Abstract
Background/Objectives: Helminth infections, particularly prevalent in low- and middle-income countries, have been extensively studied for their effects on human health. With the emergence of new infectious diseases like SARS-CoV-2 and Ebola, their impact on disease outcomes become more apparent. While individual studies have explored the impact of helminth co-infections on disease severity and vaccine efficacy, the findings are often inconsistent and context-dependent. Furthermore, the long-term effects of helminth-mediated immunosuppression on vaccine efficacy and its broader implications for co-infections in endemic regions remain not fully understood. Methods: This systematic review conducted in line with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines synthesizes the current evidence, identifies patterns, and highlights areas needing further research, offering a cohesive understanding of the topic. PubMed, Scopus, Google Scholar, and Cochrane Library were searched to include studies published from 2003 to February 2025. Results: Co-infection reveals a dual role of helminths in modulating immune responses, with both beneficial and detrimental interactions reported across studies. It may confer benefits against respiratory viral infections by muting hyper-inflammation associated with the severity of conditions like COVID-19, Influenza, and RSV. However, they can exacerbate disease outcomes in most bacteria and blood-borne viral conditions by impairing immune functions, such as neutrophil recruitment and antibody response, leading to more severe infections and higher viral loads. The stage of helminth infection also appears critical, with early-stage infections sometimes offering protection, while late-stage infections may worsen disease outcomes. Helminth infection can also negatively impact vaccine efficacy by suppressing B cell activity, reducing antibody levels, and decreasing vaccine effectiveness against infectious diseases. This immunosuppressive effect may persist after deworming, complicating efforts to restore vaccine efficacy. Maternal helminth infections also significantly influence neonatal immunity, affecting newborn vaccine responses. Conclusions: There is a need for targeted interventions and further research in helminth-endemic regions to mitigate the adverse effects on vaccine efficacy and improve public health outcomes.
1. Introduction
Parasitic worms, collectively known as helminths, have shared a complex and enduring relationship with humans that stretches back millennia [1,2]. Fossilized evidence, such as a Schistosoma mansoni egg discovered in the remains of a 6200-year-old human skeleton, underscores this long-standing coexistence [3]. These parasites, classified into three major groups—nematodes, trematodes, and cestodes—have co-evolved with their hosts, driving an evolutionary arms race that has profoundly shaped both their survival strategies and the human immune response [4]. Today, helminths remain a significant public health concern, particularly in sub-Saharan Africa [5], where they infect an estimated 1.5 billion people and contribute to 85% of the global burden of neglected tropical diseases (NTDs) [6]. By inducing malnutrition, anemia, and chronic illnesses, they exacerbate poverty cycles and strain health systems [7]. Beyond their direct health impacts, they are renowned for their ability to manipulate the host immune system with profound implications on vaccine efficacy and successes in endemic regions. Their skewed immune responses toward anti-inflammatory Th2 and regulatory pathways are known to suppress pro-inflammatory Th1 and Th17 responses [8,9,10,11]. This immunomodulation allows them to establish chronic infections and influences the outcomes of co-infections [12].
Studies examining the impact of helminth infections on viral diseases in endemic areas where coinfections are particularly common have yielded varied results, reflecting the complexity of these interactions [13]. They significantly alter immune responses, potentially benefiting or hindering the host’s ability to combat bacterial and viral pathogens [14,15]. They have been shown to suppress hyperinflammatory responses, mitigating the severity of respiratory virus infections including SARS-CoV-2, Influenza, and RSV (Respiratory Syncytial Virus). We have previously demonstrated that helminths’ seropositivity correlates inversely with Th1 and Th17 cytokines and severe COVID-19 [16]. However, the opposite side has potential disadvantages, as helminth infections were associated with diminished antibody response, reduced vaccine efficacy, increased susceptibility to concurrent infections, and potential interference with tumor immunosurveillance [17,18,19,20,21,22,23,24,25,26,27,28]. While deworming has clear benefits for reducing helminth-associated morbidity, its modulatory effect is resilient and persists in the host even after deworming, posing a new challenge of vaccination timing post-deworming [13]. This dynamic relationship has not only influenced the human immune landscape and susceptibility to infectious diseases but also non-communicable diseases (NCDs), including type 2 diabetes, allergic diseases, and autoimmune infections. Sub-Saharan Africa, where helminth infections are widespread, has reported surprisingly lower incidence and less severe COVID-19 outcomes, raising questions about the role of helminths in modulating immune responses to the virus. Although this might be associated with the lower testing capacity or the younger demography of the continent, recent studies showed a negative association between Ascaris lumbricoides seropositivity and COVID-19 severity in endemic countries [29]. Moreover, research has demonstrated that helminth antigens from Ascaris lumbricoides, Onchocerca volvulus, and Brugia malayi differentially modulate the activation of the CD4+ and CD8+ T lymphocytes of convalescent COVID-19 patients in vitro, highlighting their potential to influence viral immunity [30]. Despite these findings, the broader implications of helminth co-infections on viral immunity and vaccine efficacy, including COVID-19, remain poorly understood [31]
Building on this foundation, this present review aims to make a synthesis of recent research on helminth coinfections and their impact on disease outcomes and vaccine efficacy, thereby facilitating a thorough comprehension of how these interactions may affect health outcomes and shape the immune response. This exploration not only sheds light on the intricate dynamics of helminth–host–pathogen interactions but also offers a promising avenue for rethinking approaches to disease prevention and treatment in areas where these parasites are endemic.
2. Materials and Methods
A comprehensive literature review was conducted with adherence to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines (Table S1) to summarize the findings on the influence of helminths on communicable and NCD outcomes as well as vaccine efficacy.
2.1. Protocol Registration
The protocol for conducting this systematic review was registered and approved at the International Prospective Register of Systematic Reviews, PROSPERO registration number CRD42024556697.
2.2. Searches
The search encompassed multiple electronic bibliographic databases and aggregators, including Scopus, PubMed, Google Scholar, and Cochrane Library. Additionally, the reference list of included studies was screened for relevant articles. The search strategy was structured around key concepts essential to the review: helminth co-infection, disease dynamics, vaccination success, and infectious and non-infectious diseases. Each key term was defined by a range of synonyms and adapted for suitability across different databases. Boolean operators such as “AND” and “OR” were used to optimize the search to find articles relevant to the search question. The timeframe from 2003 to February 2025 was selected to capture recent advancements in understanding helminth co-infection. The search terms targeted studies reporting the interaction between helminths and other disease conditions and vaccine efficacy. The search did not impose any language restrictions.
2.3. Study Selection Procedures
The inclusion and exclusion criteria were established using the participants, intervention/exposure, comparator, and outcome (PICO) framework, as outlined below.
2.3.1. Participants/Population
This review included studies conducted at the population or cohort level, regardless of the administrative scale (district, regional, or national) on individuals with bacterial infections, viral infections, NCD, or vaccinated individuals.
2.3.2. Intervention(s)/Exposure
The primary exposure of interest was the co-occurrence of helminth infections with viral or bacterial infections or non-communicable diseases (NCDs), or vaccine administration.
2.3.3. Comparator(s)/Control
The comparator group consisted of individuals with mono-exposure (without helminth co-infection) to viral or bacterial infections or non-communicable diseases (NCDs) or vaccines.
2.3.4. Main Outcome
The primary objectives of this review focused on clinical effects (disease severity), immunological effects (cytokine expression, T cell activation, antibody production), and vaccine efficacy in coinfection. A narrative summary of the key findings from the selected articles is provided, highlighting reported disease outcomes and immune parameters.
2.4. Eligibility Criteria
- Studies reporting on immune responses and/or disease outcomes in individuals with helminth co-infections.
- Population-level, cohort-based, or facility-based studies conducted at any administrative level (district, regional, or national).
- Studies including a comparator group assessing immune responses and/or disease outcomes in participants without helminth exposure or infection.
2.5. Study Inclusion/Exclusion
Two independent investigators applied eligibility criteria to select studies for inclusion in this review. Disagreements were resolved through discussion, with a third reviewer consulted to reach consensus when necessary. Studies were included if they provided the following: data on immune responses and/or disease outcomes in scenarios involving helminths with viral or bacterial infections, vaccine efficacy, or non-communicable diseases (NCDs) under mono-infection and co-infection conditions; a documented methodology for evaluating immune responses and/or disease severity; a defined study population size; and observed outcomes related to disease severity and/or immune response. Studies were excluded if helminth infection status was not assessed or was only evaluated post-vaccination, or if a comparative control group was not included.
2.6. Data Extraction
The following data were extracted: author(s), publication year, immune response, clinical outcome, and study type (human, animal model); type of population (children, adults, pregnant women); and type of coinfection (virus, bacteria, NCD). Mendeley Desktop Version 1.19.8 was used to identify duplicate records.
2.7. Measures of Effect
This review’s primary objective was to estimate the immune response and/or disease outcome (severity) as reported in primary studies. This review encompassed both observational and intervention studies.
2.8. Article Screening
The review process began with the identification and removal of duplicates. Titles and abstracts of the retrieved articles were then screened to determine eligibility for inclusion in human and animal studies. In the third stage, full texts of articles identified as relevant during the second stage were thoroughly reviewed. At each stage, two reviewers independently assessed the articles, with a third reviewer resolving any disagreements through discussion to reach a consensus. Studies were included if they compared immune responses, disease outcomes, or vaccine efficacy between helminth-infected and uninfected groups, or between groups treated and untreated with anthelmintics. Additionally, the helminth status of participants had to be laboratory-confirmed prior to vaccination, with the subsequent measurement of immunological outcomes.
2.9. Data Analysis and Quality Assessment
Immune responses and disease outcomes were organized in a tabular format and analyzed narratively, with the findings synthesized into thematic narratives. Data from relevant articles were extracted from texts, tables, and figures into a custom Microsoft Excel tool. Extracted data included study type and participant characteristics; details on viral infections, bacterial infections, and non-communicable diseases; vaccines; helminth species or anthelmintic treatments; and clinical or immunological outcomes. The quality of the studies was assessed using the Effective Public Health Practice Project (EPHPP) tool, which rated studies as strong, moderate, or weak. This assessment was based on an eight-component checklist: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, and analyses. Two independent reviewers used this tool to assess the quality of each study and any disagreement was settled by a third reviewer.
3. Results
The eligibility and screening process for the articles included in this review is comprehensively detailed in Figure 1. This figure not only presents the inclusion and exclusion criteria but also provides an explanation of the reasons for excluding specific studies.
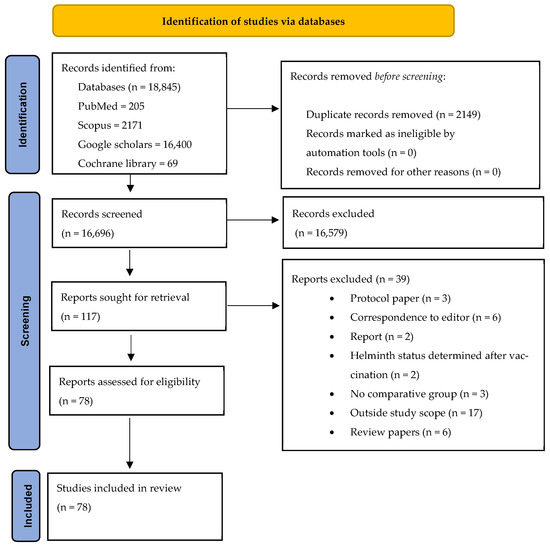
Figure 1.
PRISMA flowchart.
A total of 78 published articles were selected for inclusion in the review. Of these, 32/78 of the studies involved animal models, whereas 46/78 studies involved human subjects (Figure 2). Within the subset of human studies, 5/46 investigated the impact of maternal helminth infection on the immune responses of offspring. The remaining 41/46 studies assessed the effects of direct helminth exposure on other infections and vaccine outcomes. A total of 59 of all included 78 studies investigated the effects of helminths on infectious and non-infectious disease outcomes, with these studies being further categorized based on the type of co-infection: 29/59 on viral co-infections, 10/59 on bacterial co-infections, and 20/59 on non-communicable disease co-infections (Figure 2). Furthermore, 19/78 of the included studies examined the influence of helminths on vaccine efficacy. This group consisted of ten (10) human studies and nine (9) animal studies. The studies collectively reported on vaccine efficacy against several diseases, including COVID-19, Ebola, tuberculosis, Influenza, tetanus, typhoid fever, malaria, Human Papillomavirus infection (HPV), yellow fever, measles, diphtheria, and HBV infection (Figure 2).
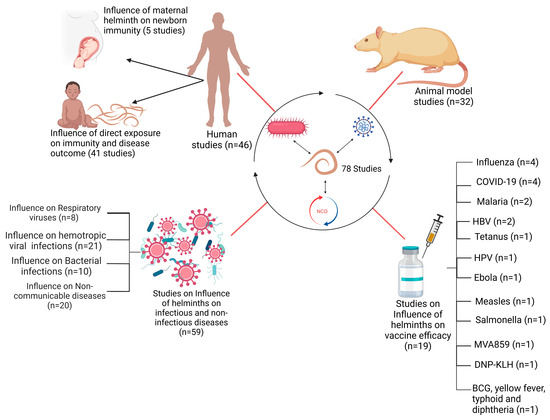
Figure 2.
Schematic representation of studies included in this review.
3.1. Helminth and Emerging Respiratory Viruses
Several reviewed articles assessed the interaction between helminths and emerging viral disease infections. Most studies focused on the interaction between helminths and SARS-CoV-2, followed by Respiratory Syncytial Virus (RSV) and Influenza virus. The interaction between helminth infections and respiratory viruses reveals a complex but potentially beneficial relationship in terms of viral disease outcomes (Table 1). Most reviewed studies suggested a potential modulatory interaction between helminths and respiratory viruses, leading to less severe respiratory disease outcomes in individuals who were co-infected [29]. Studies indicate that patients with parasitic helminths and protozoans like Entamoeba spp., Hymenolepis nana, Schistosoma mansoni, Trichuris trichiura, and Ascaris lumbricoides are less likely to experience severe COVID-19 [29]. Most viral infections, including SARS-CoV-2 and MERS-CoV, have been associated with hyperinflammation and the down-regulation of Th2 cytokines, leading to severe disease outcomes and high fatality rates [32,33]. The modulatory effect of helminths appears to be mediated by helminth antigens, which dampen the immune response by reducing pro-inflammatory response while increasing the anti-inflammatory response. Higher levels of Ascaris antibodies were associated with asymptomatic SARS-CoV-2 infection and reduced risk of severe disease, likely due to lower systemic inflammation [29]. In the case of Influenza, most animal model studies seem to converge that Heligmosomoides polygyrus bakeri (Hpb) infection in mice leads to better survival and less weight loss, attributed to enhanced lung immune responses, lower viral loads, and reduced inflammation [34]. However, the outcome of the interaction between filarial infections and Influenza is stage-dependent; early infections lead to less severe symptoms, while late-stage infections worsen disease outcomes [35]. Like COVID-19, H. polygyrus co-infection with RSV (Respiratory Syncytial Virus) results in less severe disease and lung inflammation in mice, driven by an increase in monocytes in the blood and lungs through type 1 interferon signaling, with severity mitigation requiring normal microbiota but not a Th2 response [36,37]. Reduced severity of respiratory viruses seems to be primarily associated with a skewed Th2 response associated with chronic helminth infection. In addition to the skewed helminth Th2 response, helminth-derived products such as cystatins, omega-1 glycoprotein, and ES-62 are potent immune modulators and can potentially affect host immune responses and viral disease outcomes [38]. These products, largely produced by many helminths, including filarial nematodes, can interfere with NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) pathway activation, inhibiting proinflammatory cytokines (Figure 3). NF-κB and IRF3 (Interferon Regulatory Factor 3) are key players in the innate immune response, particularly in response to viral infections, where they promote the Th1 response. NF-κB activation is stimulated by proinflammatory cytokines like TNF-α and IL-1, which bind to cell receptors and initiate a signaling cascade, leading to IκB (Inhibitor of Nuclear Factor kappa B) degradation and NF-κB activation [39]. Helminths, however, can suppress or alternatively activate this signaling pathway by secreting immunomodulatory molecules that prevent IκB degradation and block NF-κB nuclear translocation or promote the transcription of Th2 molecules, thereby reducing inflammation. Inhibition of Toll-like receptor (TLR) signaling by helminths can also lead to the same effect [38,39]. Helminth-derived products containing glycoprotein also have the potential to interfere with TLR pathways. ES-62 modulates TLR pathways, particularly TLR4, to disrupt downstream NF-κB activation by interfering with kinases and transcription factors [40]. This molecule secreted by Fasciola hepatica inhibits TLR3 and TLR4, affecting TRIF (TIR domain-containing adapter-inducing interferon-β) and MyD88 (Myeloid Differentiation Primary Response 88)-signaling pathways. These strategies reduce NF-κB activation and cytokine production, promoting an anti-inflammatory environment [41,42]. ES-62 can also induce a semi-mature phenotype in dendritic cells (DCs), decreasing the expression of MHC II molecules on their surface. This reduction in MHC II expression promotes the differentiation of regulatory T cells (Tregs), which in turn suppresses Th1 and Th17 cells. Cystatins and glycans, both powerful immune modulators, promote M2 macrophage polarization, which enhances the production of Th2 cytokines that are anti-inflammatory. Glycans can also directly suppress IL-12 production, further supporting an anti-inflammatory response. Additionally, TGF-β mimics and miRNAs act as potent modulators: TGF-β mimics engage TGF-β receptors on Tregs and macrophages, promoting Treg expansion and M2 macrophage polarization, which further drives IL-10 and TGF-β production. These cytokines inhibit neutrophil extracellular traps (NETs), leading to a more anti-inflammatory environment and the suppression of Th1 and Th17 cytokines such as IFN-γ, TNF-α, IL-17, and IL-12. Meanwhile, miRNAs support the proliferation of both Tregs and B regulatory (Breg) cells, inhibit effector T cells (particularly Th1 and Th17), and modulate B cell activation (Figure 3).
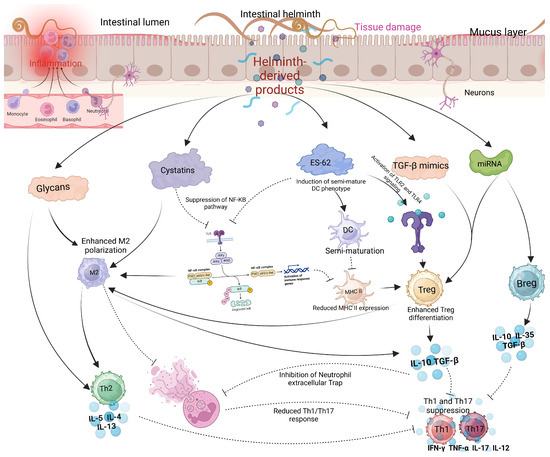
Figure 3.
Modulatory effects of helminth-derived products on immune cells. Helminths produce several immunomodulatory molecules, including glycans, cystatins, ES-62, TGF-β mimics, and miRNAs. Glycans and cystatins promote M2 macrophage polarization, while cystatins and ES-62 suppress the NF-κB pathway, reducing the activity of Th1 and Th17 cells. Additionally, TGF-β mimics and miRNAs enhance the activation of Tregs and Bregs, further inhibiting Th1 and Th17 responses (created using BioRender.com).
Helminth-induced immune modulation may provide benefits in mitigating severe outcomes of respiratory viral infections, but the resulting immune imbalance could have unintended consequences on vaccine efficacy for viral diseases. This raises the need for vaccines to incorporate adjuvants that compensate for helminth-induced immunosuppression, ensuring durable and effective protection in helminth-endemic countries. Helminth-derived molecules such as ES-62 and glycans offer valuable templates for synthetic adjuvant design, as they interfere with innate immune pathways like NF-κB and TLR signaling [43,44]. These mechanisms, while potentially dampening traditional vaccine responses, provide critical insights into developing novel adjuvants that can modulate immune responses in a more balanced manner. Previous studies have shown that rOv-ASP-1, a protein derived from the Onchocerca volvulus parasite, serves as an effective adjuvant, enhancing the efficacy of vaccines composed of proteins or synthetic peptides [45].
Mimicking helminth products like ES-62, which regulate inflammatory pathways, could enable the synthesis of vaccine molecules that enhance antigen-specific responses while simultaneously reducing adverse inflammation. This dual functionality is particularly advantageous in creating vaccines that are both effective and safer, with reduced side effects. Such approaches could include combining helminth-inspired adjuvants with immune stimulants, such as Toll-like receptor (TLR) agonists, to promote Th1 responses while the helminth-derived components mitigate excessive inflammation [46]. Alternatively, derivatives of helminth molecules such as glutathione S-transferase from Fasciola hepatica (nFhGST) could be synthesized to retain their ability to modulate NF-κB or TLR pathways while avoiding complete suppression of immune activation [43]. Structural modifications of helminth-derived molecules could also be employed to selectively retain their anti-inflammatory effects while reducing their capacity to suppress critical immune responses, such as Th1 or Th17 pathways. This strategy would ensure a balanced immune activation, preserving the ability to generate robust vaccine-induced immunity. The goal is to leverage the immunosuppressive capabilities of helminth-derived molecules in a selective manner, minimizing harmful inflammation without compromising the immune responses necessary for protection and vaccine efficacy. Advanced techniques, including molecular docking, bioinformatics, and synthetic biology, can play a pivotal role in achieving this delicate balance, paving the way for innovative and efficient vaccine designs.
The consensus among most studies points to the potential impact of helminth coinfection in altering the course of respiratory viral diseases, including SARS-CoV-2, RSV, and Influenza, through immune modulation and gut microbiome interactions [47], potentially leading to milder disease outcomes. This modulatory effect is likely driven by anti-inflammatory pathways, reduced pro-inflammatory responses, microbiome support, and the interruption of TLR and NF-κB pathways, suggesting a potential role for helminth-induced immunomodulation and a balanced gut microbiome in mitigating severe respiratory outcomes, although the extent of modulation varies with the type of helminth, the stage of helminth infection, and the specific respiratory virus involved. This highlights a complex interplay between helminthic infections and respiratory viruses, which warrants further investigation to fully understand the underlying mechanisms and implications for disease management.

Table 1.
Study characteristics of some included studies on helminth–respiratory virus interaction.
Table 1.
Study characteristics of some included studies on helminth–respiratory virus interaction.
| Study | Type of Study | Year of Publication | Helminth or Anthelmintic Treatment Involved | Respiratory Disease Condition Investigated | Main Findings ↓↑ | Ref. |
|---|---|---|---|---|---|---|
| Helminth Infection and COVID-19 Severity in Ethiopia | Human | 2021 | Helminths and protozoa | COVID-19 | ↓ COVID-19 severity. | [48] |
| Helminth Antigens and SARS-CoV-2 Response | Human (In vitro) | 2022 | Helminth antigens | COVID-19 | ↓ SARS-CoV-2-reactive CD4+ T cells, ↑ IL-10, ↓ IFNγ and TNFα levels. | [30] |
| H. polygyrus and Influenza Virus | Mice | 2023 | H. polygyrus bakeri (Hpb) | Influenza | ↓ weight loss, ↑ immune responses in the lungs, ↓ viral loads, and ↓ inflammation in lungs. | [34] |
| H. polygyrus Infection and RSV | Mice (BALB/c) | 2017 | H. polygyrus | Respiratory Syncytial Virus | ↑ monocytes in the blood and lungs driven by IFN-I signaling, ↓ viral load. | [36] |
| Filarial Infection Stage and Influenza | Mice (BALB/c) | 2022 | Filarial infection | Influenza | early-stage infection reduced symptoms, middle-stage infections offered less protection, and late-stage infections worsened symptoms and ↑ viral loads. | [35] |
| H. polygyrus and RSV | Mice (BALB/c) | 2024 | H. polygyrus | Respiratory Syncytial Virus | ↓ RSV severity, ↓ lung inflammation. | [37] |
| Ascaris Lumbricoides and COVID-19 Severity in Benin | Human | 2023 | Ascaris lumbricoides | COVID-19 | ↓ risk of severe COVID-19, ↓ systemic pro-inflammatory markers. | [29] |
| Helminth and SARS-CoV-2 | Human | 2025 | A. lumbricoides, S. ratti, A. viteae | COVID-19 | ↓ COVID-19 severity, ↓ inflammatory cytokines, ↓ SARS-CoV-2 antibodies | [16] |
This table represents the key findings of some selected studies on the interaction between helminth and respiratory virus infections. It also includes the type of study, year of publication, the main findings, and references. ↑ stands for increase; ↓ stands for decrease.
3.2. Helminths and Blood-Borne Viral Co-Infections
Co-infection of helminths with blood-borne viral infections, including Hepatitis B Virus (HBV), Human Papillomavirus (HPV), herpes simplex virus (HSV), Human T-lymphotropic Virus (HTLV), Vaccinia virus, Puumala hantavirus (PUUV), and Human Immunodeficiency Virus (HIV), have been studied in human and animal models. Contrary to the generally observed beneficial effect of helminths on respiratory virus infection outcome, their interaction with blood-borne viruses often reveals a detrimental relationship, leading to worsened clinical presentations, including increased viral loads, enlarged spleens, and more severe illness [49,50,51,52]. This exacerbation is often due to the helminths’ promotion of a type 2 immune response, which modulates the type 1 responses necessary for controlling viral clearance [53]. Few studies report no effect on disease outcome while very rare cases report a beneficial effect. In the context of human viral infections, helminth infections like those caused by Ascaris and Trichuris have been linked to higher viral loads and lower CD4+ counts in HIV-infected individuals, complicating HIV management [51]. Research on lymphatic filariasis (LF) and HIV co-infection reveals mixed findings. While few studies report no significant differences in LF prevalence or worm burden between HIV-positive and HIV-negative individuals [54], others highlight that lymphatic filariasis increases HIV susceptibility and incidence, particularly in individuals with microfilariae [55,56], suggesting that helminth-driven immune modulation increases vulnerability to viral infections. Deworming against helminth infections led to a decrease in serum IgE and improvement in antiviral immune response, viral clearance, and reduced viral severity in HIV patients [49]. Hookworm infection was also found to be associated with a heightened risk of sexually transmitted infections (STIs), bacterial vaginosis (BV), candidiasis, HPV, and viral load [57,58], likely due to Th2-biased immune responses. Similar trends were observed with soil-transmitted helminths (STH), which increased HPV prevalence in endemic regions. In the female reproductive tract, helminth-induced immune changes exacerbated HPV susceptibility and bacterial infections. Although some studies involving Schistosoma mansoni indicate a nuanced impact on HBV, showing improved immune responses during acute infection phases without worsening chronic HBV [59], most human research supports that helminth co-infections with blood-borne viruses generally worsen viral disease outcomes [50,60,61]. The findings in an animal model also demonstrated that mice co-infected with helminths (Ascaris and Nippostrongylus braziliensis (Nb)) and viruses (Vaccinia virus and HSV-2, respectively) exhibited more severe disease, increased viral loads, and reduced CD8+ T cells than those infected with the virus alone [62]. Mechanistic findings revealed that Nippostrongylus braziliensis (Nb) modulates the immune response in mice co-infected with HSV-2 by imprinting a type 2 immune signature in the female genital tract (FGT) [63]. Nb infection induced epithelial stress and the release of IL-33, which activated innate lymphoid cells (ILC2s) and inflammatory monocytes to produce IL-5, driving eosinophil recruitment and persistence in the FGT. This eosinophil influx exacerbated HSV-2-induced epithelial necrosis, genital pathology, and ulceration without altering viral replication [63]. The mechanism involved IL-5-driven eosinophilic inflammation, with raised levels of IL-5 and reduced antiviral IFN-γ responses impairing epithelial MHC expression and antiviral immunity. Depleting IL-5 reduced eosinophil infiltration and pathology, confirming its role. Notably, the exacerbated pathology is independent of IL-4 receptor alpha (Il4rα) signaling, highlighting the centrality of IL-5 and eosinophilic inflammation in Nb-enhanced HSV-2 pathogenesis [63]. Thus, helminth infections frequently complicate systemic viral disease management and underscore the need for integrated treatment strategies for co-infected individuals. Table 2 provides supporting information on the interaction between helminths and blood-borne viral infections.
Comparing disease outcomes in co-infections with respiratory versus blood-borne viruses reveals intriguing differences despite both being viral infections. Respiratory viruses, including recent SARS-CoV-2, often lead to outcomes heavily influenced by the level of inflammation, commonly referred to as a cytokine storm [64,65]. Effective management of these conditions focuses on controlling this inflammatory response [66,67,68], and helminth infections, which promote a Th2 immune response, might potentially mitigate the cytokine storm by suppressing the Th1 immune response, leading to better health outcomes [29,69]. However, this benefit comes with a trade-off, the suppression of Th1 immune response by helminths can suppress antibody production and the neutralization potential necessary for viral clearance. Despite this, the cytokine storm remains the major predictor of disease outcome, and even modest levels of antibodies are typically sufficient to clear the virus. In contrast, blood-borne viruses, such as HIV, HBV, HPV, and HTLV, exhibit a distinct pathogenic mechanism. Blood-borne retroviruses such as HIV and HTLV integrate their RNA genomes into the host DNA, resulting in chronic infections with persistent viral replication [70]. This integration disrupts normal cellular processes and causes ongoing immune system damage. HIV targets and destroys CD4+ T cells, which are crucial for an effective immune response, leading to severe immunodeficiency and an increased risk of opportunistic infections and cancers [71]. Additionally, retroviruses evade immune detection through rapid mutations and immune evasion strategies, further exacerbating disease severity. Consequently, the management of retroviral infections often does not focus on cytokine storm mitigation, as in respiratory viruses, but on viral clearance, which depends on enhanced Th1-mediated T cell and antibody responses. However, in the context of helminth co-infection with retroviruses, their suppressive effect on Th1 responses induces impaired antibody production and neutralization against systemic viruses, potentially worsening overall health outcomes rather than alleviating symptoms, as seen with respiratory viral infection [50,53,62].
In conclusion, while helminth co-infections may modulate the immune system, leading to better health outcomes in respiratory virus conditions like SARS-CoV-2, RSV, and Influenza by dampening inflammatory cytokine storms through skewed Th2 immune modulation, this same immune suppression can lead to worsened clinical outcomes in systemic viral infections, such as HIV, HTLV, HBV, HPV, and HSV, where the Th1 responses are essential for viral clearance and immune defense, thus potentially leading to poorer health outcomes and increased viral load (Figure 4).
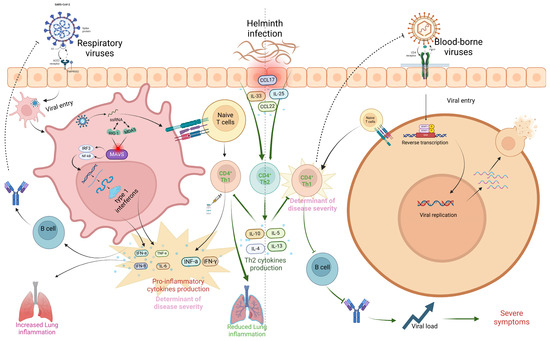
Figure 4.
Influence of helminths on SARS-CoV-2 and HIV infection outcome. The virus enters the host cell via endocytosis after binding to the ACE2 receptor, facilitated by host proteases like TMPRSS2. Following fusion, viral RNA is released into the cytoplasm, where it is detected by pattern recognition receptors (PRRs) such as RIG-I and MDA5. This activates the MAVS signaling complex, which induces the production of type 1 interferons (IFN-α, IFN-β) and pro-inflammatory cytokines through NF-κB and IRF3/IRF7 pathways. Dendritic cells present viral antigens to naïve CD4+ T cells, promoting Th1 cell differentiation via IL-12. Th1 cells secrete IFN-γ, enhancing macrophage activity and CD8+ T cell activation. This creates a feedback loop of interferon production, which, if unregulated, can contribute to cytokine storms. Helminth co-infection suppresses Th1 cells, reducing cytokine production and mitigating the cytokine storm. In the case of HIV, the virus binds to the CD4 receptor, fuses with the host cell, and releases viral RNA. Reverse transcription converts viral RNA into DNA, which is integrated into the host genome. This leads to the production of new viral particles, which spread infection by targeting CD4+ T cells. Helminth co-infection further suppresses CD4+ T cells, reducing immune response and increasing viral load, leading to severe outcomes (created using BioRender.com).

Table 2.
Study characteristics of some included studies on helminth–systemic virus interaction.
Table 2.
Study characteristics of some included studies on helminth–systemic virus interaction.
| Study | Type of Study | Year of Publication | Helminth or Anthelmintic Treatment | Systemic Viral Condition Investigated | Main Findings ↓↑ | Ref. |
|---|---|---|---|---|---|---|
| Ascaris and Vaccinia Virus | Mice | 2017 | Ascaris infection | Vaccinia Virus (VACV) | ↑ severity, ↑ viral loads, ↑ lung inflammation, ↓ CD8+ T cells. | [62] |
| Helminth Infection and HIV Immune Response | Human | 2014 | A. lumbricoides, T. trichiura | HIV | ↑ CCR5 expression on CD4 or CD8 T cells, ↑ HIV susceptibility, ↓ HLA-DR+ CD8 T cells. | [61] |
| HIV and LF Interaction and treatment in Tanzania | Human | 2016 | Wucheriria bancrofti | HIV | No significant difference in LF prevalence or worm burden was observed between HIV-positive and HIV-negative individuals. | [54] |
| LF and HIV Incidence in Tanzania | Human | 2016 | Wuchereria bancrofti | HIV | HIV incidence was significantly higher among individuals with LF compared to those without. | [55] |
| LF and HIV Risk by Microfilariae in Tanzania | Human | 2023 | Wuchereria bancrofti | HIV | Individuals with microfilariae-producing W. bancrofti had a significantly higher HIV incidence than those without. | [56] |
| Hookworm and HPV Co-Infection | Human | 2022 | Hookworm (Ancylostoma duodenale) | HPV | Hookworm infection was associated with a mixed type 1/type 2 immune response in the vaginal tract and an increased risk and viral load of HPV infection. | [58] |
| Hookworm and FRTIs in Rural Togo | Human | 2021 | Hookworm | HPV and FRTIs (Female Reproductive Tract Infections) | Hookworm infection increased the risk of HPV infections and was associated with sexually transmitted infections (STIs), bacterial vaginosis (BV), and candidiasis. | [57] |
| STH and HPV in Peru | Human | 2016 | STH | HPV | Women with STH infections in the Peruvian Amazon had a 60% higher HPV prevalence compared to uninfected women. A Th2 immune profile was observed in cervical fluid of helminth-infected women. | [72] |
| Nippostrongylus braziliensis Infection Alters FGT Immunity | Mice | 2021 | Nippostrongylus braziliensis | HSV-2 | Co-infection led to enhanced genital ulceration, necrosis, reduced MHC expression, and impaired IFN-γ response without affecting viral load. | [63] |
| Helminth and HIV | In-vitro | 2016 | Helminth proteins (BmA and ES-62) | HIV | Neither BmA nor ES-62 affected overall HIV-1 replication in CD4+ T cells or dendritic cell function. | [73] |
| L. sigmodontis and Retroviral Infection | Mice (C57BL/6) | 2016 | L. sigmodontis | Retroviral infection | ↑ viral load, larger spleens, more severe illness, ↓ virus-neutralizing antibodies, ↑ viral loads. | [50] |
| Puumala hantavirus and Heligmosomum mixtum | Mice | 2014 | Heligmosomum mixtum | Puumala hantavirus (PUUV) | ↓ immune responses, ↑ viral loads. | [60] |
| S. mansoni and HBV | Mice | 2020 | Schistosoma mansoni | Hepatitis B Virus (HBV) | ↑ antiviral response to HBV, ↑ IFN-γ. | [59] |
| Helminth Infections in HIV-patients | Human | 2015 | Deworming | HIV | ↓ symptoms, ↓serum IgE | [49] |
| Helminth Infections and Herpesvirus Interactions | Mice (C57BL6/) | 2014 | general helminth infections | Murine g-herpesvirus | ↑ IL-4, ↑ viral replication and reactivation. | [74] |
| Schistosoma and Geohelminth Infections with β-cell Function and Insulin Resistance (Tanzania) | Human | 2022 | Schistosoma and Geohelminth Infections | HIV/Insulin | Schistosoma infection ↑ β-cell function in HIV-uninfected participants. In HIV-infected individuals not on ART, Schistosoma and geohelminth infections ↓ β-cell function, and caused insulin resistance. | [75] |
| Impact of Schistosomiasis Treatment on HIV Susceptibility in Women (Uganda) | Human | 2019 | S. mansoni infection and treatment | HIV | Schistosomiasis treatment ↓ HIV entry into cervical and blood CD4+ T cells, with effects lasting up to two months. The treatment led to immune activation but also ↑ IFN-I pathway activity, which potentially ↓ HIV susceptibility by inhibiting HIV entry. | [76] |
| Helminth-induced immunomodulation and its effect on antiviral immunity | Mice | 2014 | Trichinella spiralis | Murine Norovirus (MNV), CW3 strain | ↓ antiviral immunity independently of changes in the microbiota. The impairment was mediated by STAT6-dependent alternative macrophage activation, with the molecule Ym1 contributing to ↓ antiviral response. Neutralization of Ym1 partially restores antiviral immunity. | [77] |
| HIV and helminth Co-Infections in China | Human | 2013 | Intestinal helminths and protozoa (Blastocystis hominis, Cryptosporidium spp.) | HIV | Co-infection with helminths led to a shift in Th1–Th2 balance similar to HIV infection, potentially accelerating AIDS progression. | [53] |
| Helminth-HIV Co-Infection in South Africa | Human | 2011 | A. lumbricoides, Trichuris trichiura (diagnosed via coproscopy and IgE levels) | HIV | Individuals with both helminth egg excretion and high Ascaris-specific IgE had dysregulated immune cells, higher viral loads, and increased immune activation. Those with egg excretion but low IgE had a modified Th2 response with better HIV-related immune profiles | [51] |
| Helminth Co-Infection and HTLV-1 Immune Modulation | Human | 2005 | Strongyloides stercoralis, Schistosoma mansoni | Human T cell Lymphotropic Virus Type 1 (HTLV-1) | HTLV-1 carriers co-infected with helminths had lower IFN-γ levels and reduced activation of CD8+ and CD4+ T cells compared to HTLV-1 carriers without helminths. IL-5 and IL-10 levels were higher in co-infected individuals | [52] |
This table represents the key findings of some selected studies on the interaction between helminths and non-respiratory virus infections. It also includes the type of study, year of publication, the main findings, and references. ↑ stands for increase; ↓ stands for decrease.
3.3. Helminth and Bacterial Infections
Helminths and bacterial coinfections are often common, and studies have demonstrated the co-infection of helminths with bacterial infections in tropical and subtropical regions, affecting millions of people globally [78,79]. In many tropical and subtropical areas where helminths are highly prevalent, bacterial infections, such as those caused by Mycobacterium, Salmonella, or Helicobacter pylori, are also prevalent [79]. Coinfection can lead to complex interactions between the immune system and pathogens. Helminths often modulate the host’s immune response, skewing it toward an anti-inflammatory, Th2-dominated response, which can affect the body’s ability to effectively fight off infections, including bacterial infections, with unintended effects on vaccine efficacy [80]. The effect of helminth infections on bacterial diseases can vary widely depending on the type of bacterial pathogen, the nature of the helminth infection, and the immune status of the host. The interaction between helminth infections and bacterial pathogens, particularly Mycobacterium species, has been highly studied, and it reveals a multifaceted relationship that can influence disease outcomes in various ways. Most reviewed studies suggest that helminth co-infections may exacerbate bacterial infections by impairing crucial immune responses [80,81,82]. Studies on Heligmosomoides polygyrus co-infection with Salmonella in mice highlight how helminths can reduce neutrophil recruitment to the infection site, which is vital for controlling bacterial spread. This impaired immune response led to more severe intestinal inflammation and poorer overall outcomes, indicating that helminth infections may hinder the body’s ability to effectively combat bacterial pathogens [81]. However, the immunological basis of this association requires further expansion. Similarly, co-infection with Schistosoma mansoni or Nippostrongylus brasiliensis with mycobacterial infection in mice could reduce Mincle expression in macrophages, affecting immune responses to mycobacterial infections and vaccines [80]. Deworming with albendazole 400 mg/day for 3 days was shown to reduce eosinophil counts and IL-10 levels in helminth-TB co-infected patients, showing a reversal of helminth-induced immune suppression, implying that removing helminths can restore normal immune function and improve the host’s ability to control bacterial infections [83]. This finding supports the idea that in some cases, helminth infections may need to be addressed as part of a broader strategy to manage bacterial diseases effectively. Additionally, the impact of maternal helminth infections on neonatal immunity adds another layer of complexity. Studies on the human population show altered immune responses in newborns born from helminth-infected mothers, such as higher IgE levels and modified TB-specific antibody responses, suggesting that maternal helminth infections can have long-lasting effects on the offspring’s immune system [82]. These alterations could influence how effectively vaccines work and how the infant’s immune system responds to infections later in life.
Conversely, few studies observed that helminth infections might mitigate disease severity in certain contexts, involving some bacterial infections. Chronic L. sigmodontis infection has been found to improve sepsis outcomes caused by E. coli by reducing inflammation and enhancing bacterial clearance, and it also affects macrophage function through Wolbachia and TLR2 signaling [84]. Strongyloides stercoralis infection was also associated with reduced TB severity in some patients [85]. However, more investigations are needed to unravel the immunological basis of this association. This suggests that the immunomodulatory effects of helminths, which typically induce a Th2-dominated immune response, might help mitigate the immune response to some bacterial infections, potentially leading to less severe disease [86]. These findings are consistent with the notion that in chronic bacterial infections, where inflammation can cause significant tissue damage, the anti-inflammatory effects of helminths might be beneficial [87].
While helminth infections may often worsen bacterial disease outcomes by impairing essential immune responses necessary for bacterial clearance, they may also mitigate severity in some scenarios by reducing harmful inflammation in some chronic infections. The variability in outcomes underscores the importance of in-depth and future research exploring the interaction between helminths and bacterial infection that may be context-dependent, including the type of helminth, the specific bacterial infection, and the host’s immune status, as well as the importance of maternal health in shaping neonatal immune outcomes. Therefore, tailored approaches, including the strategic use of deworming, may be necessary to optimize disease management in populations where helminth and bacterial co-infections are prevalent. Table 3 provides supporting information on the interaction between helminths and bacterial infections.

Table 3.
Study characteristics of some included studies on helminth–bacterial interaction.
3.4. Helminths and Non-Communicable Diseases
Non-communicable diseases (NCDs), such as cardiovascular diseases, diabetes, cancer, and chronic respiratory diseases, are major global health challenges, particularly in low- and middle-income countries [91,92]. These conditions are primarily driven by lifestyle factors like poor diet, lack of physical activity, smoking, and environmental factors. In many endemic regions where helminths are prevalent, there is also a growing concern of NCDs, especially diabetes. Infection with parasitic worms like roundworms and hookworms can affect the progression and management of NCDs. While they may reduce inflammation associated with NCDs, they can also impair the host’s ability to regulate metabolic processes and fight chronic diseases effectively. Understanding the interplay between helminths and NCDs is crucial for developing integrated health strategies in regions where both conditions coexist. The interaction between helminth infections and non-communicable diseases reveals a complex dynamic that can have both beneficial and detrimental outcomes, depending on the specific helminth and the context of the infection. Several studies suggest that co-infections with helminths may offer some benefits in managing certain NCDs by modulating the immune system.
In metabolic disorders like type 2 diabetes (T2D), helminth infections and derived products appear to offer some benefits in managing such conditions. Studies on H. polygyrus showed improved glucose control, reduced insulin resistance, and fat accumulation in diabetic mice, suggesting that helminth-induced immune modulation could be a promising therapeutic approach for managing T2D [93] (Figure 5). Furthermore, filarial infections have been linked to reduced inflammation and improved insulin sensitivity, with regions experiencing an increase in T2D incidence following the elimination of filarial diseases [94,95]. This suggests that chronic helminth infections may play a role against the development of metabolic dysfunctions by regulating inflammation.
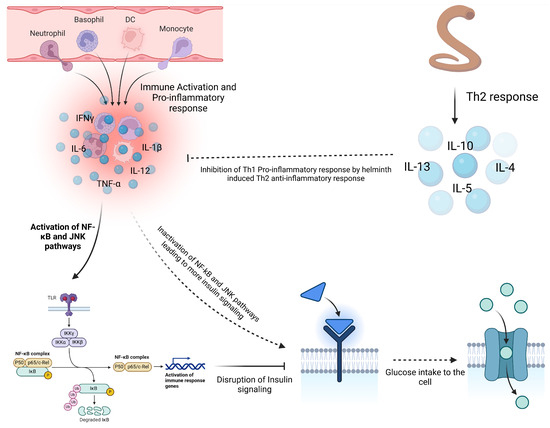
Figure 5.
Influence of helminth on insulin resistance. Chronic inflammation in obesity contributes to the development of type 2 diabetes (T2D) by promoting insulin resistance. Pro-inflammatory cytokines like TNF-α and IL-6 activate pathways such as NF-κB and JNK, disrupting insulin signaling and reducing glucose uptake. In obese individuals, macrophages infiltrate adipose tissue, further enhancing inflammation and worsening insulin resistance. Co-infection with helminths helps mitigate this inflammation through Th2 anti-inflammatory cytokines, which inactivate the NF-κB and JNK pathways, restoring insulin signaling and glucose uptake and reducing T2D risk (created using BioRender.com).
In terms of allergic conditions, helminths have shown potential in controlling allergic responses. Early-life infection with H. polygyrus in mice led to chronic mild inflammation but a controlled allergic response later in life, supporting the idea that helminth infections can regulate immune responses and reduce the likelihood of allergic inflammation [96]. Similarly, N. brasiliensis infections reduced allergic responses, particularly when the infection occurred weeks before allergen exposure, with the protective effect linked to IL-10 [97]. Chronic infection with Schistosoma mansoni also induces a type 2 immune response characterized by elevated interleukin-10 (IL-10) production, which modulates both type 1 and type 2 immune responses, reducing the risk of autoimmune diseases like multiple sclerosis, diabetes, and Crohn’s disease, as well as allergic conditions such as asthma [98,99,100]. The hygiene hypothesis suggests that helminths suppress bystander immune responses to allergens, explaining the lower prevalence of asthma in developing regions compared to industrialized countries. Epidemiological and experimental studies demonstrate an inverse association between schistosomiasis and allergy severity, with reduced asthma pathology and poor skin prick test reactivity in endemic areas, and protection was linked to IgE production [101]. In animal models, schistosome infections suppress allergic airway inflammation (AAI) when induced during the patent phase of infection, which involves egg production by fecund female worms [102]. This suppression is mediated by infection-induced CD4+Foxp3+ regulatory T cells (Tregs), as their depletion reverses the protective effect, leading to aggravated AAI. The suppression effect is lost after anti-helminthic treatment, likely due to delayed Treg reconstitution. These findings suggest that helminth-derived products, particularly during the egg-producing phase, could be harnessed to develop therapies targeting Treg induction for managing immune-mediated diseases like asthma [102]. The modulatory effect of helminths in allergy and autoimmune diseases extends beyond the direct impact on the host; the offspring of mice mothers infected with Schistosoma mansoni during specific immune phases exhibited a reduced likelihood of developing allergic airway inflammation, highlighting the potential long-term protective effects of maternal helminth infections [103]. In Gabonese mothers, placentas showed a significantly lower gene expression of VDR, Foxp3, IL-10, and Cyp27b1 compared to German mothers (non-endemic), suggesting that prenatal helminth exposure may impair immune system development, leading to altered immune responses in offspring [104]. These findings align with the “hygiene hypothesis”, which suggests that decreased exposure to infections may increase susceptibility to allergic diseases.
Helminth infections have also shown promise in treating autoimmune diseases and neurodegenerative conditions. Trichinella spiralis modulates immune responses to favor a Th2 and regulatory environment, potentially reducing inflammation and providing therapeutic benefits for autoimmune conditions [105]. In neurodegenerative diseases, co-infection with H. polygyrus reduces prion accumulation and extends survival in mice with prion disease, indicating that helminth-induced immune modulation could slow disease progression [106]. However, helminth infections are not without risks. While they may offer immune protection and modulation, clearing the infection can reverse these benefits, as seen in Strongyloides stercoralis infections in people with T2D. Once the infection was treated, the reduced inflammation and microbial leakage benefits were lost [107], raising concerns about the long-term management of such co-infections. Furthermore, chronic mild inflammation induced by helminths may have unintended consequences, particularly if it persists or interacts with other inflammatory conditions.
Overall, co-infections with helminths appear to offer a range of benefits for managing NCDs, particularly in regulating immune responses to reduce inflammation, improve metabolic outcomes, and control allergic and autoimmune conditions (Figure 5). However, the potential risks, particularly in terms of chronic inflammation or the loss of benefits following helminth clearance, must be carefully considered. These suggest that helminth-based therapies or controlled immune modulation could be a valuable tool in managing NCDs, though further research is needed to balance the risks and benefits of such approaches. Table 4 provides supporting information on the interaction between helminths and non-communicable diseases.

Table 4.
Study characteristics of included studies on helminth–non-communicable disease interaction.
3.5. Helminths and Vaccine Efficacy
Vaccines are biological preparations designed to provide immunity against infectious diseases. They contain antigens, derived from or mimicking pathogens, which stimulate the immune system to recognize and fight infections. The primary purpose of vaccines is to “train the immune system” to develop a memory of the pathogen, allowing for a faster and more effective response upon future exposure. The basic mechanism of action involves the activation of the body’s adaptive type 1 immune response, leading to the production of antibodies by B cells and the activation of T cells to destroy infected cells. Vaccines typically elicit a humoral immune response (antibody production) and a cell-mediated immune response (T cell activation). However, the efficacy of vaccines can be influenced by coinfections, including infections with helminths. The interaction between helminths and vaccine efficacy reveals a complex picture where helminths can both impair and sometimes have negligible effects on vaccine responses. Evidence from various reviewed studies indicates that helminth infections generally tend to be detrimental to vaccine efficacy. In mice infected with Schistosoma mansoni, the TD158 HIV-1 vaccine’s immune response was significantly weakened, suggesting that helminth infections can reduce the effectiveness of vaccines targeting HIV-1 [115]. Similarly, chronic infection with Schistosoma japonicum decreased the immune response to the HBV vaccine, though deworming with praziquantel improved this response, indicating that helminth infections can compromise vaccine efficacy but may be mitigated with appropriate treatment [20].
In the case of the BCG vaccine, deworming before vaccination enhanced immune responses, evidenced by increased levels of IFN-γ and IL-12. This indicates that helminths can decrease BCG vaccine efficacy by promoting an immunosuppressive environment through elevated TGF-β levels [17]. Similarly, Heligmosomoides polygyrus infection impaired the immune response to the Pfs25 DNA malaria vaccine. Although it did not affect the irradiated sporozoite vaccine, deworming improved responses to the Pfs25 vaccine, reinforcing the idea that helminth infections can diminish the efficacy of certain malaria vaccines but that this can be addressed through deworming [116]. For Influenza vaccines, mice with helminth infections showed impaired antibody responses to both seasonal and H1N1 Influenza vaccines. This impairment was associated with IL-10-producing regulatory T cells. Blocking IL-10 was found to partially restore vaccine responses, suggesting that targeting IL-10 might improve vaccine effectiveness in individuals with helminth infections [117]. The impact of helminths on COVID-19 vaccines has also been significant. Infection with helminths including Trichinella spiralis, Schistosoma mansoni, and Heligmosomoides polygyrus was found to reduce the efficacy of the COVID-19 mRNA vaccine, as evidenced by lower levels of antibodies, fewer splenic germinal center B cells, and lower T cell response [118,119,120]. Treatment with albendazole partially reversed these effects, indicating that chronic helminth infections can impair vaccine responses and deworming might help restore some level of efficacy [120]. However, deworming might not restore vaccine efficacy in all instances. Chronic worm infections were found to completely suppress the immune response to DNP-KLH and NIP-Ficoll vaccines, reducing B cells and antibody levels. Even after deworming, immune suppression persisted, requiring stronger vaccination strategies [13].
On the other hand, some vaccines have demonstrated resilience to helminth infections. The HPV-16/18 vaccine demonstrated high antibody levels in all participants regardless of malaria or helminth infections, and malaria-infected participants even showed slightly higher antibody levels, though the reasons for this observation are not yet clear [121]. Moreover, chronic helminth infections did not affect the effectiveness of the malaria vaccine, with similar immune responses observed in both infected and uninfected mice [122]. Furthermore, while maternal helminth infections initially led to lower antibody levels against tetanus in cord blood, vaccine responses in infants at 9-12 months were similar to those of infants born from uninfected mothers [123].
Overall, while few vaccines can retain effectiveness in the presence of helminth infections, the general trend indicates that helminth infections weaken vaccine-induced immune responses, particularly through mechanisms such as the increased production of immunosuppressive cytokines and disruptions in immune balance. This underscores the need for targeted strategies, such as pre-treatment with deworming medications or tailored vaccination approaches, to optimize vaccine protection in populations affected by helminths. Table 5 provides supporting information on the interaction between helminths and vaccine efficacy.

Table 5.
Study characteristics of some included studies on helminth–vaccine efficacy interaction.
4. Conclusions
The outcome of helminth co-infection is highly diverse and context-specific. It could be either beneficial or detrimental. This variability depends on several factors, including the type and stage of helminth infection, the host’s immune status, and the pathogen involved in the co-infection. Helminths can sometimes reduce inflammation and improve outcomes in some bacterial and viral infections, like respiratory viruses including SARS-CoV-2, RSV, and Influenza, as well as bacterial sepsis by E. coli. They could also be beneficial in managing some non-communicable diseases such as T2D, asthma, and metabolic disorders. Still, they may also exacerbate illness in other cases, increasing viral loads or impairing immune responses. Additionally, helminth co-infection typically diminishes vaccine efficacy, with long-lasting effects even after deworming, particularly in maternal infections affecting neonatal immunity and vaccine response.
5. Limitations
Many studies included in this review are regionally biased, primarily conducted in areas where helminth infections are endemic, such as low- and middle-income countries. This focus on specific regions may limit the generalizability of conclusions, as immunological responses and environmental factors differ significantly across geographic locations. Methodological inconsistencies further complicated synthesis, with variations in diagnostic techniques, helminth species, and outcome measures. Some studies emphasized clinical outcomes, while others focused on immunological biomarkers, which may not always directly correlate. Moreover, different studies utilized different guidelines to score disease outcome/severity, which may bring some inconsistencies in the synthesis of the results. Additionally, the diversity in the study designs ranging from observational studies and cross-sectional analyses to animal models limits the robustness of comparisons, as each approach has distinct limitations. Observational and cross-sectional studies are informative but lack causative insights, while animal models may not fully replicate human immune responses to co-infections. A scarcity of longitudinal data was also notable; without long-term studies, it is challenging to understand the sustained effects of helminth co-infections on disease progression. These limitations underscore the importance of future studies with standardized methodologies, broader geographic representation, and longitudinal tracking to better understand the nuanced effects of helminth co-infections across various disease outcomes.
6. Recommendation and Future Research Directions
Routine deworming programs utilizing anthelmintics can significantly reduce the burden of helminth infections and restore immune function before vaccination. Therefore, developing vaccination programs that specifically account for helminth infections is crucial. However, it is important to recognize that the immunomodulatory effects of helminths may persist even after deworming, leading to a lingering state of immune suppression whose duration remains largely unclear in the human population. This uncertainty underscores the necessity for further research aimed at clarifying how long the immunosuppressive effects of helminths last. Such studies are crucial for determining the optimal timing for vaccination following anthelmintic treatment, as understanding the duration of immune suppression can help enhance vaccine efficacy and overall health outcomes in populations affected by helminth infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13050436/s1, Table S1: PRISMA 2020 Checklist. Reference [134] is cited in the Supplementary Materials.
Author Contributions
Conceptualization: B.A.N.F., L.B.D., M.O., T.A. and A.Y.D.; Methodology: B.A.N.F., G.A., D.A.M., L.B.D., T.A., A.H., M.O., J.M., M.R., K.A., J.M.G. and A.Y.D.; Resources: A.Y.D., T.A., L.B.D., M.O. and A.H.; Original draft preparation: B.A.N.F., G.A. and J.M.; Review and editing: L.B.D., T.A., M.O., D.A.M., M.R., K.A., A.H. and A.Y.D.; Visualization/illustration: B.A.N.F. and G.A.; Supervision: L.B.D., M.O., T.A., A.H. and A.Y.D.; Project administration: L.B.D., A.H., M.O. and A.Y.D.; Funding acquisition: L.B.D., A.H., M.O. and A.Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the German West African Center for Global Health and Pandemic Prevention (GWAC), the TAKeOFF project, and the German Ministry of Education and Research (Bundesministerium fur Bildung und Forschung—BMBF) under an agreement with Gesellschaft fur Internationale Zusammenarbeit (GIZ) through agreement number 81204851. The funders were neither involved in the writing of the review nor in the decision to submit the article for publication.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Acknowledgments
Our thanks are offered to the following institutions for their infrastructural and administrative support in helping us carry out this work: Kwame Nkrumah University of Science and Technology (KNUST), Kumasi Center for Collaborative Research in Tropical Medicine (KCCR), and Bonn University Hospital (UKB), Institute of Medical Microbiology, Immunology, and Parasitology (IMMIP).
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Capewell, P.; Cooper, A.; Clucas, C.; Weir, W.; MacLeod, A. A Co-Evolutionary Arms Race: Trypanosomes Shaping the Human Genome, Humans Shaping the Trypanosome Genome. Parasitology 2015, 142 (Suppl. S1), S108–S119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eyck, H.J.F.; Brown, G.P.; Rollins, L.A.; Shine, R. In an Arms Race between Host and Parasite, a Lungworm’s Ability to Infect a Toad Is Determined by Host Susceptibility Not Parasite Preference. Biol. Lett. 2022, 18, 35259944. [Google Scholar] [CrossRef]
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric Schistosomiasis Parasite Found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.A. Helminths: Structure, Classification, Growth, and Development. In Medical Microbiology; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Shiferaw, M.B.; Zegeye, A.M.; Mengistu, A.D. Helminth Infections and Practice of Prevention and Control Measures among Pregnant Women Attending Antenatal Care at Anbesame Health Center, Northwest Ethiopia. BMC Res. Notes 2017, 10, 274. [Google Scholar] [CrossRef]
- World Health Organization. Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 20 February 2023).
- Edward, R.E.; Fox, R.S.; Barnes, R.D. Invertebrate Zoology: A Functional Evolutionary Approach, 7th ed.; Brooks/Cole: Belmont, CA, USA, 2004; ISBN 978-0-03-025982-1. [Google Scholar]
- Massacand, J.C.; Stettler, R.C.; Meier, R.; Humphreys, N.E.; Grencis, R.K.; Marsland, B.J.; Harris, N.L. Helminth Products Bypass the Need for TSLP in Th2 Immune Responses by Directly Modulating Dendritic Cell Function. Proc. Natl. Acad. Sci. USA 2009, 106, 13968–13973. [Google Scholar] [CrossRef]
- Maizels, R.M.; Yazdanbakhsh, M. Immune Regulation by Helminth Parasites: Cellular and Molecular Mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Maizels, R.M.; Pearce, E.J.; Artis, D.; Yazdanbakhsh, M.; Wynn, T.A. Regulation of Pathogenesis and Immunity in Helminth Infections. J. Exp. Med. 2009, 206, 2059–2066. [Google Scholar]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef]
- Fenton, A. Dances with Worms: The Ecological and Evolutionary Impacts of Deworming on Coinfecting Pathogens. Parasitology 2013, 140, 1119–1132. [Google Scholar] [CrossRef]
- Haben, I.; Hartmann, W.; Breloer, M. Nematode-Induced Interference with Vaccination Efficacy Targets Follicular T Helper Cell Induction and Is Preserved after Termination of Infection. PLoS Negl. Trop. Dis. 2014, 8, e3170. [Google Scholar] [CrossRef]
- Lazm, A.M.; Ajeel, N.A.; Abbood, A.S. A Survey Study on the Relationship between Helminthes Infection and Other Microorganism Infection. World J. Adv. Res. Rev. 2022, 15, 505–508. [Google Scholar] [CrossRef]
- Schlosser-Brandenburg, J.; Midha, A.; Mugo, R.M.; Ndombi, E.M.; Gachara, G.; Njomo, D.; Rausch, S.; Hartmann, S. Infection with Soil-Transmitted Helminths and Their Impact on Coinfections. Front. Parasitol. 2023, 2, 1–18. [Google Scholar] [CrossRef]
- Armel, B.; Fogang, N.; Meyer, J.; Debrah, L.B.; Owusu, M.; Agyei, G.; Mensah, D.A.; Boateng, J.; Mensah, J.O.; Klarmann-schulz, U.; et al. Helminth Seropositivity Inversely Correlated with Th1 and Th17 Cytokines and Severe COVID-19. Vaccines 2025, 13, 252. [Google Scholar] [CrossRef]
- Elias, D.; Britton, S.; Aseffa, A.; Engers, H.; Akuffo, H. Poor Immunogenicity of BCG in Helminth Infected Population Is Associated with Increased in Vitro TGF-β Production. Vaccine 2008, 26, 3897–3902. [Google Scholar] [CrossRef]
- Elias, D.; Britton, S.; Kassu, A.; Akuffo, H. Chronic Helminth Infections May Negatively Influence Immunity against Tuberculosis and Other Diseases of Public Health Importance. Expert. Rev. Anti Infect. Ther. 2007, 5, 475–484. [Google Scholar] [CrossRef]
- Elias, D.; Akuffo, H.; Pawlowski, A.; Haile, M.; Schön, T.; Britton, S. Schistosoma mansoni Infection Reduces the Protective Efficacy of BCG Vaccination against Virulent Mycobacterium tuberculosis. Vaccine 2005, 23, 1326–1334. [Google Scholar] [CrossRef]
- Chen, L.; Liu, W.-Q.; Lei, J.-H.; Guan, F.; Li, M.-J.; Song, W.-J.; Li, Y.-L.; Wang, T. Chronic Schistosoma Japonicum Infection Reduces Immune Response to Vaccine against Hepatitis B in Mice. PLoS ONE 2012, 7, e51512. [Google Scholar] [CrossRef]
- Guan, F.; Hou, X.; Nie, G.; Xiao, Y.; Zhang, Q.; Liu, W.Q.; Li, Y.L.; Lei, J.H. Effect of Trichinella Spiralis Infection on the Immune Response to HBV Vaccine in a Mouse Model. Foodborne Pathog. Dis. 2013, 10, 882–887. [Google Scholar] [CrossRef]
- Apiwattanakul, N.; Thomas, P.G.; Iverson, A.R.; McCullers, J.A. Chronic Helminth Infections Impair Pneumococcal Vaccine Responses. Vaccine 2014, 32, 5405–5410. [Google Scholar] [CrossRef]
- Riner, D.K.; Ndombi, E.M.; Carter, J.M.; Omondi, A.; Kittur, N.; Kavere, E.; Korir, H.K.; Flaherty, B.; Karanja, D.; Colley, D.G. Schistosoma mansoni Infection Can Jeopardize the Duration of Protective Levels of Antibody Responses to Immunizations against Hepatitis B and Tetanus Toxoid. PLoS Negl. Trop. Dis. 2016, 10, e0005180. [Google Scholar] [CrossRef]
- Alvarez-Larrotta, C.; Arango, E.M.; Carmona-Fonseca, J. Negative Immunomodulation by Parasitic Infections in the Human Response to Vaccines. J. Infect. Dev. Ctries. 2018, 12, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Gent, V.; Waihenya, R.; Kamau, L.; Nyakundi, R.; Ambala, P.; Kariuki, T.; Ochola, L. An Investigation into the Role of Chronic Schistosoma mansoni Infection on Human Papillomavirus (HPV) Vaccine Induced Protective Responses. PLoS Negl. Trop. Dis. 2019, 13, e0007704. [Google Scholar] [CrossRef]
- Tweyongyere, R.; Nassanga, B.R.; Muhwezi, A.; Odongo, M.; Lule, S.A.; Nsubuga, R.N.; Webb, E.L.; Cose, S.C.; Elliott, A.M. Effect of Schistosoma mansoni Infection and Its Treatment on Antibody Responses to Measles Catch-up Immunisation in Pre-School Children: A Randomised Trial. PLoS Negl. Trop. Dis. 2019, 13, e0007157. [Google Scholar] [CrossRef] [PubMed]
- Natukunda, A.; Zirimenya, L.; Nassuuna, J.; Nkurunungi, G.; Cose, S.; Elliott, A.M.; Webb, E.L. The Effect of Helminth Infection on Vaccine Responses in Humans and Animal Models: A Systematic Review and Meta-Analysis. Parasite Immunol. 2022, 44, e12939. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Diamond, M.S.; Thackray, L.B. Helminth–Virus Interactions: Determinants of Coinfection Outcomes. Gut Microbes 2021, 13, 1961202. [Google Scholar] [CrossRef]
- Adjobimey, T.; Meyer, J.; Hennenfent, A.; Bara, A.J.; Lagnika, L.; Kocou, B.; Adjagba, M.; Laleye, A.; Hoerauf, A.; Parcina, M. Negative Association between Ascaris Lumbricoides Seropositivity and Covid-19 Severity: Insights from a Study in Benin. Front. Immunol. 2023, 14, 1233082. [Google Scholar] [CrossRef]
- Adjobimey, T.; Meyer, J.; Terkeš, V.; Parcina, M.; Hoerauf, A. Helminth Antigens Differentially Modulate the Activation of CD4+ and CD8+ T Lymphocytes of Convalescent COVID-19 Patients in Vitro. BMC Med. 2022, 20, 241. [Google Scholar] [CrossRef]
- Sinha, A.; Pati, S.; Sahoo, P.K. Investigating Immunological Interaction between Lymphatic Filariasis and COVID-19 Infection: A Preliminary Evidence. Hum. Vaccin. Immunother. 2021, 17, 5150–5152. [Google Scholar] [CrossRef]
- Alosaimi, B.; Hamed, M.E.; Naeem, A.; Alsharef, A.A.; AlQahtani, S.Y.; AlDosari, K.M.; Alamri, A.A.; Al-Eisa, K.; Khojah, T.; Assiri, A.M.; et al. MERS-CoV Infection Is Associated with Downregulation of Genes Encoding Th1 and Th2 Cytokines/Chemokines and Elevated Inflammatory Innate Immune Response in the Lower Respiratory Tract. Cytokine 2019, 126, 154895. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Ho, Y.C. SARS-CoV-2: A Storm Is Raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef]
- Tai, C.; Xie, J.-X.; Lin, J.-S. Enteric Helminth Infection Confers Protection against Lethal Respiratory Influenza Virus Infection. J. Immunol. 2023, 210, 156.01. [Google Scholar] [CrossRef]
- Hardisty, G.R.; Knipper, J.A.; Fulton, A.; Hopkins, J.; Dutia, B.M.; Taylor, M.D. Concurrent Infection with the Filarial Helminth Litomosoides Sigmodontis Attenuates or Worsens Influenza a Virus Pathogenesis in a Stage-Dependent Manner. Front. Immunol. 2022, 12, 819560. [Google Scholar] [CrossRef]
- McFarlane, A.J.; McSorley, H.J.; Davidson, D.J.; Fitch, P.M.; Errington, C.; Mackenzie, K.J.; Gollwitzer, E.S.; Johnston, C.J.C.; MacDonald, A.S.; Edwards, M.R.; et al. Enteric Helminth-Induced Type I Interferon Signaling Protects against Pulmonary Virus Infection through Interaction with the Microbiota. J. Allergy Clin. Immunol. 2017, 140, 1068–1078.e6. [Google Scholar] [CrossRef]
- Burgess, M.O.; Janas, P.; Berry, K.; Mayr, H.; Mack, M.; Jenkins, S.J.; Bain, C.C.; McSorley, H.J.; Schwarze, J. Helminth Induced Monocytosis Conveys Protection from Respiratory Syncytial Virus Infection in Mice. Allergy 2024, 79, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Navarro, S.; Wangchuk, P.; Wilson, D.; Daly, N.L.; Loukas, A. Identifying the Immunomodulatory Components of Helminths. Parasite Immunol. 2015, 37, 293–303. [Google Scholar] [CrossRef]
- Zakeri, A.; Hansen, E.P.; Andersen, S.D.; Williams, A.R.; Nejsum, P. Immunomodulation by Helminths: Intracellular Pathways and Extracellular Vesicles. Front. Immunol. 2018, 9, 2349. [Google Scholar] [CrossRef]
- Melendez, A.J.; Harnett, M.M.; Pushparaj, P.N.; Wong, W.F.; Tay, H.K.; McSharry, C.P.; Harnett, W. Inhibition of FcεRI-Mediated Mast Cell Responses by ES-62, a Product of Parasitic Filarial Nematodes. Nat. Med. 2007, 13, 1375–1381. [Google Scholar] [CrossRef]
- Doonan, J.; Lumb, F.E.; Pineda, M.A.; Tarafdar, A.; Crowe, J.; Khan, A.M.; Suckling, C.J.; Harnett, M.M.; Harnett, W. Protection against Arthritis by the Parasitic Worm Product ES-62, and Its Drug-like Small Molecule Analogues, Is Associated with Inhibition of Osteoclastogenesis. Front. Immunol. 2018, 9, 336903. [Google Scholar] [CrossRef]
- Al-Riyami, L.; Pineda, M.A.; Rzepecka, J.; Huggan, J.K.; Khalaf, A.I.; Suckling, C.J.; Scott, F.J.; Rodgers, D.T.; Harnett, M.M.; Harnett, W. Designing Anti-Inflammatory Drugs from Parasitic Worms: A Synthetic Small Molecule Analogue of the Acanthocheilonema Viteae Product ES-62 Prevents Development of Collagen-Induced Arthritis. J. Med. Chem. 2013, 56, 9982–10002. [Google Scholar] [CrossRef]
- Aguayo, V.; Valdés Fernandez, B.N.; Rodríguez-Valentín, M.; Ruiz-Jiménez, C.; Ramos-Benítez, M.J.; Méndez, L.B.; Espino, A.M. Fasciola Hepatica GST Downregulates NF-ΚB Pathway Effectors and Inflammatory Cytokines While Promoting Survival in a Mouse Septic Shock Model. Sci. Rep. 2019, 9, 2275. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Zheng, H.; Ma, Q.; Chen, K.; Li, H. Anti-Inflammatory Effects of Helminth-Derived Products: Potential Applications and Challenges in Diabetes Mellitus Management. J. Inflamm. Res. 2024, 17, 11789–11812. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Barker, S.J.; MacDonald, A.J.; Yu, Y.; Cao, L.; Li, J.; Parhar, R.; Heck, S.; Hartmann, S.; Golenbock, D.T.; et al. Recombinant Ov-ASP-1, a Th1-Biased Protein Adjuvant Derived from the Helminth Onchocerca Volvulus, Can Directly Bind and Activate Antigen-Presenting Cells. J. Immunol. 2009, 182, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Anuradha, R.; Bethunaickan, R. TLR Specific Immune Responses against Helminth Infections. J. Parasitol. Res. 2017, 2017, 6865789. [Google Scholar] [CrossRef]
- Afful, P.; Abotsi, G.K.; Adu-Gyamfi, C.O.; Benyem, G.; Katawa, G.; Kyei, S.; Arndts, K.; Ritter, M.; Asare, K.K. Schistosomiasis–Microbiota Interactions: A Systematic Review and Meta-Analysis. Pathogens 2024, 13, 906. [Google Scholar] [CrossRef]
- Wolday, D.; Gebrecherkos, T.; Arefaine, Z.G.; Kiros, Y.K.; Gebreegzabher, A.; Tasew, G.; Abdulkader, M.; Abraha, H.E.; Desta, A.A.; Hailu, A.; et al. Effect of Co-Infection with Intestinal Parasites on COVID-19 Severity: A Prospective Observational Cohort Study. eClinicalMedicine 2021, 39, 101054. [Google Scholar] [CrossRef]
- Mulu, A.; Anagaw, B.; Gelaw, A.; Ota, F.; Kassu, A.; Yifru, S. Effect of Deworming on Th2 Immune Response during HIV-Helminths Co-Infection. J. Transl. Med. 2015, 13, 236. [Google Scholar] [CrossRef]
- Dietze, K.K.; Dittmer, U.; Koudaimi, D.K.; Schimmer, S.; Reitz, M.; Breloer, M.; Hartmann, W. Filariae-Retrovirus Co-Infection in Mice Is Associated with Suppressed Virus-Specific IgG Immune Response and Higher Viral Loads. PLoS Negl. Trop. Dis. 2016, 10, e000517. [Google Scholar] [CrossRef]
- Mkhize-Kwitshana, Z.L.; Taylor, M.; Jooste, P.; Mabaso, M.L.H.; Walzl, G. The Influence of Different Helminth Infection Phenotypes on Immune Responses against HIV in Co-Infected Adults in South Africa. BMC Infect. Dis. 2011, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Porto, A.F.; Santos, S.B.; Muniz, A.L.; Basílio, V.; Rodrigues, W.; Neva, F.A.; Dutra, W.O.; Gollob, K.J.; Jacobson, S.; Carvalho, E.M. Helminthic Infection Down-Regulates Type 1 Immune Responses in Human T Cell Lymphotropic Virus Type 1 (HTLV-1) Carriers and Is More Prevalent in HTLV-1 Carriers than in Patients with HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. J. Infect. Dis. 2005, 191, 612–618. [Google Scholar] [CrossRef]
- Tian, L.G.; Wang, T.P.; Lv, S.; Wang, F.F.; Guo, J.; Yin, X.M.; Cai, Y.C.; Dickey, M.K.; Steinmann, P.; Chen, J.X. HIV and Intestinal Parasite Co-Infections among a Chinese Population: An Immunological Profile. Infect. Dis. Poverty 2013, 2, 18. [Google Scholar] [CrossRef]
- Kroidl, I.; Saathoff, E.; Maganga, L.; Clowes, P.; Maboko, L.; Hoerauf, A.; Makunde, W.H.; Haule, A.; Mviombo, P.; Pitter, B.; et al. Correction: Prevalence of Lymphatic Filariasis and Treatment Effectiveness of Albendazole/Ivermectin in Individuals with HIV Co-Infection in Southwest-Tanzania. PLoS Negl. Trop. Dis. 2016, 10, e0004967. [Google Scholar] [CrossRef]
- Kroidl, I.; Saathoff, E.; Maganga, L.; Makunde, W.H.; Hoerauf, A.; Geldmacher, C.; Clowes, P.; Maboko, L.; Hoelscher, M. Effect of Wuchereria Bancrofti Infection on HIV Incidence in Southwest Tanzania: A Prospective Cohort Study. Lancet 2016, 388, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Mnkai, J.; Ritter, M.; Maganga, L.; Maboko, L.; Olomi, W.; Clowes, P.; Minich, J.; Lelo, A.E.; Kariuki, D.; Debrah, A.Y.; et al. Increased HIV Incidence in Wuchereria Bancrofti Microfilaria Positive Individuals in Tanzania. Pathogens 2023, 12, 387. [Google Scholar] [CrossRef]
- Holali Ameyapoh, A.; Katawa, G.; Ritter, M.; Tchopba, C.N.; Tchadié, P.E.; Arndts, K.; Kamassa, H.E.; Mazou, B.; Amessoudji, O.M.; N’djao, A.; et al. Hookworm Infections and Sociodemographic Factors Associated with Female Reproductive Tract Infections in Rural Areas of the Central Region of Togo. Front. Microbiol. 2021, 12, 738894. [Google Scholar] [CrossRef]
- Omondi, M.A.; Kamassa, E.H.; Katawa, G.; Tchopba, C.N.; Vogelbusch, C.; Parcina, M.; Tchadié, E.P.; Amessoudji, O.M.; Arndts, K.; Karou, S.D.; et al. Hookworm Infection Associates with a Vaginal Type 1/Type 2 Immune Signature and Increased HPV Load. Front. Immunol. 2022, 13, 1009968. [Google Scholar] [CrossRef]
- Loffredo-Verde, E.; Bhattacharjee, S.; Malo, A.; Festag, J.; Kosinska, A.D.; Ringelhan, M.; Rim Sarkar, S.; Steiger, K.; Heikenwaelder, M.; Protzer, U.; et al. Dynamic, Helminth-Induced Immune Modulation Influences the Outcome of Acute and Chronic Hepatitis B Virus Infection. J. Infect. Dis. 2020, 221, 1448–1461. [Google Scholar] [CrossRef] [PubMed]
- Guivier, E.; Galan, M.; Henttonen, H.; Cosson, J.F.; Charbonnel, N. Landscape Features and Helminth Co-Infection Shape Bank Vole Immunoheterogeneity, with Consequences for Puumala Virus Epidemiology. Heredity 2014, 112, 274–281. [Google Scholar] [CrossRef]
- Chachage, M.; Podola, L.; Clowes, P.; Nsojo, A.; Bauer, A.; Mgaya, O.; Kowour, D.; Froeschl, G.; Maboko, L.; Hoelscher, M.; et al. Helminth-Associated Systemic Immune Activation and HIV Co-Receptor Expression: Response to Albendazole/Praziquantel Treatment. PLoS Negl. Trop. Dis. 2014, 8, e2755. [Google Scholar] [CrossRef]
- Gazzinelli-Guimarães, P.H.; de Freitas, L.F.D.; Gazzinelli-Guimarães, A.C.; Coelho, F.; Barbosa, F.S.; Nogueira, D.; Amorim, C.; Dhom-Lemos, L.d.C.; Oliveira, L.M.; da Silveira, A.B.; et al. Concomitant Helminth Infection Downmodulates the Vaccinia Virus-Specific Immune Response and Potentiates Virus-Associated Pathology. Int. J. Parasitol. 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Chetty, A.; Darby, M.G.; Vornewald, P.M.; Martín-Alonso, M.; Filz, A.; Ritter, M.; McSorley, H.J.; Masson, L.; Smith, K.; Brombacher, F.; et al. Il4ra-Independent Vaginal Eosinophil Accumulation Following Helminth Infection Exacerbates Epithelial Ulcerative Pathology of HSV-2 Infection. Cell Host Microbe 2021, 29, 579–593.e5. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical Value of Immune-Inflammatory Parameters to Assess the Severity of Coronavirus Disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. [Analysis of Clinical Features of 29 Patients with 2019 Novel Coronavirus Pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E005. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, J.; Liu, X.; Liu, Y.; Li, F.; Liu, M.; Chiu, S.; Jin, X. Helminth Alleviates COVID-19-Related Cytokine Storm in an IL-9-Dependent Way. mBio 2024, 15, e0090524. [Google Scholar] [CrossRef]
- Piatak, M.; Saag, M.S.; Yang, L.C.; Clark, S.J.; Kappes, J.C.; Luk, K.C.; Hahn, B.H.; Shaw, G.M.; Lifson, J.D. High Levels of HIV-1 in Plasma During All Stages of Infection Determined By Competitive PCR. Science 1993, 259, 1749–1754. [Google Scholar] [CrossRef]
- Cooper, A.; García, M.; Petrovas, C.; Yamamoto, T.; Koup, R.A.; Nabel, G.J. HIV-1 Causes CD4 Cell Death through DNA-Dependent Protein Kinase during Viral Integration. Nature 2013, 498, 376–379. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Marks, M.; Kosek, M.; Huang, C.; Cabrera, L.; Olortegui, M.P.; Medrano, A.M.; Trigoso, D.R.; Qureshi, S.; Bardales, G.S.; et al. Soil-Transmitted Helminth Infections Are Associated with an Increase in Human Papillomavirus Prevalence and a T-Helper Type 2 Cytokine Signature in Cervical Fluids. J. Infect. Dis. 2016, 213, 723–730. [Google Scholar] [CrossRef]
- Mouser, E.E.I.M.; Pollakis, G.; Yazdanbakhsh, M.; Harnett, W.; de Jong, E.C.; Paxton, W.A. Brugia Malayi Antigen (BmA) Inhibits HIV-1 Trans-Infection but Neither BmA nor ES-62 Alter HIV-1 Infectivity of DC Induced CD4+ Th-Cells. PLoS ONE 2016, 11, e0146527. [Google Scholar] [CrossRef]
- Reese, T.A.; Wakeman, B.S.; Choi, H.S.; Hufford, M.M.; Huang, S.C.; Zhang, X.; Buck, M.D.; Jezewski, A.; Kambal, A.; Liu, C.Y.; et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 2014, 345, 573–577. [Google Scholar]
- PrayGod, G.; Filteau, S.; Range, N.; Ramaiya, K.; Jeremiah, K.; Rehman, A.M.; Krogh-Madsen, R.; Friis, H.; Faurholt-Jepsen, D. The Association of Schistosoma and Geohelminth Infections with β-Cell Function and Insulin Resistance among HIV-Infected and HIV-Uninfected Adults: A Cross-Sectional Study in Tanzania. PLoS ONE 2022, 17, e0262860. [Google Scholar] [CrossRef]
- Yegorov, S.; Joag, V.; Galiwango, R.M.; Good, S.V.; Mpendo, J.; Tannich, E.; Boggild, A.K.; Kiwanuka, N.; Bagaya, B.S.; Kaul, R. Schistosoma mansoni Treatment Reduces HIV Entry into Cervical CD4+ T Cells and Induces IFN-I Pathways. Nat. Commun. 2019, 10, 2296. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.C.; Monticelli, L.A.; Nice, T.J.; Sutherland, T.E.; Siracusa, M.C.; Hepworth, M.R.; Tomov, V.T.; Kobuley, D.; Tran, S.V.; Bittinger, K.; et al. Virus-Helminthcoinfection Reveals a Microbiota-Independent Mechanism of Immunomodulation. Science 2014, 345, 578–582. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.N.; Tantular, I.S.; Kusumaningrum, D.; Kusumaratna, R.K. Asymptomatic Intestinal Helminth Co-Infection among Pulmonary Tuberculosis Patients in Urban Surabaya: A Preliminary Study. J. Biomedika dan Kesehat. 2020, 3, 112–118. [Google Scholar] [CrossRef]
- Alemu, G.; Mama, M. Intestinal Helminth Co-Infection and Associated Factors among Tuberculosis Patients in Arba Minch, Ethiopia. BMC Infect. Dis. 2017, 17, 68. [Google Scholar] [CrossRef]
- Schick, J.; Altunay, M.; Lacorcia, M.; Marschner, N.; Westermann, S.; Schluckebier, J.; Schubart, C.; Bodendorfer, B.; Christensen, D.; Alexander, C.; et al. IL-4 and Helminth Infection Downregulate MINCLE-Dependent Macrophage Response to Mycobacteria and Th17 Adjuvanticity. eLife 2023, 12, 1–24. [Google Scholar] [CrossRef]
- Su, L.; Su, C.W.; Qi, Y.; Yang, G.; Zhang, M.; Cherayil, B.J.; Zhang, X.; Shi, H.N. Coinfection with an Intestinal Helminth Impairs Host Innate Immunity against Salmonella enterica Serovar Typhimurium and Exacerbates Intestinal Inflammation in Mice. Infect. Immun. 2014, 82, 3855–3866. [Google Scholar] [CrossRef]
- Gebreegziabiher, D.; Desta, K.; Desalegn, G.; Howe, R.; Abebe, M. The Effect of Maternal Helminth Infection on Maternal and Neonatal Immune Function and Immunity to Tuberculosis. PLoS ONE 2014, 9, e93429. [Google Scholar] [CrossRef]
- Abate, E.; Elias, D.; Getachew, A.; Alemu, S.; Diro, E.; Britton, S.; Aseffa, A.; Stendahl, O.; Schön, T. Effects of Albendazole on the Clinical Outcome and Immunological Responses in Helminth Co-Infected Tuberculosis Patients: A Double Blind Randomised Clinical Trial. Int. J. Parasitol. 2015, 45, 133–140. [Google Scholar] [CrossRef]
- Gondorf, F.; Berbudi, A.; Buerfent, B.C.; Ajendra, J.; Bloemker, D.; Specht, S.; Schmidt, D.; Neumann, A.L.; Layland, L.E.; Hoerauf, A.; et al. Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages. PLoS Pathog. 2015, 11, e1004616. [Google Scholar] [CrossRef] [PubMed]
- George, P.J.; Kumar, N.P.; Sridhar, R.; Hanna, L.E.; Nair, D.; Banurekha, V.V.; Nutman, T.B.; Babu, S. Coincident Helminth Infection Modulates Systemic Inflammation and Immune Activation in Active Pulmonary Tuberculosis. PLoS Negl. Trop. Dis. 2014, 8, e3289. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, E.; Kazura, J.W.; Hazlett, F.E.; Boom, W.H. Modulation of Murine Cytokine Responses to Mycobacterial Antigens by Helminth-Induced T Helper 2 Cell Responses. J. Immunol. 1993, 151, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Maslowski, K.M.; Mosconi, I.; Guenat, N.; Marsland, B.J.; Harris, N.L. IL-1β Suppresses Innate IL-25 and IL-33 Production and Maintains Helminth Chronicity. PLoS Pathog. 2013, 9, e1003531. [Google Scholar] [CrossRef]
- Anuradha, R.; Munisankar, S.; Bhootra, Y.; Dolla, C.; Kumaran, P.; Nutman, T.B.; Babu, S. Anthelmintic Therapy Modifies the Systemic and Mycobacterial Antigen-Stimulated Cytokine Profile in Helminth-Latent Mycobacterium tuberculosis Coinfection. Infect. Immun. 2017, 85, e00973-16. [Google Scholar] [CrossRef]
- Biraro, I.A.; Egesa, M.; Toulza, F.; Levin, J.; Cose, S.; Joloba, M.; Smith, S.; Dockrell, H.M.; Katamba, A.; Elliott, A.M. Impact of Co-Infections and BCG Immunisation on Immune Responses Among Household Contacts of Tuberculosis Patients in a Ugandan Cohort. PLoS ONE 2014, 9, e111517. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Dolla, C.; Menon, P.A.; Nutman, T.B.; Babu, S. Helminth Coinfection Alters Monocyte Activation, Polarization, and Function in Latent Mycobacterium tuberculosis Infection. J. Immunol. 2020, 204, 1274–1286. [Google Scholar] [CrossRef]
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 9 September 2024).
- Yazdanyar, A.; Newman, A.B. The Burden of Cardiovascular Disease in the Elderly: Morbidity, Mortality, and Costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef]
- Morimoto, M.; Azuma, N.; Kadowaki, H.; Abe, T.; Suto, Y. Regulation of Type 2 Diabetes by Helminth-Induced Th2 Immune Response. J. Vet. Med. Sci. 2016, 78, 1855–1864. [Google Scholar] [CrossRef]
- Aravindhan, V.; Mohan, V.; Surendar, J.; Rao, M.M.; Pavankumar, N.; Deepa, M.; Rajagopalan, R.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Decreased Prevalence of Lymphatic Filariasis among Diabetic Subjects Associated with a Diminished Pro-Inflammatory Cytokine Response (CURES 83). PLoS Negl. Trop. Dis. 2010, 4, 2–7. [Google Scholar] [CrossRef]
- Aravindhan, V.; Mohan, V.; Surendar, J.; Rao, M.M.; Ranjani, H.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Decreased Prevalence of Lymphatic Filariasis among Subjects with Type-1 Diabetes. Am. J. Trop. Med. Hyg. 2010, 83, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Miller, M.M.; Reinhardt, R.L. Neonatal Helminth Infection and Chronic Type 2 Inflammation Promotes Immune Suppression through FcγRIIb. J. Immunol. 2023, 210, 82.04. [Google Scholar] [CrossRef]
- Wohlleben, G.; Trujillo, C.; Müller, J.; Ritze, Y.; Grunewald, S.; Tatsch, U.; Erb, K.J. Helminth Infection Modulates the Development of Allergen-Induced Airway Inflammation. Int. Immunol. 2004, 16, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Jr, M.M.; Figueiredo, J.; Almeida, M.; Matos, M.; Araujo, M. Schistosoma mansoni Infection Is Associated with a Reduced Course of Asthma. J. Allergy Clin. Immunol. 2003, 111, 947–951. [Google Scholar]
- Zaccone, P.; Burton, O.; Miller, N.; Jones, F.; Dunne, D. Schistosoma mansoni Egg Antigens Induce Treg That Participate in Diabetes Prevention in NOD Mice. Eur. J. Immunol. 2009, 39, 1098–1107. [Google Scholar] [PubMed]
- Smits, H.; Hammad, H.; van Nimwegen, M.; Soullie, T.; Willart, M. Protective Effect of Schistosoma mansoni Infection on Allergic Airway Inflammation Depends on the Intensity and Chronicity of Infection. J. Allergy Clin. Immunol. 2007, 120, 932–940. [Google Scholar]
- Cardoso, L.S.; Oliveira, S.C.; Araujo, M.I. Schistosoma mansoni Antigens as Modulators of the Allergic Inflammatory Response in Asthma. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Layland, L.E.; Straubinger, K.; Ritter, M.; Loffredo-Verde, E.; Garn, H.; Sparwasser, T.; Prazeres da Costa, C. Schistosoma mansoni-Mediated Suppression of Allergic Airway Inflammation Requires Patency and Foxp3+ Treg Cells. PLoS Negl. Trop. Dis. 2013, 7, e2379. [Google Scholar] [CrossRef]
- Straubinger, K.; Paul, S.; Prazeres Da Costa, O.; Ritter, M.; Buch, T.; Busch, D.H.; Layland, L.E.; Prazeres Da Costa, C.U. Maternal Immune Response to Helminth Infection during Pregnancy Determines Offspring Susceptibility to Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2014, 134, 1271–1279.e10. [Google Scholar] [CrossRef]
- Ludwig, E.; Harder, J.; Lacorcia, M.; Honkpehedji, Y.J.; Nouatin, O.P.; van Dam, G.J.; Corstjens, P.L.A.M.; Sartono, E.; Esen, M.; Lobmaier, S.M.; et al. Placental Gene Expression and Antibody Levels of Mother-Neonate Pairs Reveal an Enhanced Risk for Inflammation in a Helminth Endemic Country. Sci. Rep. 2019, 9, 15776. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Wang, Y.; Zhan, B.; Gu, Y.; Cheng, Y.; Zhu, X. Excretory/Secretory Products from Trichinella Spiralis Adult Worms Ameliorate DSS-Induced Colitis in Mice. PLoS ONE 2014, 9, e96454. [Google Scholar] [CrossRef]
- Sánchez-Quintero, A.; Bradford, B.M.; Maizels, R.; Donaldson, D.S.; Mabbott, N.A. Effect of Co-Infection with a Small Intestine-Restricted Helminth Pathogen on Oral Prion Disease Pathogenesis in Mice. Sci. Rep. 2019, 9, 6674. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Menon, P.A.; Dolla, C.; Nutman, T.B.; Babu, S. Helminth Mediated Attenuation of Systemic Inflammation and Microbial Translocation in Helminth-Diabetes Comorbidity. Front. Cell Infect. Microbiol. 2020, 10, 431. [Google Scholar] [CrossRef]
- Meijlink, K.J. Can a Gut Helminth Parasite Influence Th2 Inflammatory Responses in the Skin? Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2017. [Google Scholar] [CrossRef]
- Tahapary, D.L.; De Ruiter, K.; Martin, I.; Brienen, E.A.T.; Van Lieshout, L.; Cobbaert, C.M.; Soewondo, P.; Djuardi, Y.; Wiria, A.E.; Houwing-Duistermaat, J.J.; et al. Effect of Anthelmintic Treatment on Insulin Resistance: A Cluster-Randomized, Placebo-Controlled Trial in Indonesia. Clin. Infect. Dis. 2017, 65, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Sanya, R.E.; Webb, E.L.; Zziwa, C.; Kizindo, R.; Sewankambo, M.; Tumusiime, J.; Nakazibwe, E.; Oduru, G.; Niwagaba, E.; Nakawungu, P.K.; et al. The Effect of Helminth Infections and Their Treatment on Metabolic Outcomes: Results of a Cluster-Randomized Trial. Clin. Infect. Dis. 2020, 71, 601–613. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Munisankar, S.; Thiruvengadam, K.; Menon, P.A.; Dolla, C.; Nutman, T.B.; Babu, S. Impact of Helminth Infection on Metabolic and Immune Homeostasis in Non-Diabetic Obesity. Front. Immunol. 2020, 11, 2195. [Google Scholar] [CrossRef]
- Namara, B.; Nash, S.; Lule, S.A.; Akurut, H.; Mpairwe, H.; Akello, F.; Tumusiime, J.; Kizza, M.; Kabagenyi, J.; Nkurunungi, G.; et al. Effects of Treating Helminths during Pregnancy and Early Childhood on Risk of Allergy-Related Outcomes: Follow-up of a Randomized Controlled Trial. Pediatr. Allergy Immunol. 2017, 28, 784–792. [Google Scholar] [CrossRef]
- Pierce, D.R.; McDonald, M.; Merone, L.; Becker, L.; Thompson, F.; Lewis, C.; Ryan, R.Y.M.; Hii, S.F.; Zendejas-Heredia, P.A.; Traub, R.J.; et al. Effect of Experimental Hookworm Infection on Insulin Resistance in People at Risk of Type 2 Diabetes. Nat. Commun. 2023, 14, 4503. [Google Scholar] [CrossRef]
- Sanya, R.E.; Nkurunungi, G.; Spaans, R.H.; Nampijja, M.; O’Hara, G.; Kizindo, R.; Oduru, G.; Nakawungu, P.K.; Niwagaba, E.; Abayo, E.; et al. The Impact of Intensive Versus Standard Anthelminthic Treatment on Allergy-Related Outcomes, Helminth Infection Intensity, and Helminth-Related Morbidity in Lake Victoria Fishing Communities, Uganda: Results from the LaVIISWA Cluster-Randomized Trial. Clin. Infect. Dis. 2019, 68, 1665–1674. [Google Scholar] [CrossRef]
- Da’Dara, A.A.; Lautsch, N.; Dudek, T.; Novitsky, V.; Lee, T.H.; Essex, M.; Harn, D.A. Helminth Infection Suppresses T-Cell Immune Response to HIV-DNA-Based Vaccine in Mice. Vaccine 2006, 24, 5211–5219. [Google Scholar] [CrossRef]
- Noland, G.S.; Chowdhury, D.R.; Urban, J.F.; Zavala, F.; Kumar, N. Helminth Infection Impairs the Immunogenicity of a Plasmodium Falciparum DNA Vaccine, but Not Irradiated Sporozoites, in Mice. Vaccine 2010, 28, 2917–2923. [Google Scholar] [CrossRef]
- Hartmann, W.; Brunn, M.L.; Stetter, N.; Gagliani, N.; Muscate, F.; Stanelle-Bertram, S.; Gabriel, G.; Breloer, M. Helminth Infections Suppress the Efficacy of Vaccination against Seasonal Influenza. Cell Rep. 2019, 29, 2243–2256.e4. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Karl, C.E.; Ying, B.; Liang, C.-Y.; Garcia-Salum, T.; Santana, A.C.; Ten-Caten, F.; Joseph, F., Jr.; Elbashir, S.M.; Edwards, D.K.; et al. Intestinal Helminth Infection Impairs Vaccine-Induced T Cell Responses and Protection against SARS-CoV-2 in Mice. Sci. Transl. Med. 2024, 16, eado1941. [Google Scholar] [CrossRef]
- Oshiba, S.; Harba, N.; El Melegy, M.; El Gammal, S.; Hegazy, A.; Abokhalil, N. Effects of COVID-19 Vaccine on Experimentally Infected Mice with Schistosoma mansoni. J. Biosci. Appl. Res. 2023, 9, 138–160. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, W.; Gong, Y.; Zhang, J.; Yu, Y.; Zhang, J.; Liu, M.; Guan, F.; Lei, J. Trichinella Spiralis Infection Inhibits the Efficacy of RBD Protein of SARS-CoV-2 Vaccination via Regulating Humoral and Cellular Immunity. Vaccines 2024, 12, 729. [Google Scholar] [CrossRef]
- Brown, J.; Baisley, K.; Kavishe, B.; Changalucha, J.; Andreasen, A.; Mayaud, P.; Gumodoka, B.; Kapiga, S.; Hayes, R.; Watson-Jones, D. Impact of Malaria and Helminth Infections on Immunogenicity of the Human Papillomavirus-16/18 AS04-Adjuvanted Vaccine in Tanzania. Vaccine 2014, 32, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.H.; Gazzinelli-guimaraes, P.H.; Howard, J.; Barnafo, E.; Alani, N.A.H.; Muratova, O.; Mccormack, A.; Kelnhofer, E.; Urban, J.F.; Narum, D.L.; et al. Chronic Helminth Infection Does Not Impair Immune Response to Malaria Transmission Blocking Vaccine Pfs230D1-EPA/Alhydrogel Ò in Mice. Vaccine 2019, 37, 1038–1045. [Google Scholar] [CrossRef]
- Flügge, J.; Adegnika, A.A.; Honkpehedji, Y.J.; Sandri, T.L.; Askani, E.; Manouana, G.P.; Loembe, M.M.; Brückner, S.; Duali, M.; Strunk, J.; et al. Impact of Helminth Infections during Pregnancy on Vaccine Immunogenicity in Gabonese Infants. Vaccines 2020, 8, 381. [Google Scholar] [CrossRef]
- Zhang, Y.; Hardy, L.C.; Kapita, C.M.; Hall, J.A.; Arbeeva, L.; Campbell, E.; Urban, J.F.; Belkaid, Y.; Nagler, C.R.; Iweala, O.I. Intestinal Helminth Infection Impairs Oral and Parenteral Vaccine Efficacy. J. Immunol. 2023, 211, 389–402. [Google Scholar] [CrossRef]
- Muir, R.; Metcalf, T.; Fourati, S.; Bartsch, Y.; Kyosiimire-Lugemwa, J.; Canderan, G.; Alter, G.; Muyanja, E.; Okech, B.; Namatovu, T.; et al. Schistosoma mansoni Infection Alters the Host Pre-Vaccination Environment Resulting in Blunted Hepatitis B Vaccination Immune Responses. PLoS Negl. Trop. Dis. 2023, 17, e0011089. [Google Scholar] [CrossRef]
- Stetter, N.; Hartmann, W.; Brunn, M.L.; Stanelle-Bertram, S.; Gabriel, G.; Breloer, M. A Combination of Deworming and Prime-Boost Vaccination Regimen Restores Efficacy of Vaccination Against Influenza in Helminth-Infected Mice. Front. Immunol. 2021, 12, 784141. [Google Scholar] [CrossRef]
- Hartmann, W.; Brunn, M.L.; Stetter, N.; Gabriel, G.; Breloer, M. Pre-Existing Helminth Infection Impairs the Efficacy of Adjuvanted Influenza Vaccination in Mice. PLoS ONE 2022, 17, e0266456. [Google Scholar] [CrossRef]
- Wajja, A.; Kizito, D.; Nassanga, B.; Nalwoga, A.; Kabagenyi, J.; Kimuda, S.; Galiwango, R.; Mutonyi, G.; Vermaak, S.; Satti, I.; et al. The Effect of Current Schistosoma mansoni Infection on the Immunogenicity of a Candidate TB Vaccine, MVA85A, in BCG-Vaccinated Adolescents: An Open-Label Trial. PLoS Negl. Trop. Dis. 2017, 11, e0005440. [Google Scholar] [CrossRef]
- Brückner, S.; Agnandji, S.T.; Berberich, S.; Bache, E.; Fernandes, J.F.; Schweiger, B.; Loembe, M.M.; Engleitner, T.; Lell, B.; Mordmüller, B.; et al. Effect of Antihelminthic Treatment on Vaccine Immunogenicity to a Seasonal Influenza Vaccine in Primary School Children in Gabon: A Randomized Placebo-Controlled Trial. PLoS Negl. Trop. Dis. 2015, 9, e0003768. [Google Scholar] [CrossRef] [PubMed]
- Nash, S.; Mentzer, A.J.; Lule, S.A.; Kizito, D.; Smits, G.; van der Klis, F.R.M.; Elliott, A.M. The Impact of Prenatal Exposure to Parasitic Infections and to Anthelminthic Treatment on Antibody Responses to Routine Immunisations given in Infancy: Secondary Analysis of a Randomised Controlled Trial. PLoS Negl. Trop. Dis. 2017, 11, e0005213. [Google Scholar] [CrossRef]
- Victoria, L. Pre-Vaccination Schistosoma mansoni and Hookworm Infections Are Associated with Altered Vaccine Immune Responses: A Longitudinal Analysis among Adolescents Living in Helminth-Endemic Islands of Lake Victoria, Uganda. Front. Immunol. 2024, 69, 1460183. [Google Scholar] [CrossRef]
- Nouatin, O.; Mengue, J.B.; Dejon-Agobé, J.C.; Fendel, R.; Ibáñez, J.; Ngoa, U.A.; Edoa, J.R.; Adégbité, B.R.; Honkpéhédji, Y.J.; Zinsou, J.F.; et al. Exploratory Analysis of the Effect of Helminth Infection on the Immunogenicity and Efficacy of the Asexual Blood-Stage Malaria Vaccine Candidate Gmz2. PLoS Negl. Trop. Dis. 2021, 15, e0009361. [Google Scholar] [CrossRef]
- Id, H.B.; Lhomme, E.; Sure, M.; Robinson, C.; Bockstal, V.; Valea, I.; Somda, S.; Tinto, H.; Lacabaratz, C.; Meda, N.; et al. Helminth Exposure and Immune Response to MVA-BN-Filo Ebola Vaccine Regimen. PLoS Negl. Trop. Dis. 2024, 118, e0011500. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).