.19, −23.28 | <0.001 | −19.91 | −26.48, −13.34 | <0.001 | −22.46 | −32.05, −12.88 | <0.001 |
| 2 doses | −23.59 | −32.97, −14.22 | <0.001 | −17.88 | −28.14, −7.62 | 0.001 | −25.37 | −43.48, −7.26 | 0.008 |
| 3 doses | −19.6 | −38.30, −0.89 | 0.04 | −13.71 | −33.08, 5.66 | 0.2 | −26.72 | −37.80, −15.64 | <0.001 |
| TGAb: | |||||||||
| HPV vaccination | Beta | 95% CI | p-value | Beta | 95% CI | p-value | Beta | 95% CI | p-value |
| No | - | - | - | - | - | - | |||
| 1 dose | −7.26 | −10.34, −4.18 | <0.001 | −5.64 | −10.14, −1.14 | 0.015 | −7.7 | −14.17, −1.23 | 0.022 |
| 2 doses | −5.76 | −9.12, −2.41 | 0.001 | −5.08 | −10.47, 0.30 | 0.064 | −6.99 | −14.61, 0.64 | 0.071 |
| 3 doses | −5.93 | −9.89, −1.97 | 0.004 | −4.53 | −9.56, 0.50 | 0.076 | −7.24 | −14.36, −0.11 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Ye, L.; Chen, M.; Liu, H.; Miao, J. Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study. Vaccines 2025, 13, 490. https://doi.org/10.3390/vaccines13050490
Yin Y, Ye L, Chen M, Liu H, Miao J. Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study. Vaccines. 2025; 13(5):490. https://doi.org/10.3390/vaccines13050490
Chicago/Turabian StyleYin, Yifan, Liang Ye, Min Chen, Hao Liu, and Jingkun Miao. 2025. "Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study" Vaccines 13, no. 5: 490. https://doi.org/10.3390/vaccines13050490
APA StyleYin, Y., Ye, L., Chen, M., Liu, H., & Miao, J. (2025). Association Between Human Papillomavirus Vaccination and the Risk of Hashimoto’s Thyroiditis: A Cross-Sectional Study. Vaccines, 13(5), 490. https://doi.org/10.3390/vaccines13050490





