Safety and Efficacy of Vaccination During Lactation: A Comprehensive Review of Vaccines for Maternal and Infant Health Utilizing a Large Language Model Citation Screening System
Abstract
:1. Introduction
Objectives
2. Methods
2.1. Eligibility Criteria
- ▪
- Lactating women receiving a vaccine during the postpartum period;
- ▪
- Studies reporting on vaccine safety, efficacy, immune response, or transfer of vaccine components to human milk;
- ▪
- Randomized controlled trials, prospective and retrospective cohort studies, cross-sectional studies, case reports, and case series;
- ▪
- Peer-reviewed original research articles.
- ▪
- Studies investigating vaccines targeting non-infectious agents;
- ▪
- Studies not reporting on vaccines administered during the postpartum period;
- ▪
- Studies with insufficient data on outcomes of interest;
- ▪
- Editorials, letters, legal cases, interviews;
- ▪
- Non-English language studies (or no English translation version available).
2.2. Search Strategy
2.3. Selection Process
- Initial screening: In this phase, titles and abstracts were screened by a reviewer (S.J.M.) using Rayyan, a web-based automation tool [10]. This step aimed to eliminate clearly irrelevant studies based on pre-defined eligibility criteria.
- LLM-assisted screening: For the secondary screening phase, we employed GPT-4 (version 40314), a LLM publicly accessible since June 2023. A custom-built system, created in collaboration with an artificial intelligence graduate (J.Z.), integrated the LLM into the screening process. This system was implemented in Python (version 3.8.17) and accessed via OpenAI’s application programming interface (API).
- Full-text assessment: Finally, a full-text assessment was completed by a reviewer (S.J.M.) for studies categorized as ‘inclusion’ or ‘maybe’ by the LLM to ensure they met all inclusion criteria.
2.3.1. LLM-Assisted Screening
- Creating a python script with a command prompt in Visual Studio Code (version 1.80) to enable the LLM to independently access and screen all eligible studies (Appendix B).
- For each study, the LLM evaluated the title and abstract using a detailed prompt (see Section 2.3.2).
- The LLM provided a score (1–10 scale) for each eligibility criterion, along with a rationale for each score.
- Based on these individual scores, the LLM gave an overall decision (inclusion, exclusion, or maybe) and a final score (1–10 scale). Appendix C contains an example of the LLM-citation screening output file, which includes the graded assessments and final decisions.
- All LLM decisions were manually reviewed by a human reviewer to ensure accuracy.
2.3.2. Prompt Engineering
- Evaluate each eligibility criterion (population, intervention, outcome, and publication type) separately.
- Provide a one-sentence explanation and a relevance score (1–10 scale) for each criterion.
- Give a final decision and overall score (1–10 scale) based on the individual criterion assessments.
2.3.3. Limitations and Challenges to LLM Utilization
2.4. Data Collection/Items
- 1.
- Safety in Lactating Individuals
- 2.
- Safety in Infants
- 2.1.
- Viral shedding/Transmission vaccine components
- 2.2.
- Adverse reactions in infants
- 3.
- Immunogenicity in Lactating Individuals
- 4.
- Infant Immunity Through Human Milk
- 4.1.
- Human milk immune response
- 4.2.
- Infant immune response
- 5.
- Vaccine Effectiveness
- 5.1.
- Effectiveness in lactating individuals
- 5.2.
- Effectiveness in infants
Outcome Definitions
2.5. Study Risk of Bias Assessment
2.6. Effect Measures
2.7. Data Synthesis
2.8. Reporting Bias Assessment
2.9. Software and Data Management
- Rayyan: Used for initial screening of titles and abstracts;
- OpenAI: GPT-4 (version 40314) was used as LLM;
- Python (version 3.8.17): Script enabling a LLM to access data and execute the command using an API;
- Visual Studio Code (version 1.80): Used to develop and run the LLM-screening command;
- CSV files: Used for data storage and transfer between software packages
- Zotero (versions 6.0.20–6.0.37): Used for reference management throughout the review process;
- Microsoft Excel (version 16.88): Used for data extraction and creation of summary tables.
3. Results
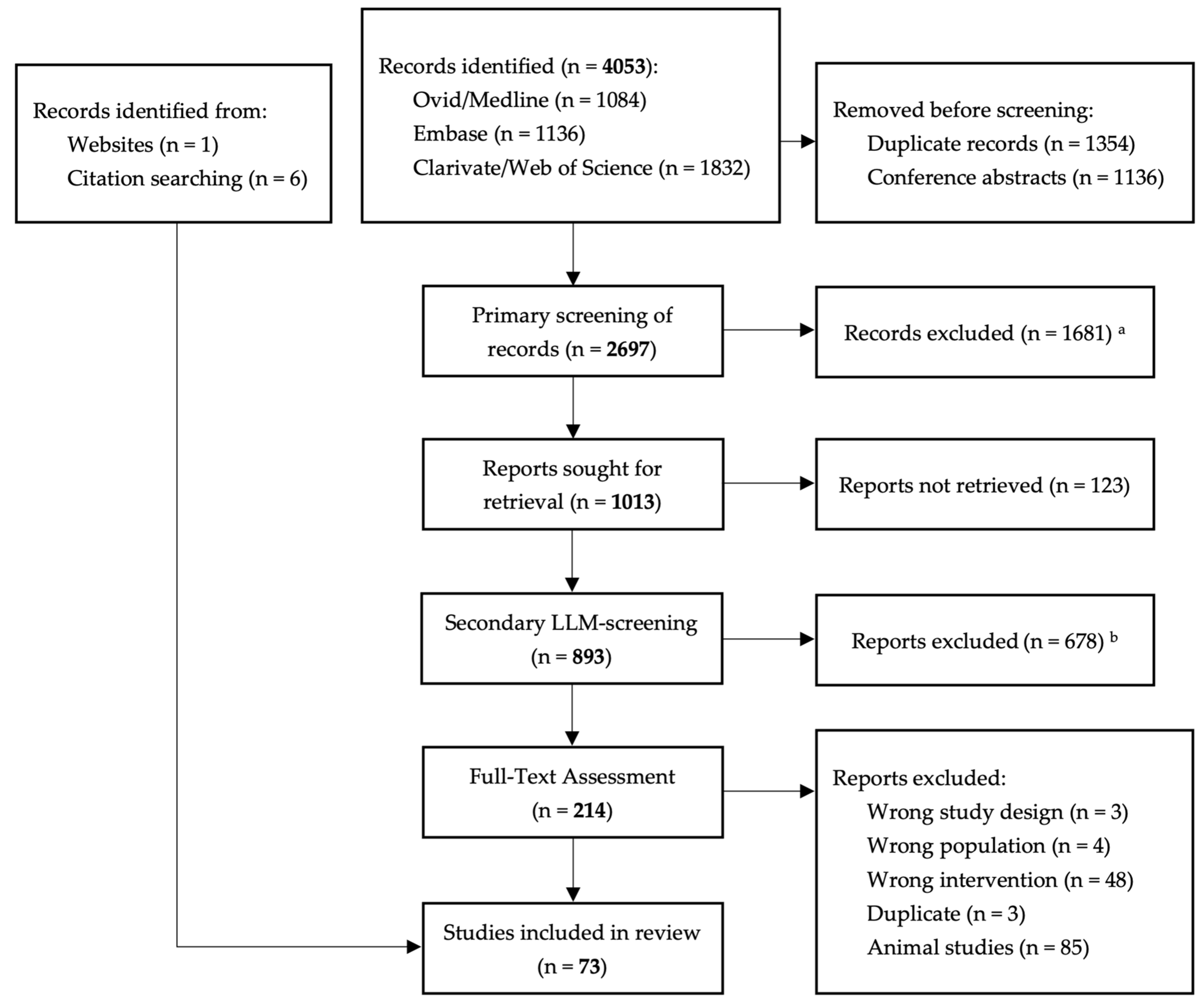
3.1. Results of the Search
3.2. Included Studies
3.3. Cholera Vaccine
3.3.1. Background
3.3.2. Included Studies
3.3.3. Outcomes
3.3.4. Quality of the Evidence
3.3.5. Conclusion
3.4. Typhoid Vaccine (Typhoid Fever)
3.4.1. Background
3.4.2. Included Studies
3.4.3. Outcomes
3.4.4. Quality of the Evidence
3.4.5. Conclusion
3.5. Influenza Vaccine (Flu)
3.5.1. Background
3.5.2. Included Studies
3.5.3. Outcomes
3.5.4. Quality of the Evidence
3.5.5. Conclusion
3.6. Pertussis Vaccine (Whooping Cough)
3.6.1. Background
3.6.2. Included Studies
3.6.3. Outcomes
3.6.4. Quality of the Evidence
3.6.5. Conclusion
3.7. Pneumococcal Vaccine
3.7.1. Background
3.7.2. Included Studies
3.7.3. Outcomes
3.7.4. Quality of the Evidence
3.7.5. Conclusion
3.8. Polio Vaccine
3.8.1. Background
3.8.2. Included Studies
3.8.3. Outcomes
3.8.4. Quality of the Evidence
3.8.5. Conclusion
3.9. Rabies Vaccine
3.9.1. Background
3.9.2. Included Studies
3.9.3. Outcomes
3.9.4. Quality of the Evidence
3.9.5. Conclusion
3.10. Rotavirus Vaccine
3.10.1. Background
3.10.2. Included Studies
3.10.3. Outcomes
3.10.4. Quality of the Evidence
3.10.5. Conclusion
3.11. Rubella Vaccine
3.11.1. Background
3.11.2. Included Studies
3.11.3. Outcomes
3.11.4. Quality of the Evidence
3.11.5. Conclusion
3.12. Varicella Vaccine (Chickenpox)
3.12.1. Background
3.12.2. Included Studies
3.12.3. Outcomes
3.12.4. Quality of the Evidence
3.12.5. Conclusion
3.13. Variola Virus Vaccine (Smallpox)
3.13.1. Background
3.13.2. Included Studies
3.13.3. Outcomes
3.13.4. Quality of the Evidence
3.13.5. Conclusion
3.14. Yellow Fever Vaccine
3.14.1. Background
3.14.2. Included Studies
3.14.3. Outcomes
3.14.4. Quality of the Evidence
3.14.5. Conclusion
3.15. COVID-19 Vaccine (SARS-CoV-2)
3.15.1. Background
3.15.2. Included Studies
3.15.3. Outcomes
3.15.4. Quality of the Evidence
3.15.5. Conclusion
3.15.6. Broader Implications for Vaccine Research
4. Discussion
4.1. Effects on Maternal Health
4.1.1. Safety
4.1.2. Immunogenicity
4.2. Effects on Infant Health
4.2.1. Safety
4.2.2. Infant Immunity Derived from Human Milk
Human Milk Immune Response
Infant Immune Response
4.2.3. Effectiveness
4.3. Contextual Considerations: Vaccine Hesitancy
4.4. Limitations
4.5. Recommendations
- Inactivated vaccines (COVID-19, cholera, influenza, pertussis, pneumococcus, poliovirus, and rotavirus) are generally safe and effective for lactating individuals and their infants. These vaccines can be administered without the need for breastfeeding interruption.
- YF vaccine: postponing vaccination is preferred; however, if vaccination is deemed necessary, breastfeeding interruption should be recommended for at least 3 weeks after vaccination due to the risk of virus transmission and potentially serious adverse consequences for the infant. Advice should be provided on maintaining milk supply and infant feeding.
- Rubella vaccine: Potential transmission of rubella virus to infants through breastmilk is suggested from observational studies, although usually without clinical consequences for the infant. Clear guidance on adverse reactions lactating participants might experience is important for expectation management.
- Rabies vaccine: Given the severe consequences of rabies infection, rabies vaccination is justified in lactating women when necessary. Healthcare providers should ensure timely vaccination and provide guidance on breastfeeding, balancing its benefits against potential risks.
- Smallpox vaccine: traditional smallpox vaccine, ACAM2000, in household members of young infants requires advice on precautionary measures to prevent tertiary contact vaccinia; advising lactating women to avoid close contact with recently vaccinated individuals against either smallpox or Mpox is appropriate.
- The varicella vaccine appears to be safe to use in lactating women without the need for breastfeeding interruption, though advice should be given that if a vesicular rash occurs following vaccination, they might consult with their healthcare provider about whether precautionary measures are needed.
4.6. Implications and Future Research
- Healthcare providers can now access consolidated evidence to guide vaccination decisions for lactating individuals
- Offers a framework for discussing benefits and risks with patients
- Findings support the general safety of most vaccines during lactation, helping address vaccine hesitancy
- Highlights the need to include lactating individuals in vaccine trials, particularly during public health emergencies
- Demonstrates the importance of standardized safety monitoring
- Well-designed clinical trials, specifically including lactating individuals
- Standardized approaches to measuring vaccine responses in maternal serum, human milk, and infants
- Long-term follow-up studies of infant outcomes
- Investigation of vaccines against emerging infectious diseases (e.g., Mpox) in lactating populations
- Studies examining the correlation between mucosal immune factors and clinical protection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CRS | Congenital rubella syndrome |
| GMT | Geometric mean titers |
| LAIV | Live-attenuated influenza vaccine |
| LLM | Large Language Model |
| MMR | Measles, mumps, rubella |
| PEP | Post-exposure prophylaxis |
| PrEP | Pre-exposure prophylaxis |
| PT | Pertussis toxoid |
| RCT | Randomized-Controlled Trial |
| VZV | Varicella zoster virus |
| WC | Whole cell |
| YF | Yellow fever |
| DTaP | Diptheria, Tetanus, acellular Pertussis (vaccine) |
| ELISA | Enzyme-linked immunosorbent assay |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| HDCV | Human diploid cell rabies vaccine |
| HRIG | Human rabies immunoglobulin |
| IgA | Immunoglobulin A |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| SIgA | Secretory Immunoglobulin A |
| IPV | Inactivated poliovirus vaccine |
| JAMA | Journal of the American Medical Association |
| NHLBI | National Heart, Lung and Blood Institute |
| NK | Natural killer (cells) |
| OPV | Oral polio vaccine |
| PCR | Polymerase chain reaction |
| RBD | Receptor-binding domain |
| RNA | Ribonucleic acid |
| mRNA | Messenger ribonucleic acid |
Appendix A. Database Searches
| Search | Ovid/Medline Query—7 July 2022 | Results |
|---|---|---|
| #5 | exp Lactation/or exp Breast Feeding/or (lactation or breast-feeding or lactating or ((breast or lactic or mammary-gland or milk) adj3 (secretion* or excretion))).ti,ab,kf. | 111,425 |
| #4 | exp Postpartum Period/or (puerper* or postpartum or post-partum or postnatal or post-natal or lactating or breast-feeding).ti,ab,kf. | 263,010 |
| #3 | exp Vaccination/or exp Vaccines/or (vaccin* or immunis* or immuniz*).ti,ab,kf. | 516,754 |
| #2 | 1 and 2 and 3 | 1552 |
| #1 | 4 not (exp Animals/not exp humans/) | 1084 |
| Search | Embase Query—7 July 2022 | Results |
|---|---|---|
| #6 | #5 NOT ‘conference abstract’/it | 1136 |
| #5 | #4 NOT ([animals]/lim NOT [humans]/lim) | 1310 |
| #4 | #1 AND #2 AND #3 | 1682 |
| #3 | ‘vaccination’/exp OR vaccin*:ti,ab,kw OR immunis*:ti,ab,kw OR immuniz*:ti,ab,kw | 589,202 |
| #2 | ‘puerperium’/exp OR puerper*:ti,ab,kw OR ‘postpartum’/exp OR postpartum:ti,ab,kw OR ‘post partum’:ti,ab,kw OR postnatal:ti,ab,kw OR ‘post natal’:ti,ab,kw OR lactating:ti,ab,kw OR ‘breast feeding’:ti,ab,kw | 331,060 |
| #1 | ‘lactation’/exp OR ‘breast feeding’/exp OR lactation:ti,ab,kw OR ‘breast feeding’:ti,ab,kw OR lactating:ti,ab,kw OR (((breast OR lactic OR ‘mammary gland’ OR milk) NEAR/3 (secretion* OR excretion)):ti,ab,kw) | 139,582 |
| Search | Web of Science Query—7 July 2022 | Results |
|---|---|---|
| #4 | #1 AND #2 AND #3 | 1832 |
| #3 | TS = (vaccin* OR immunis* OR immuniz*) | 489,429 |
| #2 | TS = (puerper* OR postpartum OR post-partum OR postnatal OR post-natal OR lactating OR breast-feeding) | 380,965 |
| #1 | TS = (lactation OR breast-feeding OR lactating OR ((breast OR lactic OR mammary-gland OR milk) NEAR/3 (secretion* OR excretion))) | 229,211 |
Appendix B. Python Script and Optimized System Prompt
- (i)
- Population: Lactating individuals and/or their offspring. Exclude articles focusing on specific diseases or conditions (such as cancer or Crohn’s disease patients) or targeting non-infectious agents (e.g., cancer vaccines). Studies encompassing both human and non-human subjects should be included.
- (ii)
- Intervention:
- -
- Vaccines administered to lactating individuals in the postpartum period.
- -
- Offspring receiving breastmilk expressed by lactating individuals vaccinated in the postpartum period.
- (iii)
- Outcome: The effect of vaccines on one or more of the following outcome categories:
- The lactating individual (examples: systemic immune response; mucosal immune response; adverse events or complications like vaccine-virus infection; side-effects; negative long-term effects; hazardous vaccine-components; etc.);
- The breastmilk (examples: breastmilk composition; excretion of vaccine components in breastmilk; excretion of vaccine-virus in breastmilk; milk supply; milk yield quality; excretion of hazardous vaccine ingredients in breastmilk, including mercury or thimerosal; etc.);
- The breastfed offspring (examples: transfer of vaccine-induced immunological factors; prevalence or incidence of vaccine-preventable infectious diseases; complications or hospital admissions; severity of the disease course in the offspring; side-effects in the offspring after administration of vaccines in lactating individuals; offspring infection with vaccine-virus; negative effects for the offspring of vaccination of their parent or indirect exposure to hazardous vaccine components such as mercury or thimerosal through breastfeeding; etc.). Include studies that focus on the indirect effects of vaccines on offspring, such as immunization through the transfer of immunological factors in breastmilk or potential harm due to exposure to hazardous vaccine components like mercury or thimerosal.
- (iv)
- Publication type or study design: do not include literature reviews, scoping reviews, editorials, legal cases or interviews. These should ALWAYS receive a hard reject.
Appendix C. Example of Output CSV for Citation Screening and Final Decisions
| Key | Population | Intervention | Outcome | Publication Type | FINAL DECISION | Output |
|---|---|---|---|---|---|---|
| Example 1 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | (i) Population: The study focuses on lactating sows and offspring, which falls within the scope of the review. 10/10 (ii) Intervention: The study investigates a novel VP2-based PPV1 subunit vaccine administered to bred pigs, including lactating sows. 10/10 (iii) Outcome: The study examines the safety of the vaccine in lactating sows and offspring, including survival rate and average daily weight gain in offspring. 10/10 (iv) Publication type or study design: The study is an experimental investigation, which is appropriate for inclusion. 10/10 FINAL DECISION: 10/10 |
| Example 2 | 3/10 | 4/10 | 3/10 | 10/10 | 3/10 | (i) Population: The article focuses on the general population in the Amazon Basin, including infants, but does not specifically mention lactating individuals or their offspring. 3/10 (ii) Intervention: The article discusses Thimerosal-containing vaccines (TCVs) as a source of mercury exposure, but does not specifically focus on vaccines administered to lactating individuals in the postpartum period or their breastfed offspring. 4/10 (iii) Outcome: The article addresses health effects associated with mercury exposure, including from TCVs, but does not specifically focus on the given outcome categories related to lactating individuals, breastmilk, or breastfed offspring. 3/10 (iv) Publication type or study design: The article appears to be a research article based on a search and selection of papers addressing mercury exposure and human health. 10/10 FINAL DECISION: 3/10 |
References
- Lokossou, G.A.G.; Kouakanou, L.; Schumacher, A.; Zenclussen, A.C. Human Breast Milk: From Food to Active Immune Response with Disease Protection in Infants and Mothers. Front. Immunol. 2022, 13, 849012. [Google Scholar] [CrossRef]
- Nicolaidou, V.; Georgiou, R.; Christofidou, M.; Felekkis, K.; Pieri, M.; Papaneophytou, C. Detection of SARS-CoV-2–Specific Antibodies in Human Breast Milk and Their Neutralizing Capacity After COVID-19 Vaccination: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 2957. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Alter, G. The multifaceted roles of breast milk antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef]
- Hebballi, N.B.; Parker, T.; Garcia, E.I.; Ferguson, D.M.; Lesser, S.; Tsao, K.; Broussard, M.; Wootton, S.H. Pertussis and influenza immunization: Perceived attitude and decision of postpartum patients. BMC Pregnancy Childbirth 2022, 22, 975. [Google Scholar] [CrossRef]
- Simmons, L.A.; Whipps, M.D.M.; Phipps, J.E.; Satish, N.S.; Swamy, G.K. Understanding COVID-19 vaccine uptake during pregnancy: ‘Hesitance’, knowledge, and evidence-based decision-making. Vaccine 2022, 40, 2755–2760. [Google Scholar] [CrossRef]
- Nichol, B.; McCready, J.L.; Steen, M.; Unsworth, J.; Simonetti, V.; Tomietto, M. Barriers and facilitators of vaccine hesitancy for COVID-19, influenza, and pertussis during pregnancy and in mothers of infants under two years: An umbrella review. PLoS ONE 2023, 18, e0282525. [Google Scholar] [CrossRef]
- Xantus, G.Z.; Burke, D.; Kanizsai, P. How to best handle vaccine decliners: Scientific facts and psychological approach. Postgrad. Med. J. 2022, 98, 626–632. [Google Scholar] [CrossRef]
- Kilich, E.; Dada, S.; Francis, M.R.; Tazare, J.; Chico, R.M.; Paterson, P.; Larson, H.J. Factors that influence vaccination decision-making among pregnant women: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0234827. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Schuurmans, D.; Bosma, M.; Xia, F.; Chi, E.; Le, Q.V.; Zhou, D. Chain-of-Thought Prompting Elicits Reasoning in Large Language Models. Adv. Neural Inf. Process. Syst. 2022, 35, 24824–24837. [Google Scholar]
- Meskó, B. Prompt Engineering as an Important Emerging Skill for Medical Professionals: Tutorial. J. Med. Internet Res. 2023, 25, e50638. [Google Scholar] [CrossRef] [PubMed]
- Giray, L. Prompt Engineering with ChatGPT: A Guide for Academic Writers. Ann. Biomed. Eng. 2023, 51, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Oami, T.; Okada, Y.; Nakada, T.-A. Performance of a Large Language Model in Screening Citations. JAMA Netw. Open 2024, 7, e2420496. [Google Scholar] [CrossRef]
- Powell, M.; Sheets, R.; McEwen, J.; Knezevic, I.; Moorthy, V. Guidelines on Clinical Evaluation of Vaccines: Regulatory Expectations; World Health Organisation: Geneva, Switzerland, 2017; p. 573. [Google Scholar]
- ICH Considerations: General Principles to Address Virus and Vector Shedding—Scientific Guideline|European Medicines Agency (EMA). 2009. Available online: https://www.ema.europa.eu/en/ich-considerations-general-principles-address-virus-vector-shedding-scientific-guideline (accessed on 8 January 2025).
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef]
- NHLBI. Study Quality Assessment Tools. National Heart, Lung, and Blood Institute (NHLBI). 2013. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 14 October 2024).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Brady, R.C.; Jackson, L.A.; Frey, S.E.; Shane, A.L.; Walter, E.B.; Swamy, G.K.; Schlaudecker, E.P.; Szefer, E.; Wolff, M.; McNeal, M.M.; et al. Randomized trial comparing the safety and antibody responses to live attenuated versus inactivated influenza vaccine when administered to breastfeeding women. Vaccine 2018, 36, 4663–4671. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; DaPra, C.; Medo, E. Influenza Vaccine Associated with the Gene Expression of T Cell Surface Markers in Human Milk. Breastfeed. Med. 2022, 17, 218–225. [Google Scholar] [CrossRef]
- Pickering, L.K.; Morrow, A.L.; Herrera, I.; O’Ryan, M.; Estes, M.K.; Guilliams, S.E.; Jackson, L.; Carter-Campbell, S.; Matson, D.O. Effect of maternal rotavirus immunization on milk and serum antibody titers. J. Infect. Dis. 1995, 172, 723–728. [Google Scholar]
- Tingle, A.J.; Mitchell, L.A.; Grace, M.; Middleton, P.; Mathias, R.; MacWilliam, L.; Chalmers, A. Randomised double-blind placebo-controlled study on adverse effects of rubella immunisation in seronegative women. Lancet 1997, 349, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Grillner, L.; Hedstrom, C.E.; Bergstrom, H. Vaccination against rubella of newly delivered women. Scand. J. Infect. Dis. 1973, 5, 237–241. [Google Scholar] [PubMed]
- Okuda, M.; Yamanaka, M.; Takahashi, T.; Ishikawa, H.; Endoh, M.; Hirahara, F. Positive rates for rubella antibody in pregnant women and benefit of post-partum vaccination in a Japanese perinatal center. J. Obstet. Gynaecol. Res. 2008, 34, 168–173. [Google Scholar] [CrossRef]
- Buimovici-Klein, E.; Hite, R.L.; Byrne, T.; Cooper, L.Z. Isolation of rubella virus in milk after postpartum immunization. J. Pediatr. 1977, 91, 939–941. [Google Scholar] [PubMed]
- Losonsky, G.A.; Fishaut, J.M.; Strussenberg, J.; Ogra, P.L. Effect of immunization against rubella on lactation products. II. Maternal-neonatal interaction. J. Infect. Dis. 1982, 145, 661–666. [Google Scholar]
- Losonsky, G.A.; Fishaut, J.M.; Strussenberg, J.; Ogra, P.L. Effect of immunization against rubella on lactation products. I. Development and characterization of specific immunologic reactivity in breast milk. J. Infect. Dis. 1982, 145, 654–660. [Google Scholar]
- Landes, R.D.; Bass, J.W.; Millunchick, E.W.; Oetgen, W.J. Neonatal rubella following postpartum maternal immunization. J. Pediatr. 1980, 97, 465–467. [Google Scholar] [CrossRef]
- Bohlke, K.; Galil, K.; A Jackson, L.; Schmid, D.; Starkovich, P.; Loparev, V.N.; Seward, J.F. Postpartum varicella vaccination: Is the vaccine virus excreted in breast milk? Obstet. Gynecol. 2003, 102 Pt 1, 970–977. [Google Scholar] [CrossRef]
- Saringkarisate, K.; Len, K.A.; Melish, M.E.; Prothero, B.K.; Ching, N. Vaccine-Strain Varicella Virus Transmitted to a Term Infant Following Maternal Postpartum Vaccination. J. Pediatric. Infect. Dis. Soc. 2022, 11, 452–453. [Google Scholar] [CrossRef]
- Kluthe, M.; Herrera, A.; Blanca, H.; Leung, J.; Bialek, S.R.; Schmid, D.S. Neonatal Vaccine-strain Varicella-zoster Virus Infection 22 Days After Maternal Postpartum Vaccination. Pediatr. Infect. Dis. J. 2012, 31, 977. [Google Scholar] [CrossRef]
- Garde, V.; Harper, D.; Fairchok, M.P. Tertiary contact vaccinia in a breastfeeding infant. JAMA 2004, 291, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Twele-Montecinos, L.; MacDonald, J.; Webster, P.; Law, B. Case report: Probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. Can. Med Assoc. J. 2011, 183, E243–E245. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Transmission of yellow fever vaccine virus through breast-feeding—Brazil, 2009. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 130–132. [Google Scholar]
- Traiber, C.; Amaral, P.C.; Ritter, V.R.F.; Winge, A. Infant meningoencephalitis probably caused by yellow fever vaccine virus transmitted via breastmilk. J. Pediatr. 2011, 87, 269–272. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Nogueira, J.S.; Porto, V.B.G.; Sato, H.K. The search for yellow fever virus vaccine in breast milk of inadvertently vaccinated women in Brazil. Rev. Inst. Med. Trop. São Paulo 2020, 62, e33. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Bashir, R.A.; Abdelrahman, D.N.; Madni, H.; El Hussein, A.R.; Elkidir, I.M.; Enan, K.A. Transmission of yellow fever vaccine virus from breast feeding mothers to their infants: Reporting of yellow fever virus (YFV) RNA detection in milk specimens. F1000Research 2022, 11, 76. [Google Scholar] [CrossRef]
- Miyazato, Y.; Terada, M.; Ujiie, M.; Saito, S.; Moriya, A.; Ando, M.; Ohmagari, N. A nationwide prospective cohort study on safety of the 17D-204 yellow fever vaccine during a vaccine shortage in Japan. J. Travel Med. 2023, 30, taac070. [Google Scholar] [CrossRef]
- Doughty, H.; Barton, D. Severe nonanaphylactic allergic reaction to the Pfizer-BioNTech COVID-19 vaccine. JAAD Case Rep. 2022, 19, 84–86. [Google Scholar] [CrossRef]
- Diogo, P.; Correia, G.; Martins, J.B.; Soares, R.; Palma, P.J.; Santos, J.M.; Gonçalves, T. Delayed Cutaneous Adverse Reaction of the AstraZeneca COVID-19 Vaccine in a Breastfed Female Infant: A Coincidence or a Rare Effect? Vaccines 2022, 10, 602. [Google Scholar] [CrossRef]
- Clemens, J.D.; Sack, D.A.; Chakraborty, J.; Rao, M.; Ahmed, F.; Harris, J.R.; van Loon, F.; Khan, M.; Yunis, M.; Huda, S.; et al. Field trial of oral cholera vaccines in Bangladesh: Evaluation of anti-bacterial and anti-toxic breast-milk immunity in response to ingestion of the vaccines. Vaccine 1990, 8, 469–472. [Google Scholar] [CrossRef]
- Hahn-Zoric, M.; Carlsson, B.; Jalil, F.; Mellander, L.; Germanier, R.; Hanson, L.A. The influence on the secretory IgA antibody levels in lactating women of oral typhoid and parenteral cholera vaccines given alone or in combination. Scand. J. Infect. Dis. 1989, 21, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Mascart-Lemone, F.; Carlsson, B.; Jalil, F.; Hahn-Zoric, M.; Duchateau, J.; Hanson, L.A. Polymeric and monomeric IgA response in serum and milk after parenteral cholera and oral typhoid vaccination. Scand. J. Immunol. 1988, 28, 443–448. [Google Scholar] [CrossRef]
- Merson, M.H.; Black, R.E.; Sack, D.A.; Svennerholm, A.M.; Holmgren, J. Maternal cholera immunisation and scecretory IgA in breast milk. Lancet 1980, 1, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, A.M.; Hanson, L.Å.; Holmgren, J.; Lindblad, B.S.; Nilsson, B.; Quereshi, F. Different secretory immunoglobulin A antibody responses to cholera vaccination in Swedish and Pakistani women. Infect. Immun. 1980, 30, 427–430. [Google Scholar] [CrossRef]
- Clemens, J.D.; Sack, D.A.; Harris, J.R.; Khan, M.R.; Chakraborty, J.; Chowdhury, S.; Rao, M.R.; VAN Loon, F.P.L.; Stanton, B.F.; Yunus, M.; et al. Breast feeding and the risk of severe cholera in rural Bangladeshi children. Am. J. Epidemiol. 1990, 131, 400–411. [Google Scholar] [CrossRef]
- Orije, M.R.P.; Larivière, Y.; A Herzog, S.; Mahieu, L.M.; Van Damme, P.; Leuridan, E.; Maertens, K. Breast Milk Antibody Levels in Tdap-Vaccinated Women After Preterm Delivery. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e1305–e1313. [Google Scholar] [CrossRef]
- De Schutter, S.; Maertens, K.; Baerts, L.; De Meester, I.; Van Damme, P.; Leuridan, E. Quantification of Vaccine-induced Antipertussis Toxin Secretory IgA Antibodies in Breast Milk: Comparison of Different Vaccination Strategies in Women. Pediatr. Infect. Dis. J. 2015, 34, e149–e152. [Google Scholar] [CrossRef]
- Finn, A.; Zhang, Q.; Seymour, L.; Fasching, C.; Pettitt, E.; Janoff, E.N. Induction of functional secretory IgA responses in breast milk, by pneumococcal capsular polysaccharides. J. Infect. Dis. 2002, 186, 1422–1429. [Google Scholar] [CrossRef]
- Svennerholm, A.M.; Hanson, L.A.; Holmgren, J. Antibody responses to live and killed poliovirus vaccines in the milk of Pakistani and Swedish women. J. Infect. Dis. 1981, 143, 707–711. [Google Scholar]
- Scrimgeour, E.M.; Mehta, F.R. Rabies in Oman: Failed postexposure vaccination in a lactating woman bitten by a fox. Int. J. Infect. Dis. 2001, 5, 160–162. [Google Scholar] [CrossRef]
- Bertrand, K.; Honerkamp-Smith, G.; Chambers, C.D. Maternal and Child Outcomes Reported by Breastfeeding Women Following Messenger RNA COVID-19 Vaccination. Breastfeed. Med. 2021, 16, 697–701. [Google Scholar] [CrossRef]
- Charepe, N.; Gonçalves, J.; Juliano, A.M.; Lopes, D.G.; Canhão, H.; Soares, H.; Serrano, E.F. COVID-19 mRNA vaccine and antibody response in lactating women: A prospective cohort study. BMC Pregnancy Childbirth 2021, 21, 632. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Bondzie, E.A.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Prahl, M.; Cassidy, A.G.; Gay, C.; Wu, A.H.; Jigmeddagva, U.; Lin, C.Y.; Gonzalez, V.J.; Basilio, E.; Chidboy, M.A.; et al. COVID-19 mRNA Vaccination in Lactation: Assessment of Adverse Events and Vaccine Related Antibodies in Mother-Infant Dyads. Front. Immunol. 2021, 12, 777103. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Henle, A.M. Increase in SARS-CoV-2 RBD-Specific IgA and IgG Antibodies in Human Milk from Lactating Women Following the COVID-19 Booster Vaccination. J. Hum. Lact. 2023, 39, 51–58. [Google Scholar] [CrossRef]
- İremli, B.G.; Şendur, S.N.; Ünlütürk, U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2600–2605. [Google Scholar] [CrossRef]
- Jakuszko, K.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Rukasz, D.; Kłak, R.; Królak-Olejnik, B.; Krajewska, M. Immune Response to Vaccination against COVID-19 in Breastfeeding Health Workers. Vaccines 2021, 9, 663. [Google Scholar] [CrossRef]
- Juncker, H.G.; Mulleners, S.J.; van Gils, M.J.; Bijl, T.P.L.; de Groot, C.J.M.; Pajkrt, D.; Korosi, A.; van Goudoever, J.B.; van Keulen, B.J. Comparison of SARS-CoV-2-specific antibodies in human milk after mrna-based COVID-19 vaccination and infection. Vaccines 2021, 9, 1475. [Google Scholar] [CrossRef]
- Kachikis, A.; Englund, J.A.; Singleton, M.; Covelli, I.; Drake, A.L.; Eckert, L.O. Short-term Reactions Among Pregnant and Lactating Individuals in the First Wave of the COVID-19 Vaccine Rollout. JAMA Netw. Open 2021, 4, e2121310. [Google Scholar] [CrossRef]
- Lee, Y.; Grubbs, G.; Ramelli, S.C.; Levine, A.R.; Bathula, A.; Saharia, K.; Purcell, M.; Singireddy, S.; Dugan, C.L.; Kirchoff, L.; et al. SARS-CoV-2 mRNA vaccine induced higher antibody affinity and IgG titers against variants of concern in post-partum vs. non-post-partum women. eBioMedicine 2022, 77, 103940. [Google Scholar] [CrossRef]
- Low, J.M.; Gu, Y.; Ng, M.S.F.; Amin, Z.; Lee, L.Y.; Ng, Y.P.M.; Shunmuganathan, B.D.; Niu, Y.; Gupta, R.; Tambyah, P.A.; et al. Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. npj Vaccines 2021, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Low, J.M.; Lee, L.Y.; Ng, Y.P.M.; Zhong, Y.; Amin, Z. Breastfeeding Mother and Child Clinical Outcomes After COVID-19 Vaccination. J. Hum. Lact. 2022, 38, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.; Pentecost, B.T.; Schoen, C.N.; Alfandari, D.; Schneider, S.S.; Baker, R.; Arcaro, K.F. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet. Gynecol. 2022, 139, 181. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.E.; Centeno, L.D.L.; Cheng, W.A.; Ruiz, C.J.M.; Lee, Y.; Congrave-Wilson, Z.; Powell, R.L.; Stellwagen, L.; Pannaraj, P.S. Human Milk SARS-CoV-2 Antibodies up to 6 Months After Vaccination. Pediatrics 2022, 149, e2021054260. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Bäuerl, C.; Mena-Tudela, D.; Aguilar-Camprubí, L.; Pérez-Cano, F.J.; Parra-Llorca, A.; Lerin, C.; Martínez-Costa, C.; Collado, M.C. Anti-SARS-CoV-2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous SARS-CoV-2 exposure: A longitudinal study. Genome Med. 2022, 14, 42. [Google Scholar] [CrossRef]
- Stafford, L.S.; Luaces, V.V.; Neu, J.; Cacho, N.; Parker, L.; Burchfield, D.; Li, N.; Larkin, J. Effect of SARS-CoV-2 vaccine on the breastmilk antibody response among lactating healthcare workers. J. Immunol. 2021, 206 (Suppl. S1), 30.15. [Google Scholar] [CrossRef]
- Valcarce, V.; Stafford, L.S.; Neu, J.; Cacho, N.; Parker, L.; Mueller, M.; Burchfield, D.J.; Li, N.; Larkin, J. Detection of SARS-CoV-2-Specific IgA in the Human Milk of COVID-19 Vaccinated Lactating Health Care Workers. Breastfeed. Med. 2021, 16, 1004–1009. [Google Scholar] [CrossRef]
- Yeo, K.T.; Chia, W.N.; Tan, C.W.; Ong, C.; Yeo, J.G.; Zhang, J.; Poh, S.L.; Lim, A.J.; Sim, K.H.; Sutamam, N.; et al. Neutralizing Activity and SARS-CoV-2 Vaccine mRNA Persistence in Serum and Breastmilk After BNT162b2 Vaccination in Lactating Women. Front. Immunol. 2022, 12, 783975. [Google Scholar] [CrossRef]
- Golan, Y.; Prahl, M.; Cassidy, A.; Lin, C.Y.; Ahituv, N.; Flaherman, V.J.; Gaw, S.L. Evaluation of Messenger RNA From COVID-19 BTN162b2 and mRNA-1273 Vaccines in Human Milk. JAMA Pediatr. 2021, 175, 1069–1071. [Google Scholar] [CrossRef]
- Young, B.E.; Seppo, A.E.; Diaz, N.; Rosen-Carole, C.; Nowak-Wegrzyn, A.; Vasquez, J.M.C.; Ferri-Huerta, R.; Nguyen-Contant, P.; Fitzgerald, T.; Sangster, M.Y.; et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs. mRNA Vaccination. JAMA Pediatr. 2022, 176, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Bueno-Llamoga, P.; Bäuerl, C.; Cortés-Macias, E.; Selma-Royo, M.; Pérez-Cano, F.; Lerin, C.; Martínez-Costa, C.; Collado, M.C. Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients 2022, 14, 2117. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Jiang, Y.; Carlson, M.; Ford, E.S.; Jani, S.; Houck, J.; Wu, X.; Jing, L.; Pecor, T.; Kachikis, A.; et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. Mucosal Immunol. 2023, 16, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; DeRiso, E.A.; Davis, C.; Bordt, E.A.; De Guzman, R.M.; Shook, L.L.; Yonker, L.M.; Fasano, A.; Akinwunmi, B.; Lauffenburger, D.A.; et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021, 13, eabi8631. [Google Scholar] [CrossRef]
- Bender, J.M.; Lee, Y.; Cheng, W.A.; Marentes Ruiz, C.J.; Pannaraj, P.S. Coronavirus Disease 2019 Vaccine Booster Effects Are Seen in Human Milk Antibody Response. Front. Nutr. 2022, 9, 898849. [Google Scholar] [CrossRef]
- Conti, M.G.; Terreri, S.; Terrin, G.; Natale, F.; Pietrasanta, C.; Salvatori, G.; Brunelli, R.; Midulla, F.; Papaevangelou, V.; Carsetti, R.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infection Versus Vaccination in Pregnancy: Implications for Maternal and Infant Immunity. Clin. Infect. Dis. 2022, 75 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef]
- Esteve-Palau, E.; Gonzalez-Cuevas, A.; Guerrero, M.E.; Garcia-Terol, C.; Alvarez, M.C.; Casadevall, D.; Diaz-Brito, V. Quantification of Specific Antibodies Against SARS-CoV-2 in Breast Milk of Lactating Women Vaccinated with an mRNA Vaccine. JAMA Netw. Open 2021, 4, e2120575. [Google Scholar] [CrossRef]
- Gonçalves, J.; Juliano, A.M.; Charepe, N.; Alenquer, M.; Athayde, D.; Ferreira, F.; Archer, M.; Amorim, M.J.; Serrano, F.; Soares, H. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep. Med. 2021, 2, 100468. [Google Scholar] [CrossRef]
- Juncker, H.G.; Mulleners, S.J.; Ruhé, E.J.; Coenen, E.R.; Bakker, S.; van Doesburg, M.; Harinck, J.E.; Rood, R.D.; Bouhuijs, J.H.; Oomen, M.; et al. Comparing the human milk antibody response after vaccination with four COVID-19 vaccines: A prospective, longitudinal cohort study in the Netherlands. eClinicalMedicine 2022, 47, 101393. [Google Scholar] [CrossRef]
- Juncker, H.G.; Mulleners, S.J.; van Gils, M.J.; de Groot, C.J.M.; Pajkrt, D.; Korosi, A.; van Goudoever, J.B.; van Keulen, B.J. The Levels of SARS-CoV-2 Specific Antibodies in Human Milk Following Vaccination. J. Hum. Lact. 2021, 37, 477–484. [Google Scholar] [CrossRef]
- Rosenberg-Friedman, M.; Kigel, A.; Bahar, Y.; Werbner, M.; Alter, J.; Yogev, Y.; Dror, Y.; Lubetzky, R.; Dessau, M.; Gal-Tanamy, M.; et al. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat. Commun. 2021, 12, 6222. [Google Scholar] [CrossRef]
- Schwartz, A.; Nir, O.; Toussia-Cohen, S.; Leibovich, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Levin, E.G.; et al. Presence of SARS-CoV-2 antibodies in lactating women and their infants following BNT162b2 messenger RNA vaccine. Am. J. Obstet. Gynecol. 2021, 225, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Young, B.E.; Li, D.; Seppo, A.; Zhou, Q.; Wiltse, A.; Nowak-Wegrzyn, A.; Murphy, K.; Widrick, K.; Diaz, N.; et al. Broad Cross-Reactive IgA and IgG against Human Coronaviruses in Milk Induced by COVID-19 Vaccination and Infection. Vaccines 2022, 10, 980. [Google Scholar] [CrossRef]
- Baird, J.K.; Jensen, S.M.; Urba, W.J.; Fox, B.A.; Baird, J.R. SARS-CoV-2 Antibodies Detected in Mother’s Milk Post-Vaccination. J. Hum. Lact. 2021, 37, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Bernal, O.; Cervantes-Luevano, K.; Flores-Acosta, G.I.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. COVID-19 Neutralizing Antibodies in Breast Milk of Mothers Vaccinated with Three Different Vaccines in Mexico. Vaccines 2022, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Low, J.M.; Gu, Y.; Ng, M.S.F.; Wang, L.W.; Amin, Z.; Zhong, Y.; MacAry, P.A. Human Milk Antibodies After BNT162b2 Vaccination Exhibit Reduced Binding Against SARS-CoV-2 Variants of Concern. Vaccines 2022, 10, 225. [Google Scholar] [CrossRef]
- Mulleners, S.J.; Juncker, H.G.; van Gils, M.J.; van Goudoever, J.B.; van Keulen, B.J. Human Milk Antibody Response After Combining Two Different COVID-19 Vaccines: Mix-and-Match. J. Hum. Lact. 2022, 38, 401–406. [Google Scholar] [CrossRef]
- Trofin, F.; Nastase, E.V.; Iancu, L.S.; Constantinescu, D.; Cianga, C.M.; Lunca, C.; Ursu, R.G.; Cianga, P.; Dorneanu, O.S. Anti-RBD IgA and IgG Response and Transmission in Breast Milk of Anti-SARS-CoV-2 Vaccinated Mothers. Pathogens 2022, 11, 286. [Google Scholar] [CrossRef]
- Yang, X.; Fox, A.; DeCarlo, C.; Norris, C.; Griffin, S.; Wedekind, S.; Flanagan, J.M.; Shenker, N.; Powell, R.L. Comparative Profiles of SARS-CoV-2 Spike-Specific Human Milk Antibodies Elicited by mRNA- and Adenovirus-Based COVID-19 Vaccines. Breastfeed. Med. 2022, 17, 638–646. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- Kanungo, S.; Azman, A.S.; Ramamurthy, T.; Deen, J.; Dutta, S. Cholera. Lancet 2022, 399, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Typhoid vaccines: WHO position paper—March 2018. Wkly. Epidemiol. Rec. 2018, 93, 153–172. [Google Scholar]
- Chaves, S.S.; Perez, A.; Farley, M.M.; Miller, L.; Schaffner, W.; Lindegren, M.L.; Sharangpani, R.; Meek, J.; Yousey-Hindes, K.; Thomas, A.; et al. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr. Infect. Dis. J. 2014, 33, 912–919. [Google Scholar] [CrossRef]
- Mertz, D.; Geraci, J.; Winkup, J.; Gessner, B.D.; Ortiz, J.R.; Loeb, M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta-analysis of observational studies. Vaccine 2017, 35, 521–528. [Google Scholar] [CrossRef]
- Jarvis, J.R.; Dorey, R.B.; Warricker, F.D.M.; Alwan, N.A.; Jones, C.E. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: Systematic review and meta-analysis. Vaccine 2020, 38, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Immunization, Infectious Disease, and Public Health Preparedness Expert Work Group. Influenza in Pregnancy: Prevention and Treatment. 2024. Available online: https://www.acog.org/clinical/clinical-guidance/committee-statement/articles/2024/02/influenza-in-pregnancy-prevention-and-treatment (accessed on 30 August 2024).
- Dattani, S.; Spooner, F.; Ritchie, H.; Roser, M. Influenza. Our World in Data. 2024. Available online: https://ourworldindata.org/influenza (accessed on 1 September 2024).
- Alain, S.; Dommergues, M.A.; Jacquard, A.C.; Caulin, E.; Launay, O. State of the art: Could nursing mothers be vaccinated with attenuated live virus vaccine? Vaccine 2012, 30, 4921–4926. [Google Scholar] [CrossRef]
- Munoz, F.M.; Patel, S.M.; Jackson, L.A.; Swamy, G.K.; Edwards, K.M.; Frey, S.E.; Petrie, C.R.; Sendra, E.A.; Keitel, W.A. Safety and immunogenicity of three seasonal inactivated influenza vaccines among pregnant women and antibody persistence in their infants. Vaccine 2020, 38, 5355–5363. [Google Scholar] [CrossRef]
- Perego, G.; Vigezzi, G.P.; Cocciolo, G.; Chiappa, F.; Salvati, S.; Balzarini, F.; Odone, A.; Signorelli, C.; Gianfredi, V. Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis. Vaccines 2021, 9, 998. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Steinhoff, M.C.; Omer, S.B.; McNeal, M.M.; Roy, E.; Arifeen, S.E.; Dodd, C.N.; Raqib, R.; Breiman, R.F.; Zaman, K. IgA and Neutralizing Antibodies to Influenza A Virus in Human Milk: A Randomized Trial of Antenatal Influenza Immunization. PLoS ONE 2013, 8, e70867. [Google Scholar] [CrossRef]
- Mbayei, S.A.; Faulkner, A.; Miner, C.; Edge, K.; Cruz, V.; Peña, S.A.; Kudish, K.; Coleman, J.; Pradhan, E.; Thomas, S.; et al. Severe Pertussis Infections in the United States, 2011–2015. Clin. Infect. Dis. 2019, 69, 218–226. [Google Scholar] [CrossRef]
- Rowe, S.L.; Tay, E.L.; Franklin, L.J.; Stephens, N.; Ware, R.S.; Kaczmarek, M.C.; Lester, R.A.; Lambert, S.B. Effectiveness of parental cocooning as a vaccination strategy to prevent pertussis infection in infants: A case-control study. Vaccine 2018, 36, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Pneumococcal Conjugate Vaccines in Infants and Children Under 5 Years of Age: WHO Position Paper—February 2019. 2019, pp. 85–104. Available online: https://www.who.int/publications/i/item/10665-310968 (accessed on 18 October 2024).
- Gierke, R.; Wodi, P.; Kobayashi, M. Chapter 17: Pneumococcal Disease. In Pinkbook: Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; CDC, 2021. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html (accessed on 19 October 2024).
- Centers for Disease Control and Prevention (CDC). Global Polio Eradication Initiative Strategic Plan, 2004. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 107–108. [Google Scholar]
- John, T.J. A developing country perspective on vaccine-associated paralytic poliomyelitis. Bull. World Health Organ. 2004, 82, 53–58. [Google Scholar] [PubMed]
- Felsenfeld, O.; Wolf, R.; Gyr, K.; Grant, L.; Dutta, N.; Zarifi, A.; Zafari, Y. Simultaneous vaccination against cholera and yellow fever. Lancet 1973, 301, 457–458. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; World Health Organization: Geneva, Switzerland, 2018; Available online: https://iris.who.int/handle/10665/272364 (accessed on 21 October 2024).
- World Health Organization. Rotavirus vaccines: WHO position paper—July 2021. Wkly. Epidemiol. Rec. 2021, 96, 19. [Google Scholar]
- Lawrence, J.; He, S.; Martin, J.; Schödel, F.; Ciarlet, M.; Murray, A.V. Safety and immunogenicity of pentavalent rotavirus vaccine in a randomized, double-blind, placebo-controlled study in healthy elderly subjects. Hum. Vaccines Immunother. 2014, 10, 2247–2254. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Red Book: 2024–2027 Report of the Committee on Infectious Diseases (33rd Edition). Vol Book Chapter: Rubella. AAP Publications. 2024. Available online: https://publications.aap.org/redbook/book/755/chapter/14081184/Rubella (accessed on 1 October 2024).
- WHO TEAM Immunization. Vaccines and Biologicals (IVB). Rubella Vaccines: WHO Position Paper—July 2020. Wkly. Epidemiol. Rec. 2020, 95. Available online: https://www.who.int/publications/i/item/WHO-WER9527 (accessed on 1 October 2024).
- Hanson, K.E.; Marin, M.; Daley, M.F.; Groom, H.C.; Jackson, L.A.; Sy, L.S.; Klein, N.P.; DeSilva, M.B.; Panagiotakopoulos, L.; Weintraub, E.; et al. Safety of measles, mumps, and rubella vaccine in adolescents and adults in the vaccine safety Datalink. Vaccine X 2023, 13, 100268. [Google Scholar] [CrossRef]
- World Health Organization. Varicella and Herpes Zoster Vaccines: WHO Position Paper; World Health Organisation: Geneva, Switzerland, 2014; pp. 265–288. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/varicella (accessed on 8 October 2024).
- Macartney, K.; McIntyre, P. Vaccines for post-exposure prophylaxis against varicella (chickenpox) in children and adults. Cochrane Database Syst Rev. 2008, 3, CD001833. [Google Scholar] [CrossRef]
- Merck & Co., Inc. Varivax [Package Insert]. 2023. Available online: https://www.merckvaccines.com/varivax/ (accessed on 27 November 2024).
- Marin, M.; Leung, J.; Gershon, A.A. Transmission of Vaccine-Strain Varicella-Zoster Virus: A Systematic Review. Pediatrics 2019, 144, e20191305. [Google Scholar] [CrossRef]
- Lawrence, R.M. Chapter 13—Transmission of Infectious Diseases Through Breast Milk and Breastfeeding. In Breastfeeding, 7th ed.; Lawrence, R.A., Lawrence, R.M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 406–473. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Mpox Considerations for People Who Are Pregnant or Breastfeeding; CDC, 2024. Available online: https://www.cdc.gov/mpox/hcp/clinical-care/pregnancy.html (accessed on 11 November 2024).
- World Health Organisation. Smallpox and mpox orthopoxviruses vaccine position paper. Wkly. Epidemiol. Rec. 2024, 99, 28. [Google Scholar]
- Jentes, E.S.; Poumerol, G.; Gershman, M.D.; Hill, D.R.; Lemarchand, J.; Lewis, R.F.; Staples, J.E.; Tomori, O.; Wilder-Smith, A.; Monath, T.P. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect. Dis. 2011, 11, 622–632. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines and vaccination against yellow fever: WHO Position Paper—June 2013. Wkly. Epidemiol. Rec. 2013, 88, 269–283. [Google Scholar]
- Fu, W.; Sivajohan, B.; McClymont, E.; Albert, A.; Elwood, C.; Ogilvie, G.; Money, D. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int. J. Gynecol. Obstet. 2022, 156, 406–417. [Google Scholar] [CrossRef]
- Muyldermans, J.; De Weerdt, L.; De Brabandere, L.; Maertens, K.; Tommelein, E. The Effects of COVID-19 Vaccination on Lactating Women: A Systematic Review of the Literature. Front. Immunol. 2022, 13, 852928. [Google Scholar] [CrossRef]
- Mulleners, S.J.; Juncker, H.G.; Ruhé, E.J.M.; Korosi, A.; van Goudoever, J.B.; van Gils, M.J.; van Keulen, B.J. Comparing the SARS-CoV-2-specific antibody response in human milk after homologous and heterologous booster vaccinations. Commun. Biol. 2023, 6, 100. [Google Scholar] [CrossRef]
- Perez, M.J.; Paul, R.; Raghuraman, N.; Carter, E.B.; Odibo, A.O.; Kelly, J.C.; Foeller, M.E. Characterizing initial COVID-19 vaccine attitudes among pregnancy-capable healthcare workers. Am. J. Obstet. Gynecol. MFM 2022, 4, 100557. [Google Scholar] [CrossRef]
- Dudley, M.Z.; A Halsey, N.; Omer, S.B.; Orenstein, W.A.; O’Leary, S.T.; Limaye, R.J.; A Salmon, D. The state of vaccine safety science: Systematic reviews of the evidence. Lancet Infect. Dis. 2020, 20, e80–e89. [Google Scholar] [CrossRef]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2023, 13, 1081107. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Augustine, R.A.; Grattan, D.R. Hormone Interactions Regulating Energy Balance During Pregnancy. J. Neuroendocr. 2010, 22, 805–817. [Google Scholar] [CrossRef]
- Khardori, R.; Adamski, A.; Khardori, N. Infection, Immunity, and Hormones/Endocrine Interactions. Infect. Dis. Clin. N. Am. 2007, 21, 601–615. [Google Scholar] [CrossRef]
- Rojas, A.; Hachey, W.; Kaur, G.; Korejwo, J.; Muhammad, R. Enhanced safety surveillance of STAMARIL® yellow fever vaccine provided under the expanded access investigational new drug program in the USA. J. Travel Med. 2023, 30, taad037. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; O’Brien, P.; Morris, E.; Draycott, T.; Lees, C.; Ladhani, S. Monkeypox vaccines in pregnancy: Lessons must be learned from COVID-19. Lancet Glob. Health 2022, 10, e1230–e1231. [Google Scholar] [CrossRef]
- Hanson, L.A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 1998, 81, 523–533, quiz 533–534, 537. [Google Scholar] [CrossRef]
- Rodrigues, F.; Ziade, N.; Jatuworapruk, K.; Caballero-Uribe, C.V.; Khursheed, T.; Gupta, L. The Impact of Social Media on Vaccination: A Narrative Review. J. Korean Med Sci. 2023, 38, e326. [Google Scholar] [CrossRef]
- Ruggeri, K.; Vanderslott, S.; Yamada, Y.; Argyris, Y.A.; Većkalov, B.; Boggio, P.S.; Fallah, M.P.; Stock, F.; Hertwig, R. Behavioural interventions to reduce vaccine hesitancy driven by misinformation on social media. BMJ 2024, 384, e076542. [Google Scholar] [CrossRef]
- World Health Organisation; Akbar, R. Ten Threats to Global Health in 2019; World Health Organisation: Geneva, Switzerland, 2019; Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 2 June 2022).
- De Brabandere, L.; Hendrickx, G.; Poels, K.; Daelemans, W.; Van Damme, P.; Maertens, K. Influence of the COVID-19 pandemic and social media on the behaviour of pregnant and lactating women towards vaccination: A scoping review. BMJ Open 2023, 13, e066367. [Google Scholar] [CrossRef]
- Razzaghi, H.; Yankey, D.; Vashist, K.; Lu, P.-J.; Kriss, J.L.; Nguyen, K.H.; Lee, J.; Ellington, S.; Polen, K.; Bonner, K.; et al. COVID-19 vaccination coverage and intent among women aged 18–49 years by pregnancy status, United States, April–November 2021. Vaccine 2022, 40, 4554–4563. [Google Scholar] [CrossRef]
- Razai, M.S.; Mansour, R.; Ravindran, P.; Freeman, S.; Mason-Apps, C.; Morris, J.; Majeed, A.; Ussher, M.; Hargreaves, S.; Oakeshott, P. Facilitators and barriers to vaccination uptake in pregnancy: A qualitative systematic review. PLoS ONE 2024, 19, e0298407. [Google Scholar] [CrossRef]
- Schmid, P.; Habersaat, K.B.; MacDonald, N.E. WHO: How to Respond to Vocal Vaccine Deniers in Public: Best Practice Guidance. 2021. Available online: https://www.who.int/europe/teams/vaccine-preventable-diseases-immunization (accessed on 16 March 2025).
- Cooper, S.; Schmidt, B.-M.; Sambala, E.Z.; Swartz, A.; Colvin, C.J.; Leon, N.; Wiysonge, C.S. Factors that influence parents’ and informal caregivers’ views and practices regarding routine childhood vaccination: A qualitative evidence synthesis. Cochrane Database Syst. Rev. 2021, 10, CD013265. [Google Scholar] [CrossRef] [PubMed]
| Vaccine (No. Studies) | Safety | Immunogenicity | |||||
|---|---|---|---|---|---|---|---|
| Adverse Reactions Mother | Vaccine Shedding | Adverse Reactions Infant | Maternal Serum | Maternal Human Milk | Infant Sample | Effectiveness for Infants | |
| Influenza (2) | RCT [21] | RCT [21] | RCT [21] | RCT [21] | RCT [21] NRS [22] | No data | No data |
| Rotavirus (1) | RCT [23] | RCT [23] | No data | RCT [23] | RCT [23] | No data | No data |
| Rubella (7) | RCT [24] NRS [25,26] CR [27] | NRS [28,29] CR [27,30] | NRS [25,28] CR [30] | NRS [25,26,29] CR [27,30] | NRS [28,29] CR [27] | NRS [25,28] CR [27,30] | No data |
| Varicella (3) | NRS [31] CR [32,33] | NRS [31] CR [32,33] | NRS [31] CR [32,33] | NRS [31] | No data | NRS [31] | No data |
| Smallpox (1) | No data | CR [34] | CR [34] | No data | No data | No data | No data |
| Yellow fever (6) | CR [35,36,37] | NRS [38] CS [39] CR [35,36] | NRS [38] CS [39] CR [35,36,37,40] | NRS [38] CS [39] | No data | CR [35,36,37] | No data |
| COVID-19 (41) | NRS A CR [41] | NRS B | NRS C CR [42] | NRS D | NRS E | NRS F | No data |
| Cholera (6) | No data | No data | No data | RCT [43,44] NRS [45,46,47] | RCT [43,44] NRS [45,46,47] | No data | NRS [48] |
| Pertussis (3) | No data | No data | No data | NRS [49] | NRS [49,50] | No data | NRS [51] |
| Pneumococcal (1) | No data | No data | No data | NRS [51] | NRS [51] | No data | No data |
| Polio (1) | No data | No data | No data | NRS [52] | NRS [52] | No data | No data |
| Typhoid (2) | No data | No data | No data | RCT [44] NRS [45] | RCT [44] NRS [45] | No data | No data |
| Rabies (1) | No data | No data | No data | CR [53] | No data | No data | CR [53] |
| Vaccine | Recommendation | Summary of Findings (GRADE) | ||
|---|---|---|---|---|
| Breastfeeding|Considerations | Lactating Mothers | Infants | ||
| Cholera | ✓ | No special considerations. |
|
|
| COVID-19 | ✓ | Prefer mRNA vaccines due to a more favorable/stable immune response. |
|
|
| Influenza | ✓ | Prefer IIV due to a more favorable immunogenicity and safety profile. |
|
|
| Pertussis | ✓ | No special considerations. |
|
|
| Pneumo-coccal | ✓ | No special considerations. |
|
|
| Polio | ✓ | Prefer IPV due to a more favorable/stable immune response. |
|
|
| Rabies | ✓✎ | Given the high mortality of rabies, timely vaccination is justified when warranted. |
|
|
| Rotavirus | ✓ | No special considerations and lack of clinical relevance: vaccine indication for infants, not for adult use. |
|
|
| Rubella | ✓✎ | Consider that while HM shedding is possible, no clinical consequences have been observed; manage expectations for potential side effects. a |
|
|
| Smallpox | ✘✎ | Given the risk of contact vaccinia, advice on precautionary measures to prevent infant exposure; also, for prevention of smallpox/Mpox, consider newer generation vaccines over ACAM2000. b |
|
|
| Typhoid | ✓ | No special considerations. |
|
|
| Varicella | ✓✎ | Safe to use without breastfeeding interruption; advise caution and medical guidance if vesicular rash occurs post-vaccination. |
|
|
| Yellow fever | ✘✎ | If possible, postpone. Otherwise, consider breastfeeding interruption for ≥ 3 weeks and provide guidance on maintaining milk supply and infant feeding. |
|
|
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity (Oral Vaccines) | RCTs | High | None | High |
| Immunogenicity (Parenteral Vaccines) | Observational | Low | Risk of bias (+1) | Moderate |
| Infant Immunity (Oral Vaccines) | RCTs | High | Indirectness (−1) | Moderate |
| Infant Immunity (Parenteral Vaccines) | Observational | Low | Indirectness (−1), Inconsistency (−1) | Very Low |
| Effectiveness in Infants | Observational | Low | Imprecision (−1), Indirectness (+1) | Low |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity in Lactating Individuals | RCT + Observational | High | Risk of bias (−1), Imprecision (−1) | Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Indirectness (−1), Imprecision (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | RCT | High | None | High |
| Safety in Infants | RCT | High | None | High |
| Immunogenicity in Lactating Individuals | RCT | High | None | High |
| Infant Immunity Through Human Milk | RCT | High | Indirectness (−1) | Moderate |
| T-cell Response in Human Milk | Cross-sectional | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity in Lactating Individuals | Observational | Low | Risk of bias (−1) | Very Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Indirectness (−1) | Very Low |
| Effectiveness in Infants | Case-control | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity in Lactating Individuals | Observational | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Imprecision (−1), Indirectness (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity in Lactating Individuals | No studies | N/A | Risk of bias (−1), Imprecision (−1), Indirectness (−1) | Very Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Imprecision (−1), Indirectness (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | No studies | N/A | N/A | No evidence |
| Immunogenicity in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Infant Immunity Through Human Milk | No studies | N/A | N/A | No evidence |
| Effectiveness | Case report | Low | Risk of bias (−1), Imprecision (−1), Indirectness (−1) | Very Low |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | RCT | High | Risk of bias (−1), Imprecision (−1) | Moderate |
| Safety in Infants | RCT | High | Risk of bias (−1), Indirectness (−1) | Moderate |
| Immunogenicity in Lactating Individuals | RCT | High | Risk of bias (−1), Imprecision (−1) | Moderate |
| Infant Immunity Through Human Milk | RCT | High | Risk of bias (−1), Indirectness (−1) | Moderate |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | RCT + Observational | High | Risk of bias (−1), Inconsistency (−1) | Moderate |
| Safety in Infants | Observational | Low | Inconsistency (−1), Large effect (+1) | Low |
| Immunogenicity in Lactating Individuals | RCT + Observational | High | Risk of bias (−1), Inconsistency (−1) | Moderate |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Inconsistency (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | Observational | Low | Imprecision (−1) | Very Low |
| Safety in Infants | Observational + Case reports | Low | Imprecision (−1), Inconsistency (−1) | Very Low |
| Immunogenicity in Lactating Individuals | Observational | Low | None (consistent findings) | Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Safety in Infants | Case report | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Immunogenicity in Lactating Individuals | No studies | N/A | N/A | No evidence |
| Infant Immunity Through Human Milk | No studies | N/A | N/A | No evidence |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | Observational + Case reports | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Safety in Infants | Observational + Case series | Low | Risk of bias (−1), Inconsistency (−1) | Very Low |
| Immunogenicity in Lactating Individuals | Observational | Low | Risk of bias (−1), Imprecision (−1) | Very Low |
| Infant Immunity Through Human Milk | Case reports | Low | Risk of bias (−1), Imprecision (−1), Indirectness (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Outcome | Study Design | Starting Level | Down/Upgrading Domains | Final Certainty |
|---|---|---|---|---|
| Safety in Lactating Individuals | Observational | Low | Risk of bias (−1) | Very Low |
| Safety in Infants | Observational | Low | Risk of bias (−1) | Very Low |
| Immunogenicity in Lactating Individuals | Observational | Low | None (consistent findings across multiple studies) | Low |
| Infant Immunity Through Human Milk | Observational | Low | Risk of bias (−1), Indirectness (−1) | Very Low |
| Effectiveness | No studies | N/A | N/A | No evidence |
| Vaccine | Study Design [ref] | Population (Group) | Mothers (n/N) | Infants (n/N) | Viral Detection (+/Total) | Vaccine Strain Confirmed | Infant Serology | Infant Symptoms | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mother, HM | Mother, Other | Infant, Sample | ||||||||
| Yellow fever | NRS [38] | Vaccinated LM | 10/11 | 0 | 0/28 | 0/30 | NT | - | NT | 0/11 |
| CS [39] | Symptomatic infants | 8 | 8 | 11/42 | 2/8 | NT | NT | 0/8 | YF-like illness | |
| CR [35] | Symptomatic infant | 1 | 1 | NT | NT | 0/1 | - | Pos. | YEL-AND-like illness | |
| CR [36] | Symptomatic infant | 1 | 1 | NT | NT | 1/2 | Infant CSF | Pos. | YEL-AND | |
| CR [37] | Symptomatic infant | 1 | 1 | NT | NT | NT | NT | Pos. | YEL-AND like illness | |
| Rubella | NRS [25] | LM | 949 | 63 | NT | NT | NT | - | Neg. | None |
| NRS [29] | LM (vaccine 1) | 4 | 0 | 3/4 | 4/4 | NT | NT | NT | NR | |
| LM (vaccine 2) | 4 | 0 | 4/4 | 4/4 | NT | NT | NT | NR | ||
| LM (vaccine 3) | 5 | 0 | 2/5 | 2/5 | NT | NT | NT | NR | ||
| NRS [28] | LM (breastfeeding) | 16 | 16 | 11/16 | 9/16 | 9/16 | NT | 4/16 | 0/16 | |
| LM (non-breastfeeding) | 10 | 10 | NT | 5/10 | 0/10 | NT | 0/10 | 0/10 | ||
| CR [27] | Symptomatic mother | 1 | 1 | 1/3 | 0/1 | 1/2 | NT | Pos. | None | |
| CR [30] | Symptomatic infant | 1 | 1 | 0/1 | 0/1 | 0/3 | - | Pos. | Rubella-like symptoms | |
| Varicella | NRS [31] | LM | 12 | 12 | 0/217 | 0/1 | 0/6 | - | 0/12 | 0/12 |
| CR [33] | Symptomatic infant | 1 | 1 | NT | NT | 1/1 | Infant rash | NT | Mild varicella disease | |
| CR [32] | Symptomatic infant | 1 | 1 | NT | 1/1 | 3/3 | Infant CSF, infant rash, maternal rash | Pos. | Extensive varicella disease | |
| Influenza | RCT [21] | LM (live vaccine) | 120/124 | 120/124 | 0/359 | 71/359 | 1/359 | Maternal nasal swabs | NT | None |
| LM (inactive vaccine) | 31/124 | 31/124 | 0/93 | 0/93 | 0/93 | None | NT | None | ||
| Rotavirus | NRS [23] | LM (vaccine) | 21 | 21 | NT | 1/55 | 0/39 | NT | NR | NR |
| LM (placebo) | 21 | 11 | NT | 0/30 | 0/21 | - | NR | NR | ||
| Smallpox | CR [34] | Symptomatic infant | 1 | 1 | NT | NT | 2/2 | Infant rash | 2/2 | Vaccinia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulleners, S.J.; Juncker, H.G.; Zuiderveld, J.; Ziesemer, K.A.; van Goudoever, J.B.; van Keulen, B.J. Safety and Efficacy of Vaccination During Lactation: A Comprehensive Review of Vaccines for Maternal and Infant Health Utilizing a Large Language Model Citation Screening System. Vaccines 2025, 13, 350. https://doi.org/10.3390/vaccines13040350
Mulleners SJ, Juncker HG, Zuiderveld J, Ziesemer KA, van Goudoever JB, van Keulen BJ. Safety and Efficacy of Vaccination During Lactation: A Comprehensive Review of Vaccines for Maternal and Infant Health Utilizing a Large Language Model Citation Screening System. Vaccines. 2025; 13(4):350. https://doi.org/10.3390/vaccines13040350
Chicago/Turabian StyleMulleners, Sien J., Hannah G. Juncker, Jan Zuiderveld, Kirsten A. Ziesemer, Johannes B. van Goudoever, and Britt J. van Keulen. 2025. "Safety and Efficacy of Vaccination During Lactation: A Comprehensive Review of Vaccines for Maternal and Infant Health Utilizing a Large Language Model Citation Screening System" Vaccines 13, no. 4: 350. https://doi.org/10.3390/vaccines13040350
APA StyleMulleners, S. J., Juncker, H. G., Zuiderveld, J., Ziesemer, K. A., van Goudoever, J. B., & van Keulen, B. J. (2025). Safety and Efficacy of Vaccination During Lactation: A Comprehensive Review of Vaccines for Maternal and Infant Health Utilizing a Large Language Model Citation Screening System. Vaccines, 13(4), 350. https://doi.org/10.3390/vaccines13040350







