Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy and Research Questions
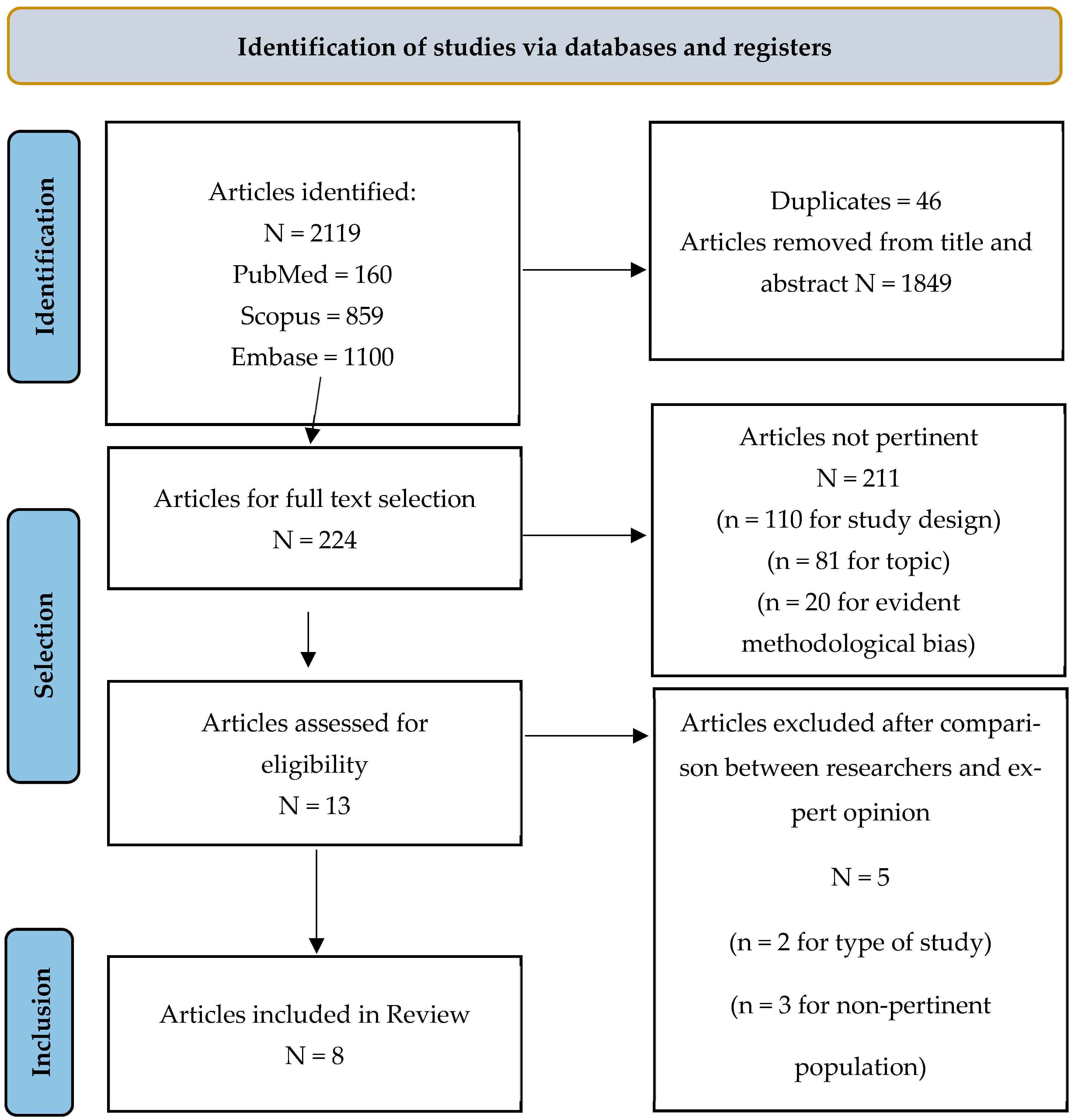
2.5. Selection Process
2.6. Data Collection Process
2.7. Data Items
2.8. Study Risk of Bias Assessment
2.9. Effect Measures
2.10. Synthesis of Results
3. Results
3.1. Characteristics of the Included Studies
3.2. Systematic Results of the Included Studies
3.2.1. HPV Vaccination Awareness and Education
3.2.2. Attitudes and Barriers Toward HPV Vaccination
3.2.3. Interventions and Success Factors in Vaccine Adoption
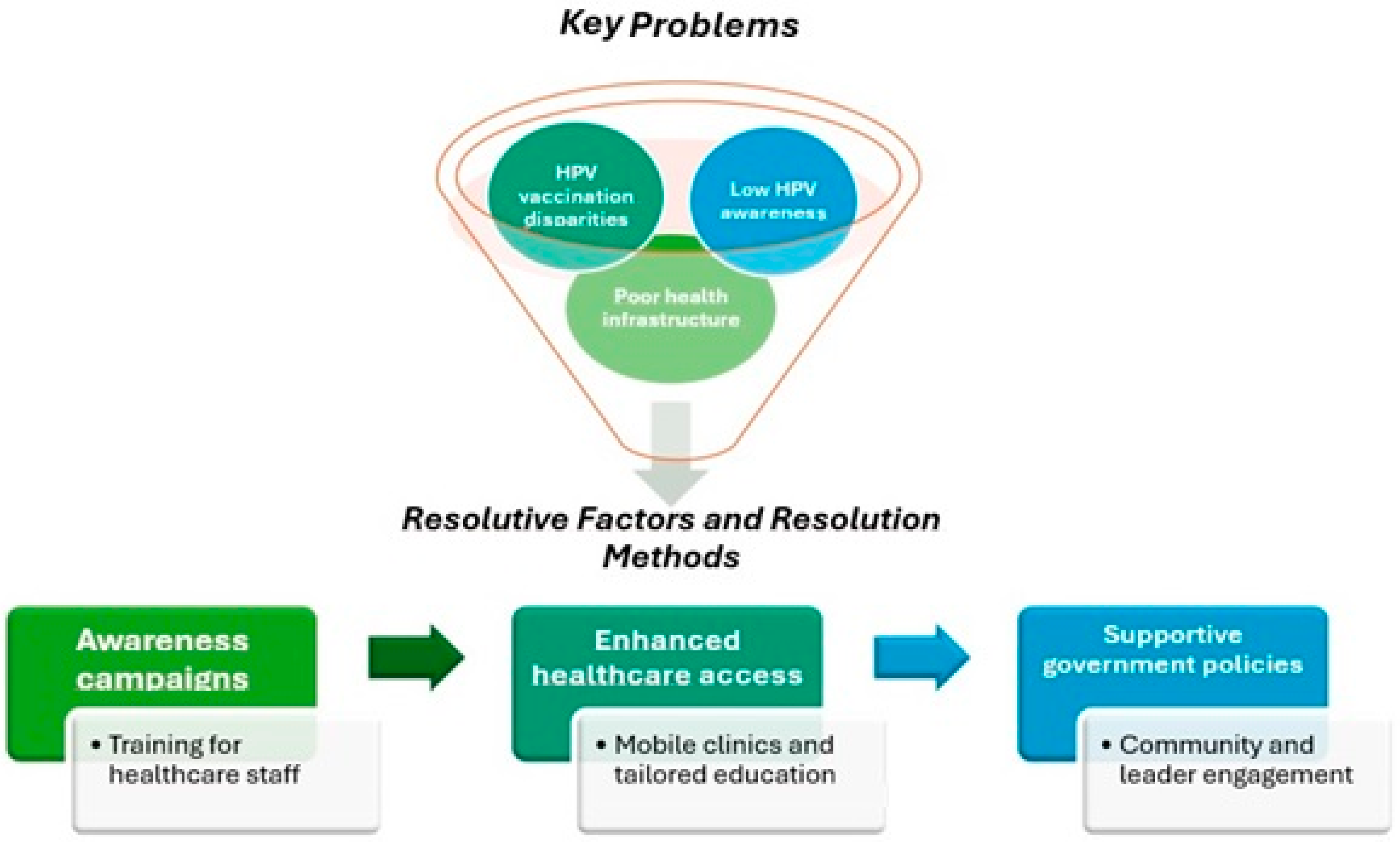
4. Discussion
4.1. Perspectives for Future Research, Clinical Practice, and Policy Raccomandations
- -
- Prioritize comprehensive public health campaigns to increase awareness of HPV and the vaccine’s importance, particularly targeting marginalized communities and underserved regions;
- -
- Invest in healthcare infrastructure and resources to ensure the effective implementation of HPV vaccination programs, focusing on areas with limited resources;
- -
- Implement policies that address the socioeconomic barriers to vaccine access, including financial support and improved transportation networks for vulnerable populations;
- -
- Foster international collaboration to share best practices and develop region-specific strategies that account for local cultural, political, and economic contexts;
- -
- Advocate for the integration of HPV vaccination into broader health policies that focus on cancer prevention, public health equity, and the improvement of healthcare delivery systems.
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Protocol Registration
References
- Wolf, J.; Kist, L.F.; Pereira, S.B.; Quessada, M.A.; Petek, H.; Pille, A. Human Papillomavirus Infection: Epidemiology, Biology, Host Interactions, Cancer Development, Prevention, and Therapeutics. Rev. Med. Virol. 2024, 34, 2537. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.E.; Becker, G.L.; Jackson, J.B.; Rysavy, M.B. Human Papillomavirus and Associated Cancers: A Review. Viruses 2024, 16, 680. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 7 April 2025).
- Alrefai, E.A.; Alhejaili, R.T.; Haddad, S.A. Human Papillomavirus and Its Association With Cervical Cancer: A Review. Cureus 2024, 16, e57432. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Human Malignancies Associated with Persistent HPV Infection. Oncol. 2024, 29, 457–464. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Q.; Zhang, Y.; Ji, Y.; Li, J.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; Zhang, Y.; et al. Global Burden of Cervical Cancer: Current Estimates, Temporal Trend and Future Projections Based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2025; in press. [Google Scholar] [CrossRef]
- Fan, X.; He, W.; Zhang, Q.; Zhang, B.; Dong, L.; Li, L.; Liu, X. Evaluation and Prediction Analysis of 3- and 5-Year Relative Survival Rates of Patients with Cervical Cancer: A Model-Based Period Analysis. Cancer Control 2024, 31, 10732748241232324. [Google Scholar] [CrossRef]
- National Cancer Institute. Cervical Cancer Prognosis and Survival Rates—NCI. Available online: https://www.cancer.gov/types/cervical/survival (accessed on 7 April 2025).
- American Cancer Society. Cervical Cancer Survival Rates|Cancer 5 Year Survival Rates. Available online: https://www.cancer.org/cancer/types/cervical-cancer/detection-diagnosis-staging/survival.html (accessed on 7 April 2025).
- Manso, L.; Ramchandani-Vaswani, A.; Romero, I.; Sánchez-Lorenzo, L.; Bermejo-Pérez, M.J.; Estévez-García, P.; Fariña-Madrid, L.; García García, Y.; Gil-Martin, M.; Quindós, M. SEOM-GEICO Clinical Guidelines on Cervical Cancer (2023). Clin. Transl. Oncol. 2024, 26, 2771–2782. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper, December 2022. Available online: https://www.who.int/publications/i/item/who-wer9750-645-672 (accessed on 9 August 2024).
- Basoya, S.; Anjankar, A. Cervical Cancer: Early Detection and Prevention in Reproductive Age Group. Cureus 2022, 14, e31312. [Google Scholar] [CrossRef]
- Pathak, P.; Pajai, S.; Kesharwani, H. A Review on the Use of the HPV Vaccine in the Prevention of Cervical Cancer. Cureus 2022, 14, 28710. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global Estimates of Incidence and Mortality of Cervical Cancer in 2020: A Baseline Analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W. Contribution of Vaccination to Improved Survivl and Health: Modelling 50 Years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.Y.; Arshad, F.; Areia, C.; Alshammari, T.M.; Alghoul, H.; Casajust, P. Current Approaches to Vaccine Safety Using Observational Data: A Rationale for the EUMAEUS (Evaluating Use of Methods for Adverse Events Under Surveillance-for Vaccines) Study Design. Front. Pharmacol. 2022, 13, 837632. [Google Scholar] [CrossRef]
- Soper, D. Reducing the Health Burden of HPV Infection through Vaccination. Infect. Dis. Obstet. Gynecol. 2006, 2006, 83084. [Google Scholar] [CrossRef]
- Mahajan, I.; Kadam, A.; McCann, L.; Ghose, A.; Wakeham, K.; Dhillon, N.S.; Stanway, S.; Boussios, S.; Banerjee, S.; Priyadarshini, A. Early Adoption of Innovation in HPV Prevention Strategies: Closing the Gap in Cervical Cancer. ecancermedicalscience 2024, 18, 1762. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; MacLure, K. A Systematic Review of Human Papillomavirus Vaccination Challenges and Strategies to Enhance Uptake. Vaccines 2024, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.G.; Barrera, M.; Holleran Steiker, L.K. Issues and Challenges in the Design of Culturally Adapted Evidence-Based Interventions. Annu. Rev. Clin. Psychol. 2010, 6, 213–239. [Google Scholar] [CrossRef]
- Barbieri, V.; Wiedermann, C.J.; Lombardo, S.; Piccoliori, G.; Gärtner, T.; Engl, A. Vaccine Hesitancy and Public Mistrust During Pandemic Decline: Findings from 2021 and 2023 Cross-Sectional Surveys in Northern Italy. Vaccines 2024, 12, 176. [Google Scholar] [CrossRef]
- World Health Organization. Immunization Coverage. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 7 April 2025).
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 4 August 2022).
- Kane, M.A.; Serrano, B.; De Sanjosé, S.; Wittet, S. Implementation of Human Papillomavirus Immunization in the Developing World. Vaccine 2012, 30, F192–F200. [Google Scholar] [CrossRef]
- Alodhialah, A.M.; Almutairi, A.A.; Almutairi, M. Assessing Barriers to Cancer Screening and Early Detection in Older Adults in Saudi Arabia: A Mixed-Methods Approach to Oncology Nursing Practice Implications. Curr. Oncol. 2024, 31, 7872–7889. [Google Scholar] [CrossRef]
- McQuaid, E.L.; Landier, W. Cultural Issues in Medication Adherence: Disparities and Directions. J. Gen. Intern. Med. 2018, 33, 200–206. [Google Scholar] [CrossRef]
- Santos, A.C.d.; Silva, N.N.T.; da Silva, I.D.C.G.; Carneiro, M.; Coura-Vital, W.; Lima, A.A. Effectiveness of HPV Vaccina-Tion in Reducing Infection among Young Brazilian Women. BMC Infect. Dis. 2025, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Waheed, D.-N.; Burdier, F.R.; Eklund, C.; Baussano, I.; Mariz, F.C.; Téblick, L. An Update on One-Dose HPV Vaccine Studies, Immunobridging and Humoral Immune Responses. A Meeting Report. Prev. Med. Rep. 2023, 35, 102368. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A.P. Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Mendeley. Available online: https://www.mendeley.com/search/ (accessed on 1 December 2024).
- Critical Appraisal Skills Programme, CASP. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 1 December 2024).
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Fokom Domgue, J.; Dille, I.; Kapambwe, S.; Yu, R.; Gnangnon, F.; Chinula, L. HPV Vaccination in Africa in the COVID-19 Era: A Cross-Sectional Survey of Healthcare Providers’ Knowledge, Training, and Recommendation Practices. Front. Public Health 2024, 12, 1343064. [Google Scholar] [CrossRef]
- Zhang, C.; Greengold, J.; Tackett, S.; Lentz, C.; Bennett, W.; McGuire, M. Evaluation of Online Educational Curriculum on HPV Vaccination Practices among Adult Primary Care Providers. BMC Med. Educ. 2023, 23, 923. [Google Scholar] [CrossRef] [PubMed]
- Sullivan-Blum, Z.C.; Brophy, M.; Didde, R.; Nagireddy, R.; Swagerty, H.; Weir, S. PrEP Patient Attitudes, Beliefs and perceived Barriers Surrounding HPV Vaccination: A Qualitative Study of Semistructured Interviews with PrEP Patients in Primary Care Clinics in Kansas and Missouri. BMJ Open 2022, 12, 058510. [Google Scholar] [CrossRef]
- Hecht, M.L.; BeLue, R.; Ray, A.; Hopfer, S.; Miller-Day, M.; Mckee, F. HPV Vaccine Intent among Adult Women Receiving Care at Community Health Centers. J. Cancer Educ. 2022, 37, 1186–1193. [Google Scholar] [CrossRef]
- Brandt, H.M.; Vanderpool, R.C.; Curry, S.J.; Farris, P.; Daniel-Ulloa, J.; Seegmiller, L. A Multi-Site Case Study of Community-Clinical Linkages for Promoting HPV Vaccination. Hum. Vaccines Immunother. 2019, 15, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.L.; Vamos, C.A.; Sappenfield, W.M.; Straub, D.M.; Daley, E.M. Relationship Status Impacts Primary Reasons for Interest in the HPV Vaccine among Young Adult Women. Vaccine 2016, 34, 3119–3124. [Google Scholar] [CrossRef]
- Crann, S.E.; Barata, P.C.; Mitchell, R.; Mawhinney, L.; Thistle, P.; Chirenje, Z.M.; Stewart, D.E. Healthcare Providers’ Perspec-Tives on the Acceptability and Uptake of HPV Vaccines in Zimbabwe. J. Psychosom. Obstet. Gynecol. 2016, 37, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Canfell, K.; Egger, S.; Velentzis, L.S.; Brown, J.D.; O’Connell, D.L.; Banks, E.; Sitas, F. Factors Related to Vaccine Uptake by Young Adult Women in the Catch-up Phase of the National HPV Vaccination Program in Australia: Results from an Observa-Tional Study. Vaccine 2015, 33, 2387–2394. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Phan, T.N.T.; Pham, T.T.; Le, T.T.; Le, H.M.; Nguyen, D.T. Urban-Rural Disparities in Acceptance of Human Papillomavirus Vaccination among Women in Can Tho, Vietnam. Ann. Ig. 2023, 35, 641–659. [Google Scholar] [CrossRef]
- Raviglione, M.C.B.; Tediosi, F.; Villa, S.; Casamitjana, N.; Plasència, A. Global Health Essentials, 1st ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Karanja-Chege, C.M. HPV Vaccination in Kenya: The Challenges Faced and Strategies to Increase Uptake. Front. Public Health 2022, 10, 802947. [Google Scholar] [CrossRef]
- Ewongwo, A.; Sahor, A.F.; Ngwa, W.; Nwachukwu, C. A Guide to Global Access to HPV Vaccination to All Women in Low- and Middle-Income Countries; a Minireview of Innovation and Equity. Front. Oncol. 2024, 14, 1380663. [Google Scholar] [CrossRef]
- Ver, A.T.; Notarte, K.I.; Velasco, J.V.; Buac, K.M.; Nazareno, J.; Lozañes, J.A. A Systematic Review of the Barriers to Implementing Human Papillomavirus Vaccination Programs in Low- and Middle-Income Countries in the Asia-Pacific. Asia Pac. J. Clin. Oncol. 2021, 17, 530–545. [Google Scholar] [CrossRef]
- Nogueira-Rodrigues, A.; Flores, M.G.; Macedo Neto, A.O.; Braga, L.A.C.; Vieira, C.M.; Sousa-Lima, R.M. HPV Vaccination in Latin America: Coverage Status, Implementation Challenges and Strategies to Overcome It. Front. Oncol. 2022, 12, 984449. [Google Scholar] [CrossRef]
- MacDonald, S.E.; Kenzie, L.; Letendre, A.; Bill, L.; Shea-Budgell, M.; Henderson, R. Barriers and Supports for Uptake of Human Papillomavirus Vaccination in Indigenous People Globally: A Systematic Review. PLOS Glob. Public Health 2023, 3, 0001406. [Google Scholar] [CrossRef]
- Islam, J.Y.; Gurbani, A.; Ramos, S.; Morgan, K.; Kim, C.J.; Richter, K.L. Health Care Provider Perceptions of Facilitators and Barriers to Human Papillomavirus Vaccination Delivery in Five Countries. Sex. Transm. Dis. 2021, 48, 557–564. [Google Scholar] [CrossRef]
- Amponsah-Dacosta, E.; Kagina, B.M.; Olivier, J. Health Systems Constraints and Facilitators of Human Papillomavirus Immunization Programmes in Sub-Saharan Africa: A Systematic Review. Health Policy Plan. 2020, 35, 701–717. [Google Scholar] [CrossRef]
- Kutz, J.M.; Rausche, P.; Gheit, T.; Puradiredja, D.I.; Fusco, D. Barriers and Facilitators of HPV Vaccination in Sub-Saharan Africa: A Systematic Review. BMC Public Health 2023, 23, 974. [Google Scholar] [CrossRef]
- Waheed, D.E.; Bolio, A.; Guillaume, D.; Sidibe, A.; Morgan, C.; Karafillakis, E. Planning, Implementation, and Sustaining High Coverage of Human Papillomavirus (HPV) Vaccination Programs: What Works in the Context of Low-Resource Countries? Front. Public Health 2023, 11, 1112981. [Google Scholar] [CrossRef]
- Hakimi, S.; Lami, F.; Allahqoli, L.; Alkatout, I. Barriers to the HPV Vaccination Program in the Eastern Mediterranean Region: A Narrative Review. J. Turk. Ger. Gynecol. Assoc. 2023, 24, 48–56. [Google Scholar] [CrossRef]
- Xu, M.A.; Choi, J.; Capasso, A.; DiClemente, R.J. Improving HPV Vaccination Uptake Among Adolescents in Low Resource Settings: Sociocultural and Socioeconomic Barriers and Facilitators. Adolesc. Health Med. Ther. 2024, 15, 73–82. [Google Scholar] [CrossRef]
- Bogdanova, A.; Andrawos, C.; Constantinou, C. Cervical Cancer, Geographical Inequalities, Prevention and Barriers in Resource Depleted Countries. Oncol. Lett. 2022, 23, 113. [Google Scholar] [CrossRef]
- Peterson, C.E.; Silva, A.; Holt, H.K.; Balanean, A.; Goben, A.H.; Dykens, J.A. Barriers and Facilitators to HPV Vaccine Uptake among US Rural Populations: A Scoping Review. Cancer Causes Control 2020, 31, 801–814. [Google Scholar] [CrossRef]
- Branda, F.; Pavia, G.; Ciccozzi, A.; Quirino, A.; Marascio, N.; Gigliotti, S. Human Papillomavirus (HPV) Vaccination: Progress, Challenges, and Future Directions in Global Immunization Strategies. Vaccines 2024, 12, 1293. [Google Scholar] [CrossRef]
- Giannone, G.; Giuliano, A.R.; Bandini, M.; Marandino, L.; Raggi, D.; Earle, W. HPV Vaccination and HPV-Related Malignancies: Impact, Strategies and Optimizations toward Global Immunization Coverage. Cancer Treat. Rev. 2022, 111, 102467. [Google Scholar] [CrossRef]
- Tsu, V.D.; LaMontagne, D.S.; Atuhebwe, P.; Bloem, P.N.; Ndiaye, C. National Implementation of HPV Vaccination Programs in Low-Resource Countries: Lessons, Challenges, and Future Prospects. Prev. Med. 2021, 144, 106335. [Google Scholar] [CrossRef]
- Guillaume, D.; Waheed, D.E.; Schleiff, M.; Muralidharan, K.K.; Vorsters, A.; Limaye, R.J. Global Perspectives of Determinants Influencing HPV Vaccine Introduction and Scale-up in Low- and Middle-Income Countries. PLoS ONE 2024, 19, 0291990. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Wang, Q.; Lai, Y.; Holloway, A. The Status and Challenges of HPV Vaccine Programme in China: An Exploration of the Related Policy Obstacles. BMJ Glob. Health 2023, 8, 012554. [Google Scholar] [CrossRef]
- Aggarwal, S.; Agarwal, P.; Gupta, N. A Comprehensive Narrative Review of Challenges and Facilitators in the Implementation of Various HPV Vaccination Program Worldwide. Cancer Med. 2024, 13, 6862. [Google Scholar] [CrossRef]
- Essa-Hadad, J.; Gorelik, Y.; Vervoort, J.; Jansen, D.; Edelstein, M. Understanding the Health System Barriers and Enablers to Childhood MMR and HPV Vaccination among Disadvantaged, Minority or Underserved Populations in Middle- and High-Income Countries: A Systematic Review. Eur. J. Public Health 2024, 34, 368–374. [Google Scholar] [CrossRef]
- Grabert, B.K.; Heisler-MacKinnon, J.; Liu, A.; Margolis, M.A.; Cox, E.D.; Gilkey, M.B. Prioritizing and Implementing HPV Vaccination Quality Improvement Programs in Healthcare Systems: The Perspective of Quality Improvement Leaders. Hum. Vaccines Immunother. 2021, 17, 3577–3586. [Google Scholar] [CrossRef]
- Guillaume, D.; Meyer, D.; Waheed, D.E.; Schlieff, M.; Muralidharan, K.; Chou, V.B.; Limaye, R. Factors influencing the prioritization of vaccines by policymakers in low- and middle-income countries: A scoping review. Health Policy Plan. 2023, 38, 363–376. [Google Scholar] [CrossRef]
- Marques, M.D.; Sheel, M. Addressing the social inequities of vaccination: An imperative to close the gap. Lancet Glob. Health 2023, 11, e173–e174. [Google Scholar] [CrossRef]
- Ekezie, W.; Awwad, S.; Krauchenberg, A.; Karara, N.; Dembiński, Ł.; Grossman, Z.; del Torso, S.; Dornbusch, H.J.; Neves, A.; Copley, S.; et al. For The ImmuHubs Consortium. Access to Vaccination among Disadvantaged, Isolated and Difficult-to-Reach Communities in the WHO European Region: A Systematic Review. Vaccines 2022, 10, 1038. [Google Scholar] [CrossRef]
- Prem, K.; Cernuschi, T.; Malvolti, S.; Brisson, M.; Jit, M. Optimal Human Papillomavirus Vaccination Strategies in the Context of Vaccine Supply Constraints in 100 Countries. eClinicalMedicine 2024, 74, 102735. [Google Scholar] [CrossRef]
- Oberlin, A.M.; Rahangdale, L.; Chinula, L.; Fuseini, N.M.; Chibwesha, C.J. Making HPV Vaccination Available to Girls Everywhere. Int. J. Gynaecol. Obstet. 2018, 143, 267–276. [Google Scholar] [CrossRef]
- Sguanci, M.; Mancin, S.; Gazzelloni, A.; Diamanti, O.; Ferrara, G.; Morales Palomares, S.; Parozzi, M.; Petrelli, F.; Cangelosi, G. The Internet of Things in the Nutritional Management of Patients with Chronic Neurological Cognitive Impairment: A Scop-Ing Review. Healthcare 2024, 13, 23. [Google Scholar] [CrossRef]
- Terzoni, S.; Ferrara, P.; Parozzi, M.; Colombani, F.; Mora, C.; Cilluffo, S.; Jeannette, V.G.; Destrebecq, A.; Pinna, B.; Lusignani, M.; et al. Nurses’ role in the management of persons with chronic urogenital pelvic pain syndromes: A scoping review. Neurourol. Urodyn. 2023, 42, 13–22. [Google Scholar] [CrossRef]
- Gazineo, D.; Parozzi, M.; Ricco, M.; Savini, S.; Ferrara, G.; Anastasi, G.; Cangelosi, G.; Godino, L.; Andreoli, D. Relational skills of nephrology and dialysis nurses in clinical care settings: A scoping review and stakeholder consultation. Nurse Educ. Pract. 2025, 82, 104229. [Google Scholar] [CrossRef]
- Mieronkoski, R.; Azimi, I.; Rahmani, A.M.; Aantaa, R.; Terävä, V.; Liljeberg, P.; Salanterä, S. The Internet of Things for Basic Nursing Care—A Scoping Review. Int. J. Nurs. Stud. 2017, 69, 78–90. [Google Scholar] [CrossRef]
- Scuri, S.; Tesauro, M.; Petrelli, F.; Argento, N.; Damasco, G.; Cangelosi, G.; Nguyen, C.T.T.; Savva, D.; Grappasonni, I. Use of an Online Platform to Evaluate the Impact of Social Distancing Measures on Psycho-Physical Well-Being in the COVID-19 Era. Int. J. Environ. Res. Public Health 2022, 19, 6805. [Google Scholar] [CrossRef]
- Lee, G.A.; Durante, A.; Baker, E.E.; Vellone, E.; Caggianelli, G.; Dellafiore, F.; Khan, M.; Khatib, R. Patients’ perceptions on the facilitators and barriers using injectable therapies in dyslipidaemia: An empirical qualitative descriptive international study. J Adv. Nurs. 2023, 79, 4687–4696. [Google Scholar] [CrossRef]
- Prado, L.; Allemann, S.; Viprey, M.; Schott, A.M.; Dediu, D.; Dima, A.L. Toward an Interdisciplinary Approach to Constructing Care Delivery Pathways from Electronic Health Care Databases to Support Integrated Care in Chronic Conditions. Systematic Review of Quantification and Visualization Methods. J. Med. Internet Res. 2023, 25, 49996. [Google Scholar] [CrossRef]
- Cangelosi, G.; Mancin, S.; Morales Palomares, S.; Pantanetti, P.; Quinzi, E.; Debernardi, G.; Petrelli, F. Impact of School Nurse on Managing Pediatric Type 1 Diabetes with Technological Devices Support: A Systematic Review. Diseases 2024, 12, 173. [Google Scholar] [CrossRef]
- Gawande, M.S.; Zade, N.; Kumar, P.; Gundewar, S.; Weerarathna, I.N.; Verma, P. The Role of Artificial Intelligence in Pandemic Responses: From Epidemiological Modeling to Vaccine Development. Mol. Biomed. 2025, 6, 1. [Google Scholar] [CrossRef] [PubMed]
| First Author/Year/Location | Type of Study | Population | Main Intervention | Main Limitations | Main Results |
|---|---|---|---|---|---|
| Fokom Domgue et al. [35], 2024, Africa | Observational, cross-sectional | 153 healthcare workers | Online survey on training, knowledge, and willingness to recommend the HPV vaccine | Representativeness bias: The study is limited to urban settings; there is limited accessibility to the vaccine. | Only 37.4% had the vaccine available in their facility; 56.2% had not received specific training; 83.2% recommended the vaccine, mainly for girls; and lack of training (28.6%) was a key reason for not recommending the HPV vaccine. |
| Zhang et al. [36], 2023, America | Observational | 223 primary care physicians | Online educational course to improve HPV vaccination practices | Data incompleteness: lack of demographic data for early participants; low response rate for the post-intervention test. | Significant increase in knowledge and confidence scores in HPV vaccination, maintained over time. |
| Sullivan-Blum et al. [37], 2022, America | Observational, qualitative | 16 PrEP patients | Semi-structured interviews to explore attitudes, beliefs, and perceived barriers | Small sample: only 16 interviews; lack of formal recording of interviews. | High vaccine acceptance if recommended by the physician and covered by insurance, with significant gaps in knowledge about the effects of HPV on men. |
| Hecht et al. [38], 2022, America | RCT | EG (n = 136) CG (n = 81) | Program based on personalized narratives to promote HPV vaccination | Lack of data on actual vaccination; limited generalizability due to the specific study population with Planned Parenthood clients. | Significant increase in vaccination intentions and self-efficacy, with 41% in the EG extremely confident of completing the vaccination cycle. |
| Brandt et al. [39], 2019, America | Observational, qualitative, multicenter | Clinical and community entities | Examination of community–clinic collaborations to promote HPV vaccination | Selection bias: study limited to known collaborations; methodological limitations in qualitative data collection techniques. | Improvement in vaccination rates through community–clinic coordinated interventions. Main barriers included poor knowledge of HPV and administrative challenges. HPV vaccination underfunded or poorly documented in medical records. |
| Thomson et al. [40], 2016, America | Observational | 1457 young unvaccinated women | Data analysis from the National Health Interview Survey 2010 | Recall bias due to the use of self-reported data; high non-response rate. | Only 31.4% of women expressed interest in the vaccine. The main reasons for non-vaccination varied significantly based on relationship status. |
| Crann et al. [41], 2016, Africa | Observational, qualitative | 15 healthcare providers | Interviews to assess knowledge and opinions on HPV and HPV vaccines | Limited initial knowledge of HPV and vaccines; non-representative sample due to convenience sampling. | Strong support for the implementation of vaccination programs despite identified barriers. Supportive, but barriers included cost, schedule, and healthcare infrastructure. |
| Canfell et al. [42], 2015, Oceania | Observational | 1139 young adult women | Self-administered questionnaire on vaccination status, sociodemographic information, and behavioral characteristics | Data reliability is compromised by self-reporting; potential overestimation of vaccine uptake due to opportunistic vaccination. | A total of 77% received at least one dose of the vaccine. Factors such as being born in Australia, being single, and living in areas with high socioeconomic status were associated with a higher vaccination rate. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cangelosi, G.; Sacchini, F.; Mancin, S.; Petrelli, F.; Amendola, A.; Fappani, C.; Sguanci, M.; Morales Palomares, S.; Gravante, F.; Caggianelli, G. Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review. Vaccines 2025, 13, 460. https://doi.org/10.3390/vaccines13050460
Cangelosi G, Sacchini F, Mancin S, Petrelli F, Amendola A, Fappani C, Sguanci M, Morales Palomares S, Gravante F, Caggianelli G. Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review. Vaccines. 2025; 13(5):460. https://doi.org/10.3390/vaccines13050460
Chicago/Turabian StyleCangelosi, Giovanni, Francesco Sacchini, Stefano Mancin, Fabio Petrelli, Antonella Amendola, Clara Fappani, Marco Sguanci, Sara Morales Palomares, Francesco Gravante, and Gabriele Caggianelli. 2025. "Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review" Vaccines 13, no. 5: 460. https://doi.org/10.3390/vaccines13050460
APA StyleCangelosi, G., Sacchini, F., Mancin, S., Petrelli, F., Amendola, A., Fappani, C., Sguanci, M., Morales Palomares, S., Gravante, F., & Caggianelli, G. (2025). Papillomavirus Vaccination Programs and Knowledge Gaps as Barriers to Implementation: A Systematic Review. Vaccines, 13(5), 460. https://doi.org/10.3390/vaccines13050460







