An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Animals
2.3. mRNA Design
2.4. Preparation and Characterization of MPhXV-mRNA
2.5. Validation of mRNA Expression
2.6. Preparation and Characterization of Mrna-3012-LNP
2.7. Mouse Vaccination
2.8. Serum-Neutralizing Antibody Assay
2.9. Challenge Experiments and Seroprotection Experiments
2.10. Evaluation of MPXV-Specific Antibody Titers
2.11. Elispot Assay
2.12. Flow Cytometry Assay
2.13. In Vivo Toxicity Evaluation
3. Results
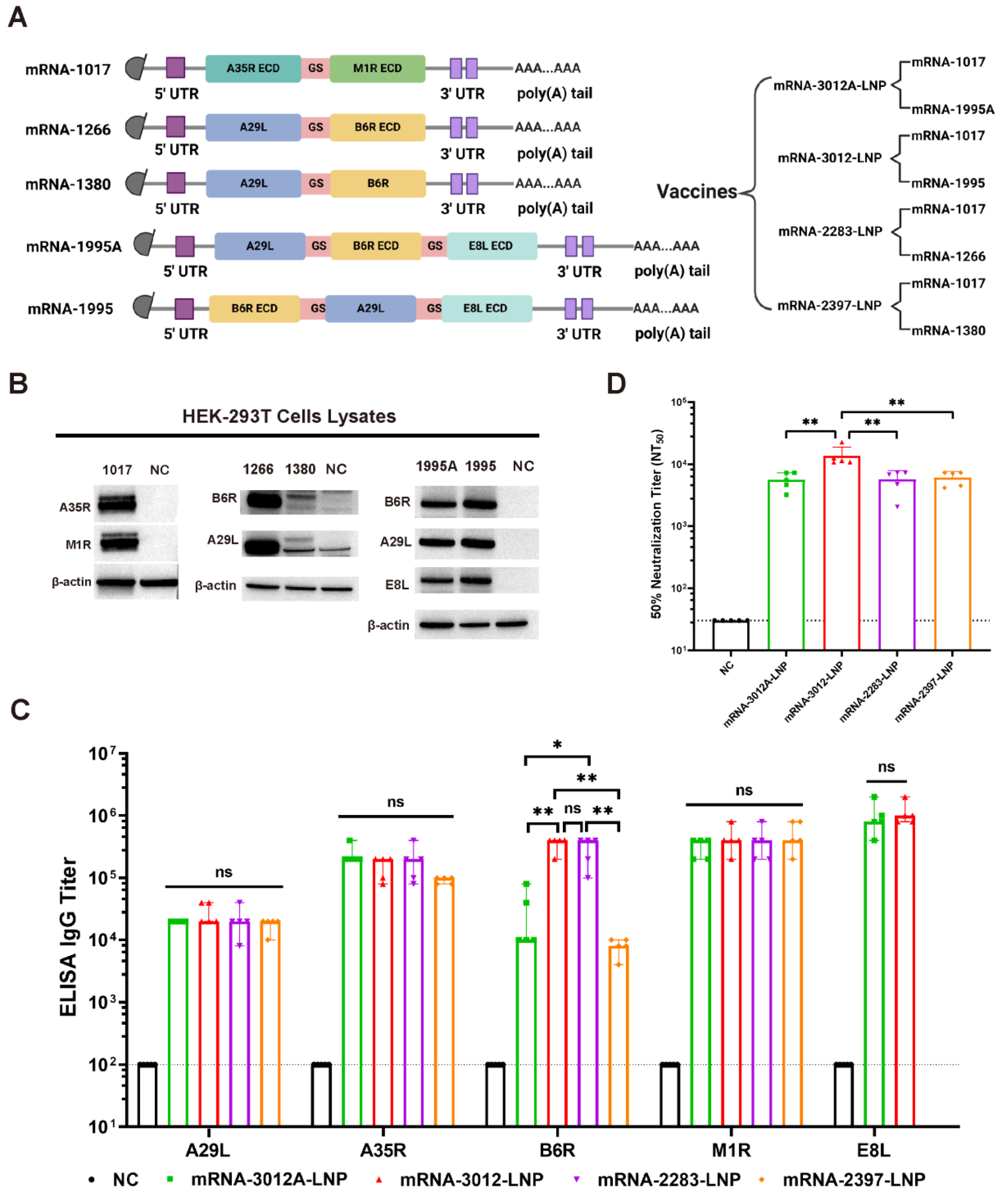
3.1. Design, Screening, and Characterization of MPXV Bivalent mRNA Vaccine Candidates
3.2. Systematic Evaluation of the Effect of mRNA-3012-LNP on Humoral Immunization in Mice In Vivo
3.3. MPXV-Specific Cellular Immune Response in Mice Vaccinated with mRNA-3012-LNP
3.4. Protection Efficacy of mRNA-3012-LNP Against VACV Challenge in Mice
3.5. Acute and Immunological Toxicity Evaluation of the mRNA-3012-LNP Vaccine in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, C.T.; Wenner, H.A. Monkeypox Virus. Bacteriol. Rev. 1973, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Second Meeting of the International Health Regulations (2005) (IHR) Emergency Committee Regarding the Multi-Country Outbreak of Monkeypox. Available online: https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox (accessed on 19 August 2024).
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef]
- Fifth Meeting of the International Health Regulations (2005) (IHR) Emergency Committee on the Multi-Country Outbreak of mpox (Monkeypox). Available online: https://www.who.int/news/item/11-05-2023-fifth-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-on-the-multi-country-outbreak-of-monkeypox-(mpox) (accessed on 6 November 2024).
- Kibungu, E.M.; Vakaniaki, E.H.; Kinganda-Lusamaki, E.; Kalonji-Mukendi, T.; Pukuta, E.; Hoff, N.A.; Bogoch, I.I.; Cevik, M.; Gonsalves, G.S.; Hensley, L.E.; et al. Clade I–Associated Mpox Cases Associated with Sexual Contact, the Democratic Republic of the Congo. Emerg. Infect. Dis. 2024, 30, 172–176. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern (accessed on 29 October 2024).
- World Health Organization. Global Mpox Trends. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 21 March 2025).
- Aden, D.; Zaheer, S.; Kumar, R.; Ranga, S. Monkeypox (Mpox) Outbreak during COVID-19 Pandemic—Past and the Future. J. Med. Virol. 2023, 95, e28701. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Pittman, P.R. Mpox (Monkeypox) in Pregnancy: Viral Clade Differences and Their Associations with Varying Obstetrical and Fetal Outcomes. Viruses 2023, 15, 1649. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; Bondy, L.; Hanage, W.P. Monkeypox Virus Infections in Humans. Clin. Microbiol. Rev. 2022, 35, e0009222. [Google Scholar] [CrossRef]
- Gessain, A.; Nakoune, E.; Yazdanpanah, Y. Monkeypox. N. Engl. J. Med. 2022, 387, 1783–1793. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, C.C.; Lee, J.C.; Chiu, C.W.; Hsueh, P.R.; Ko, W.C. A Brief on New Waves of Monkeypox and Vaccines and Antiviral Drugs for Monkeypox. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2022, 55, 795–802. [Google Scholar] [CrossRef]
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Thompson, E.; Wilhelmsen, C.; Zimmerman, M.; Ichou, M.A.; Steffen, S.E.; Schmaljohn, C.S.; Schmaljohn, A.L.; Jahrling, P.B. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J. Virol. 2004, 78, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Mucker, E.M.; Golden, J.W.; Hammerbeck, C.D.; Kishimori, J.M.; Royals, M.; Joselyn, M.D.; Ballantyne, J.; Nalca, A.; Hooper, J.W. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J. Virol. 2022, 96, e01504-21. [Google Scholar] [CrossRef]
- Jezek, Z.; Marennikova, S.S.; Mutumbo, M.; Nakano, J.H.; Paluku, K.M.; Szczeniowski, M. Human Monkeypox: A Study of 2510 Contacts of 214 Patients. J. Infect. Dis. 1986, 154, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.T.; Lawrence, S.J.; Wagner, E.; Nopora, K.; Rösch, S.; Young, P.; Schmidt, D.; Kreusel, C.; De Carli, S.; Meyer, T.P.; et al. Immunogenicity and Safety of Three Consecutive Production Lots of the Non Replicating Smallpox Vaccine MVA: A Randomised, Double Blind, Placebo Controlled Phase III Trial. PLoS ONE 2018, 13, e0195897. [Google Scholar] [CrossRef]
- Turner Overton, E.; Schmidt, D.; Vidojkovic, S.; Menius, E.; Nopora, K.; Maclennan, J.; Weidenthaler, H. A Randomized Phase 3 Trial to Assess the Immunogenicity and Safety of 3 Consecutively Produced Lots of Freeze-Dried MVA-BN® Vaccine in Healthy Adults. Vaccine 2023, 41, 397–406. [Google Scholar] [CrossRef]
- Eto, A.; Saito, T.; Yokote, H.; Kurane, I.; Kanatani, Y. Recent Advances in the Study of Live Attenuated Cell-Cultured Smallpox Vaccine LC16m8. Vaccine 2015, 33, 6106–6111. [Google Scholar] [CrossRef]
- Pischel, L.; Martini, B.A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K.M.; Grimshaw, A.A.; Malik, A.A.; et al. Vaccine Effectiveness of 3rd Generation Mpox Vaccines against Mpox and Disease Severity: A Systematic Review and Meta-Analysis. Vaccine 2024, 42, 126053. [Google Scholar] [CrossRef]
- Shchelkunova, G.A.; Shchelkunov, S.N. Smallpox, Monkeypox and Other Human Orthopoxvirus Infections. Viruses 2022, 15, 103. [Google Scholar] [CrossRef]
- Monath, T.P.; Caldwell, J.R.; Mundt, W.; Fusco, J.; Johnson, C.S.; Buller, M.; Liu, J.; Gardner, B.; Downing, G.; Blum, P.S.; et al. ACAM2000 Clonal Vero Cell Culture Vaccinia Virus (New York City Board of Health Strain)—A Second-Generation Smallpox Vaccine for Biological Defense. Int. J. Infect. Dis. 2004, 8, 31–44. [Google Scholar] [CrossRef]
- Nalca, A.; Zumbrun, E.E. ACAM2000TM: The New Smallpox Vaccine for United States Strategic National Stockpile. Drug Des. Dev. Ther. 2010, 4, 71–79. [Google Scholar]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed]
- Zaeck, L.M.; Lamers, M.M.; Verstrepen, B.E.; Bestebroer, T.M.; van Royen, M.E.; Götz, H.; Shamier, M.C.; van Leeuwen, L.P.M.; Schmitz, K.S.; Alblas, K.; et al. Low Levels of Monkeypox Virus-Neutralizing Antibodies after MVA-BN Vaccination in Healthy Individuals. Nat. Med. 2023, 29, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef] [PubMed]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Sakhatskyy, P.; Wang, S.; Chou, T.W.; Lu, S. Immunogenicity and Protection Efficacy of Monovalent and Polyvalent Poxvirus Vaccines That Include the D8 Antigen. Virology 2006, 355, 164. [Google Scholar] [CrossRef]
- Hooper, J.W.; Custer, D.M.; Thompson, E. Four-Gene-Combination DNA Vaccine Protects Mice against a Lethal Vaccinia Virus Challenge and Elicits Appropriate Antibody Responses in Nonhuman Primates. Virology 2003, 306, 181. [Google Scholar] [CrossRef]
- Sang, Y.; Zhang, Z.; Liu, F.; Lu, H.; Yu, C.; Sun, H.; Long, J.; Cao, Y.; Mai, J.; Miao, Y.; et al. Monkeypox Virus Quadrivalent mRNA Vaccine Induces Immune Response and Protects against Vaccinia Virus. Signal Transduct. Target. Ther. 2023, 8, 172. [Google Scholar] [CrossRef]
- Zuiani, A.; Dulberger, C.L.; Silva, N.S.D.; Marquette, M.; Lu, Y.-J.; Palowitch, G.M.; Dokic, A.; Sanchez-Velazquez, R.; Schlatterer, K.; Sarkar, S.; et al. A Multivalent mRNA Monkeypox Virus Vaccine (BNT166) Protects Mice and Macaques from Orthopoxvirus Disease. Cell 2024, 187, 1363–1373.e12. [Google Scholar] [CrossRef]
- Freyn, A.W.; Atyeo, C.; Earl, P.L.; Americo, J.L.; Chuang, G.-Y.; Natarajan, H.; Frey, T.R.; Gall, J.G.; Moliva, J.I.; Hunegnaw, R.; et al. An Mpox Virus mRNA-Lipid Nanoparticle Vaccine Confers Protection against Lethal Orthopoxviral Challenge. Sci. Transl. Med. 2023, 15, eadg3540. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of Protection against Mpox Following mRNA or Modified Vaccinia Ankara Vaccination in Nonhuman Primates. Cell 2024, 187, 5540–5553.e10. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Peng, L.; Han, Y.; Wang, D.; He, X.; Wang, J.; Ou, C. Lipid Nanoparticle-Based mRNA Vaccines in Cancers: Current Advances and Future Prospects. Front. Immunol. 2022, 13, 922301. [Google Scholar] [CrossRef]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.M.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. mRNA Vaccines Encoding Fusion Proteins of Monkeypox Virus Antigens Protect Mice from Vaccinia Virus Challenge. Nat. Commun. 2023, 14, 5925. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Yu, C.; Cao, Y.; Miao, Y.; Sun, H.; Zhang, Z.; Mai, J.; Wang, X.; Mao, Y.; Li, H.; et al. A Rabies mRNA Vaccine Provides a Rapid and Long-Term Immune Response in Mice. Nano Today 2023, 53, 102038. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, C.; Zhou, S.; Guo, Y.; Zuo, Q.; Ma, J.; Liu, S.; Wu, X.; Peng, Z.; Fan, T.; et al. Bioluminescent Imaging of Vaccinia Virus Infection in Immunocompetent and Immunodeficient Rats as a Model for Human Smallpox. Sci. Rep. 2015, 5, 11397. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Q.; Xie, H.; Chen, R.; Huang, W.; Liang, C.; Xiao, X.; Yu, Y.; Wang, Y. Screening and Evaluation of Potential Inhibitors against Vaccinia Virus from 767 Approved Drugs. J. Med. Virol. 2019, 91, 2016–2024. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 Neutralizing Antibody by a Pseudotyped Virus-Based Assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liu, Q.; Huang, W.; Nie, J.; Wang, Y. A Bioluminescent Imaging Mouse Model for Marburg Virus Based on a Pseudovirus System. Hum. Vaccin Immunother. 2017, 13, 1811–1817. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Long, J.; Sun, H.; Miao, Y.; Sang, Y.; Lu, H.; Yu, C.; Zhang, Z.; Wang, L.; Yang, J.; et al. Dendritic Cell-Mimicking Nanoparticles Promote mRNA Delivery to Lymphoid Organs. Adv. Sci. Weinh 2023, 10, e2302423. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA Vaccine Development. Signal Transduct. Target.Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817. [Google Scholar] [CrossRef]
- Ahmed, R.; Gray, D. Immunological Memory and Protective Immunity: Understanding Their Relation. Science 1996, 272, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From Vaccines to Memory and Back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, Z.-J.; Zhu, Y.-L.; Tang, W.; Zhou, C.; Zhao, S.-Q.; Wu, M.; Ming, T.; Deng, Y.-Q.; Chen, Q.; et al. Rational Development of Multicomponent mRNA Vaccine Candidates against Mpox. Emerg. Microbes Infect. 2023, 12, 2192815. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, J.; Wu, J.; Zhang, Z.; Long, J.; Yu, C.; Liao, Y.; Zhang, H.; Yang, J. An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus. Vaccines 2025, 13, 374. https://doi.org/10.3390/vaccines13040374
Zuo J, Wu J, Zhang Z, Long J, Yu C, Liao Y, Zhang H, Yang J. An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus. Vaccines. 2025; 13(4):374. https://doi.org/10.3390/vaccines13040374
Chicago/Turabian StyleZuo, Jun, Jiayu Wu, Zhen Zhang, Jinrong Long, Changxiao Yu, Yuqin Liao, Hongsheng Zhang, and Jing Yang. 2025. "An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus" Vaccines 13, no. 4: 374. https://doi.org/10.3390/vaccines13040374
APA StyleZuo, J., Wu, J., Zhang, Z., Long, J., Yu, C., Liao, Y., Zhang, H., & Yang, J. (2025). An Mpox Multi-Antigen-Tandem Bivalent mRNA Candidate Vaccine Effectively Protects Mice Against the Vaccinia Virus. Vaccines, 13(4), 374. https://doi.org/10.3390/vaccines13040374





