Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccines
2.2. Clinical Trial Design
2.3. Determination of Neutralizing Antibody Titers
2.4. Determination of IgG and IgA Antibody Titers
2.5. Data Selection for Pre-Blinding Analysis
2.6. Analytical Methods
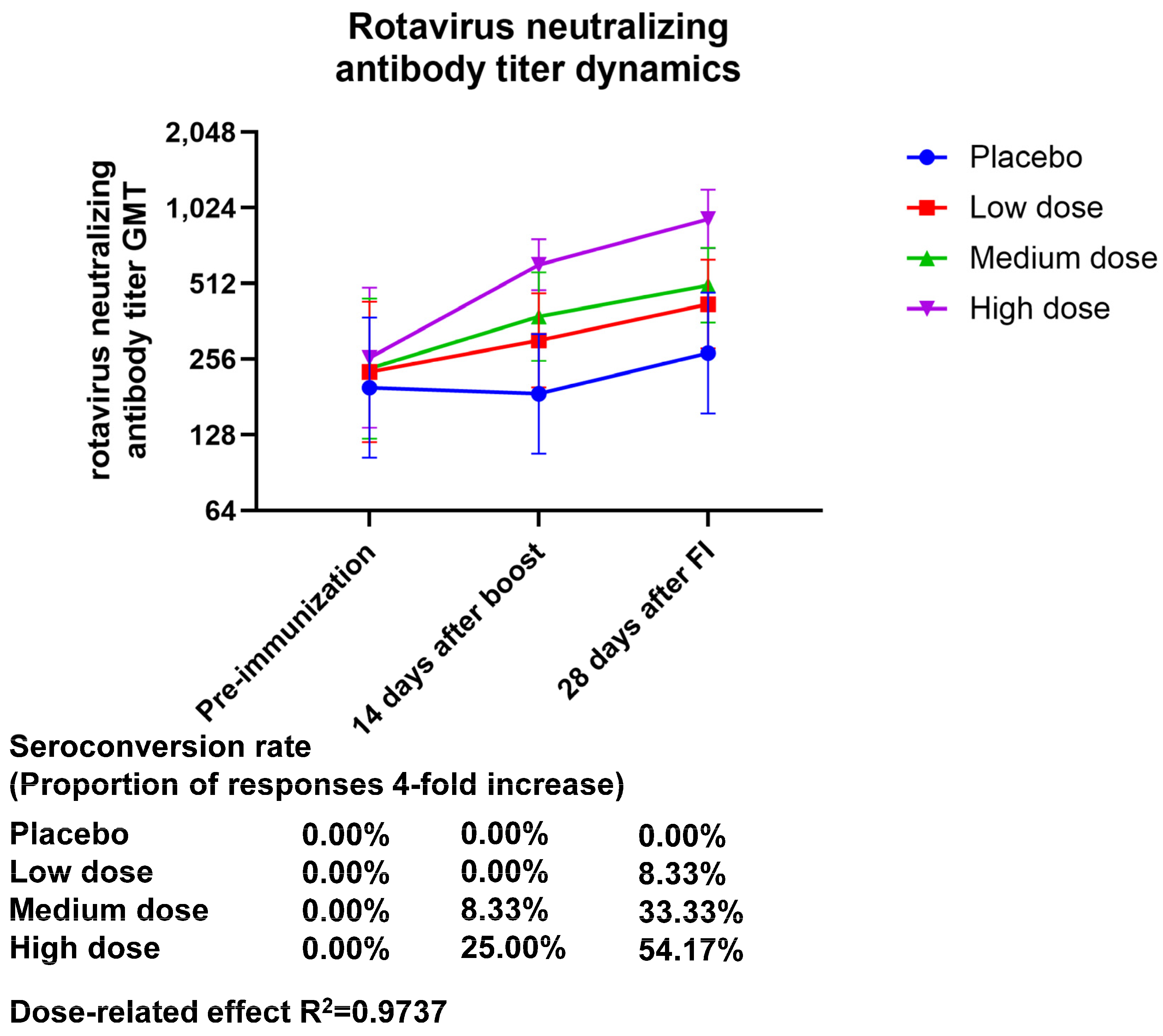
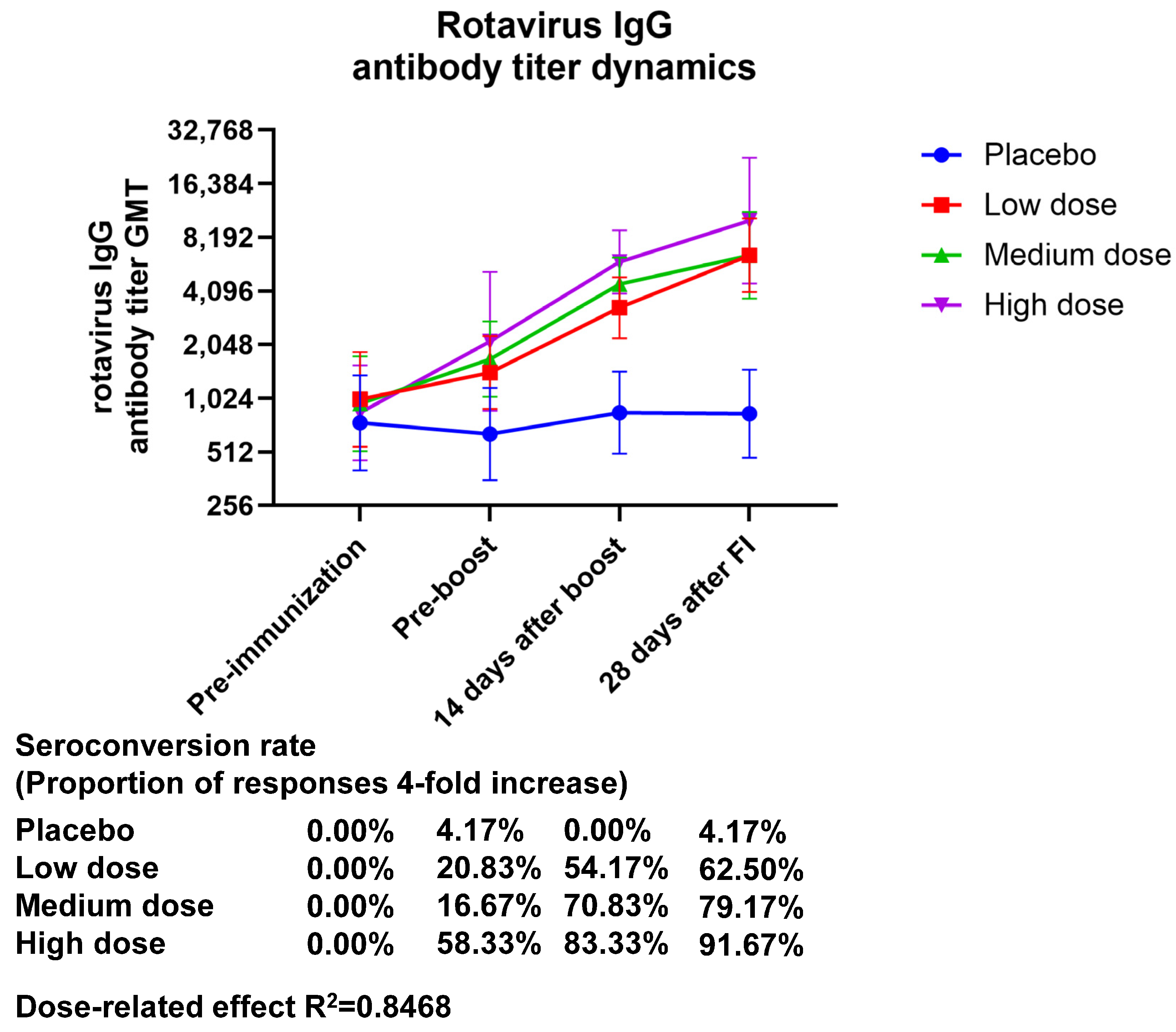
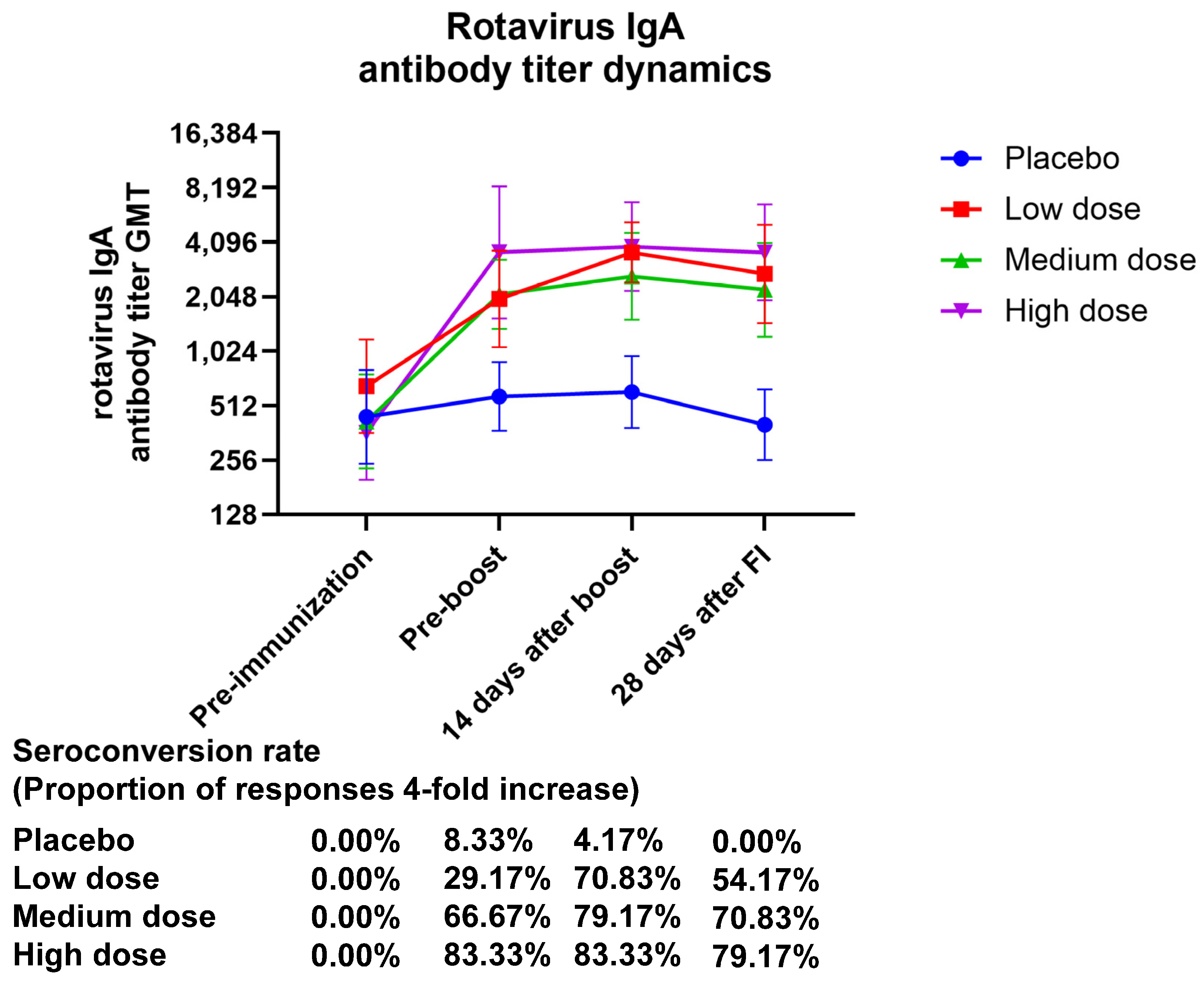
3. Results
3.1. Safety
3.2. Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Middleton, B.F.; Danchin, M.; Fathima, P.; Bines, J.E.; Macartney, K.; Snelling, T.L. Review of the health impact of the oral rotavirus vaccine program in children under 5 years in Australia: 2006–2021. Vaccine 2023, 41, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.; Pereira, P.; Benninghoff, B. Rotavirus vaccination and intussusception: A paradigm shift? Hum. Vaccines Immunother. 2021, 17, 278–282. [Google Scholar] [CrossRef]
- Bernstein, D.I. Rotavirus Vaccines-Going Strong After 15 Years. JAMA Pediatr. 2021, 175, e210356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Chen, N.; Pang, B.; Liu, M.; Cai, K.; Kobayashi, N. Surveillance of Human Rotaviruses in Wuhan, China (2019–2022): Whole-Genome Analysis of Emerging DS-1-like G8P [8] Rotavirus. Int. J. Mol. Sci. 2023, 24, 12189. [Google Scholar] [CrossRef]
- Hasso-Agopsowicz, M.; Ladva, C.N.; Lopman, B.; Sanderson, C.; Cohen, A.L.; Tate, J.E.; Riveros, X.; Henao-Restrepo, A.M.; Clark, A.; Alkorta, M.; et al. Global Review of the Age Distribution of Rotavirus Disease in Children Aged <5 Years Before the Introduction of Rotavirus Vaccination. Clin. Infect. Dis. 2019, 69, 1071–1078. [Google Scholar] [CrossRef]
- Glass, R.I.; Tate, J.E.; Jiang, B.; Parashar, U. The Rotavirus Vaccine Story: From Discovery to the Eventual Control of Rotavirus Disease. J. Infect. Dis. 2021, 224, S331–S342. [Google Scholar] [CrossRef]
- Carcamo-Calvo, R.; Munoz, C.; Buesa, J.; Rodriguez-Diaz, J.; Gozalbo-Rovira, R. The Rotavirus Vaccine Landscape, an Update. Pathogens 2021, 10, 520. [Google Scholar] [CrossRef]
- Barsoum, Z. Regional hospitalisation and seasonal variations of Pediatric rotavirus gastroenteritis pre- and post-RV vaccination: A prospective and retrospective study. World J. Pediatr. 2022, 18, 404–416. [Google Scholar] [CrossRef]
- Yu, J.; Lai, S.; Geng, Q.; Ye, C.; Zhang, Z.; Zheng, Y.; Wang, L.; Duan, Z.; Zhang, J.; Wu, S.; et al. Prevalence of rotavirus and rapid changes in circulating rotavirus strains among children with acute diarrhea in China, 2009–2015. J. Infect. 2019, 78, 66–74. [Google Scholar] [CrossRef]
- Chen, S.; Gao, S.; Li, J.; Li, J.; Duan, Z.J. Cost-benefit analysis of rotavirus vaccine included in the national immunization program in China. Vaccine 2023, 41, 547–554. [Google Scholar] [CrossRef]
- Xia, S.; Du, J.; Su, J.; Liu, Y.; Huang, L.; Yu, Q.; Xie, Z.; Gao, J.; Xu, B.; Gao, X.; et al. Efficacy, immunogenicity and safety of a trivalent live human-lamb reassortant rotavirus vaccine (LLR3) in healthy Chinese infants: A randomized, double-blind, placebo-controlled trial. Vaccine 2020, 38, 7393–7400. [Google Scholar] [CrossRef] [PubMed]
- Skansberg, A.; Sauer, M.; Tan, M.; Santosham, M.; Jennings, M.C. Product review of the rotavirus vaccines ROTASIIL, ROTAVAC, and Rotavin-M1. Hum. Vaccines Immunother. 2021, 17, 1223–1234. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, Y.; Liang, Z.; Tian, Y.; Liu, B.; Gao, Z.; Jia, L.; Chen, L.; Wang, Q. Effectiveness of Lanzhou lamb rotavirus vaccine in preventing gastroenteritis among children younger than 5 years of age. Vaccine 2019, 37, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Buyse, H.; Vinals, C.; Karkada, N.; Han, H.H. The human rotavirus vaccine Rotarix in infants: An integrated analysis of safety and reactogenicity. Hum. Vaccines Immunother. 2014, 10, 19–24. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Q.; Liu, Y.; Lv, H.; Mo, Z.; Li, F.; Yu, Q.; Jin, F.; Chen, W.; Zhang, Y.; et al. Efficacy, safety and immunogenicity of hexavalent rotavirus vaccine in Chinese infants. Virol. Sin. 2022, 37, 724–730. [Google Scholar] [CrossRef]
- Witte, D.; Handley, A.; Jere, K.C.; Bogandovic-Sakran, N.; Mpakiza, A.; Turner, A.; Pavlic, D.; Boniface, K.; Mandolo, J.; Ong, D.S.; et al. Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: A randomised, double-blind, four-arm parallel group dose-ranging study. Lancet Infect. Dis. 2022, 22, 668–678. [Google Scholar] [CrossRef]
- Singh, T.; Delannois, F.; Haguinet, F.; Molo, L.Y. Review of Over 15 Years Postmarketing Safety Surveillance Spontaneous Data for the Human Rotavirus Vaccine (Rotarix) on Intussusception. Drug Saf. 2022, 45, 155–168. [Google Scholar] [CrossRef]
- Cates, J.E.; Amin, A.B.; Tate, J.E.; Lopman, B.; Parashar, U. Do Rotavirus Strains Affect Vaccine Effectiveness? A Systematic Review and Meta-analysis. Pediatr. Infect. Dis. J. 2021, 40, 1135–1143. [Google Scholar] [CrossRef]
- Murunga, N.; Otieno, G.P.; Maia, M.; Agoti, C.N. Effectiveness of Rotarix ((R)) vaccine in Africa in the first decade of progressive introduction, 2009–2019: Systematic review and meta-analysis. Wellcome Open Res. 2020, 5, 187. [Google Scholar] [CrossRef]
- Middleton, B.F.; Jones, M.A.; Waddington, C.S.; Danchin, M.; McCallum, C.; Gallagher, S.; Leach, A.J.; Andrews, R.; Kirkwood, C.; Cunliffe, N.; et al. The ORVAC trial protocol: A phase IV, double-blind, randomised, placebo-controlled clinical trial of a third scheduled dose of Rotarix rotavirus vaccine in Australian Indigenous infants to improve protection against gastroenteritis. BMJ Open 2019, 9, e032549. [Google Scholar] [CrossRef]
- Ella, R.; Babji, S.; Ciarlet, M.; Blackwelder, W.C.; Vadrevu, K.M. A randomized, open-labelled, non-inferiority phase 4 clinical trial to evaluate the immunogenicity and safety of the live, attenuated, oral rotavirus vaccine, ROTAVAC(R) in comparison with a licensed rotavirus vaccine in healthy infants. Vaccine 2019, 37, 4407–4413. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.Y.; Yang, H.; Zhang, J.; Li, Y.F.; Hou, A.C.; Ma, L.; Sun, L.W.; Wang, C.X. Child rotavirus infection in association with acute gastroenteritis in two Chinese sentinel hospitals. Pediatr. Int. 2000, 42, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ren, S.; Chen, L.; Xue, J.; Shao, X.; Zhang, T.; Zhao, G. Rotavirus Infection in Children < 5 Years of Age in Suzhou, China, 2013-2019: Disease Burden, Genotype Distribution and Seasonality. Pediatr. Infect. Dis. J. 2022, 41, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Dong, Z.; Shen, J.; Yang, Z.; Liao, Y.; Hu, W.; Pei, S.; Shaman, J. Rotavirus Gastroenteritis Infection Among Children Vaccinated and Unvaccinated With Rotavirus Vaccine in Southern China: A Population-Based Assessment. JAMA Netw. Open 2018, 1, e181382. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, G.; Zhang, W.; Qian, F.; Li, Y.; Zhang, M.; Gu, S.; Wang, M.; Lin, F.; Hu, Y.; et al. Rotavirus infection and its genetic characterization in non-hospitalized adults with acute gastroenteritis in Shanghai, China. Arch. Virol. 2013, 158, 1671–1677. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Banyai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Banyai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, W.; Pu, J.; Liu, Y.; Huang, L.L.; Zhou, Y.; Gao, J.M.; Tan, J.B.; Liu, X.L.; Yang, J.; et al. A randomized, double-blind, placebo-controlled phase I clinical trial of rotavirus inactivated vaccine (Vero cell) in a healthy adult population aged 18-49 years to assess safety and preliminary observation of immunogenicity. Vaccine 2024, 42, 4030–4039. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). Available online: https://ich.org (accessed on 9 March 2021).
- Dang, D.A.; Nguyen, V.T.; Vu, D.T.; Nguyen, T.H.; Nguyen, D.M.; Yuhuan, W.; Baoming, J.; Nguyen, D.H.; Le, T.L.; Rotavin, M.V.T.G. A dose-escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin-M1) in Vietnamese children. Vaccine 2012, 30, A114–A121. [Google Scholar] [CrossRef]
- Luna, E.J.; Frazatti-Gallina, N.M.; Timenetsky, M.C.; Cardoso, M.R.; Veras, M.A.; Miraglia, J.L.; Escobar, A.M.; Grisi, S.J.; Raw, I.; Precioso, A.R. A phase I clinical trial of a new 5-valent rotavirus vaccine. Vaccine 2013, 31, 1100–1105. [Google Scholar] [CrossRef]
- Wu, Z.W.; Li, Q.L.; Zhou, H.S.; Duan, K.; Gao, Z.; Zhang, X.J.; Jiang, Z.J.; Hao, Z.Y.; Jin, F.; Bai, X.; et al. Safety and immunogenicity of a novel oral hexavalent rotavirus vaccine: A phase I clinical trial. Hum. Vaccines Immunother. 2021, 17, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.B.; Lim, F.S.; Quak, S.H.; Lee, B.W.; Teoh, Y.L.; Suryakiran, P.V.; Han, H.H.; Bock, H.L. Efficacy, Immunogenicity and Safety of a Human Rotavirus Vaccine RIX4414 in Singaporean Infants. Ann. Acad. Med. Singap. 2016, 45, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Huang, T.; Li, Y.; Wang, L.H.; Tao, J.; Fu, B.; Si, G.; Nong, Y.; Mo, Z.; Liao, X.; et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum. Vaccines Immunother. 2016, 12, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Huang, T.; Li, Y.; Luo, D.; Tao, J.; Fu, B.; Si, G.; Nong, Y.; Mo, Z.; Liao, X.; et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: A randomized, placebo-controlled trial in China. Hum. Vaccines Immunother. 2014, 10, 11–18. [Google Scholar] [CrossRef]
- Kanungo, S.; Chatterjee, P.; Bavdekar, A.; Murhekar, M.; Babji, S.; Garg, R.; Samanta, S.; Nandy, R.K.; Kawade, A.; Boopathi, K.; et al. Safety and immunogenicity of the Rotavac and Rotasiil rotavirus vaccines administered in an interchangeable dosing schedule among healthy Indian infants: A multicentre, open-label, randomised, controlled, phase 4, non-inferiority trial. Lancet Infect. Dis. 2022, 22, 1191–1199. [Google Scholar] [CrossRef]
- Moon, S.S.; Richter-Roche, M.; Resch, T.K.; Wang, Y.; Foytich, K.R.; Wang, H.; Mainou, B.A.; Pewin, W.; Lee, J.; Henry, S.; et al. Microneedle patch as a new platform to effectively deliver inactivated polio vaccine and inactivated rotavirus vaccine. NPJ Vaccines 2022, 7, 26. [Google Scholar] [CrossRef]
- Resch, T.K.; Wang, Y.; Moon, S.S.; Joyce, J.; Li, S.; Prausnitz, M.; Jiang, B. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. Sci. Rep. 2018, 8, 561. [Google Scholar] [CrossRef]
- Wang, Y.; Azevedo, M.; Saif, L.J.; Gentsch, J.R.; Glass, R.I.; Jiang, B. Inactivated rotavirus vaccine induces protective immunity in gnotobiotic piglets. Vaccine 2010, 28, 5432–5436. [Google Scholar] [CrossRef]
| Placebo (N = 24) | Low Dose (N = 24) | Medium Dose (N = 24) | High Dose (N = 24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | No. of Events | N (%) | p | No. of Events | N (%) | p | No. of Events | N (%) | p | No. of Events | |
| Total adverse events | 2 (8.33) | 3 | 9 (37.50) | 0.016 | 12 | 3 (12.50) | >0.999 | 5 | 1 (4.17) | >0.999 | 2 |
| Local adverse events | 1 (4.17) | 1 | 6 (25.00) | 0.102 | 6 | 1 (4.17) | >0.999 | 2 | 1 (4.17) | >0.999 | 2 |
| Systemic adverse events | 2 (8.33) | 2 | 4 (16.67) | 0.663 | 6 | 3 (12.50) | >0.999 | 3 | 0 (0.00) | 0.470 | 0 |
| Placebo (N = 24) | Low Dose (N = 24) | Medium Dose (N = 24) | High Dose (N = 24) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | No. of Events | N (%) | No. of Events | N (%) | No. of Events | N (%) | No. of Events | ||
| Total adverse reactions | Grade 1 | 2(8.33) | 3 | 8 (33.33) | 10 | 3 (12.50) | 4 | 1 (4.17) | 2 |
| Grade 2 | 0 (0.00) | 0 | 2 (8.33) | 2 | 1 (4.17) | 1 | 0 (0.00) | 0 | |
| Grade 3 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | |
| Local adverse reactions | Grade 1 | 1(4.17) | 1 | 6 (25.00) | 6 | 1 (4.17) | 1 | 1(4.17) | 2 |
| Grade 2 | 0 (0.00) | 0 | 0 (0.00) | 0 | 1 (4.17) | 1 | 0 (0.00) | 0 | |
| Grade 3 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | |
| Systemic adverse reactions | Grade 1 | 2 (8.33) | 2 | 3 (12.50) | 4 | 3 (12.50) | 3 | 0 (0.00) | 0 |
| Grade 2 | 0 (0.00) | 0 | 2 (8.33) | 2 | 0 (0.00) | 0 | 0 (0.00) | 0 | |
| Grade 3 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 | |
| Intensity | Group (N = 24) | Grade 1 N (%) | Grade 2 N (%) | Grade 3 N (%) |
|---|---|---|---|---|
| Respiratory, thoracic, and mediastinal disorders | ||||
| Cough | Placebo | 1 (4.17) | 0 (0) | 0 (0) |
| Low dose | 1 (4.17) | 0 (0) | 0 (0) | |
| Medium dose | 0 (0) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| General disorders and administration site conditions | ||||
| Pyrexia | Placebo | 0 (0) | 0 (0) | 0 (0) |
| Low dose | 1 (4.17) | 2 (8.33) | 0 (0) | |
| Medium dose | 1 (4.17) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| Fatigue | Placebo | 0 (0) | 0 (0) | 0 (0) |
| Low dose | 0 (0) | 0 (0) | 0 (0) | |
| Medium dose | 1 (4.17) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| Vaccination site erythema | Placebo | 0 (0) | 0 (0) | 0 (0) |
| Low dose | 0 (0) | 0 (0) | 0 (0) | |
| Medium dose | 0 (0) | 1 (4.17) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| Site of vaccination painful | Placebo | 1 (4.17) | 0 (0) | 0 (0) |
| Low dose | 6 (25.00) | 0 (0) | 0 (0) | |
| Medium dose | 0 (0) | 0 (0) | 0 (0) | |
| High dose | 1 (4.17) | 0 (0) | 0 (0) | |
| Vaccination site swelling | Placebo | 0 (0) | 0 (0) | 0 (0) |
| Low dose | 1 (4.17) | 0 (0) | 0 (0) | |
| Medium dose | 0 (0) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| Gastrointestinal disorders | ||||
| Constipation | Placebo | 0 (0) | 0 (0) | 0 (0) |
| Low dose | 1 (4.17) | 0 (0) | 0 (0) | |
| Medium dose | 0 (0) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) | |
| Diarrhea | Placebo | 1 (4.17) | 0 (0) | 0 (0) |
| Low dose | 1 (4.17) | 0 (0) | 0 (0) | |
| Medium dose | 1 (4.17) | 0 (0) | 0 (0) | |
| High dose | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Feng, G.; Wu, J.; Liu, X.; Pu, J.; Wang, Y.; You, W.; Yin, N.; Yi, S.; Tan, J.; et al. Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. Vaccines 2025, 13, 369. https://doi.org/10.3390/vaccines13040369
Liu Y, Feng G, Wu J, Liu X, Pu J, Wang Y, You W, Yin N, Yi S, Tan J, et al. Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. Vaccines. 2025; 13(4):369. https://doi.org/10.3390/vaccines13040369
Chicago/Turabian StyleLiu, Yan, Guangwei Feng, Jinyuan Wu, Xinling Liu, Jing Pu, Yanxia Wang, Wangyang You, Na Yin, Shan Yi, Jiebing Tan, and et al. 2025. "Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial" Vaccines 13, no. 4: 369. https://doi.org/10.3390/vaccines13040369
APA StyleLiu, Y., Feng, G., Wu, J., Liu, X., Pu, J., Wang, Y., You, W., Yin, N., Yi, S., Tan, J., Lin, X., Huang, L., Gao, J., Yu, Q., Tong, Q., Zhang, Y., Chen, R., Hu, X., Ye, J., ... Li, H. (2025). Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. Vaccines, 13(4), 369. https://doi.org/10.3390/vaccines13040369






