The Evaluation of a Porcine Circovirus Type 2 (PCV2) Intradermal Vaccine Against a PCV2 Field Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Safety Study
2.2. Efficacy Study
2.2.1. Porcine Circovirus Type 2 (PCV2) Antibody Titres
2.2.2. Monitoring of Clinical Parameters and Average Daily Gain
2.2.3. Porcine Circovirus Type 2 (PCV2) Virus Shedding Post-Challenge
2.2.4. Porcine Circovirus Type 2 (PCV2) Serum Viremia Post-Challenge
2.2.5. Necropsy
2.2.6. Immunohistochemical Staining
2.2.7. Statistical Analysis
3. Results
3.1. Safety Study
3.2. Efficacy Study
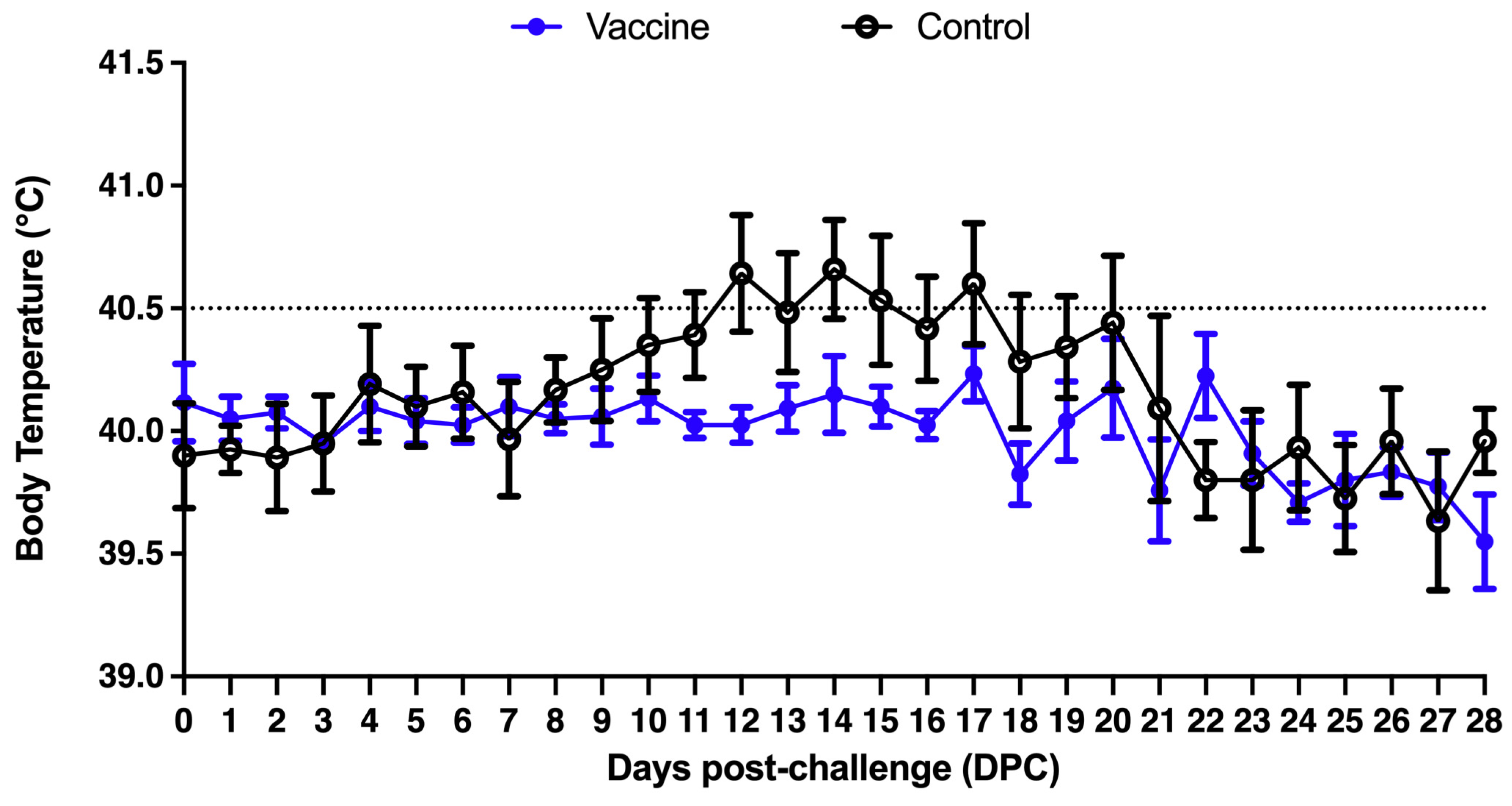
3.2.1. Clinical Manifestations and Mortality Observation Records and Body Temperature Measurement
3.2.2. Porcine Circovirus Type 2 (PCV2) Antibody Determination
3.2.3. Comparison of Clinical Observations, Mortality, Average Daily Gain, and Incidence of Stunted Pigs
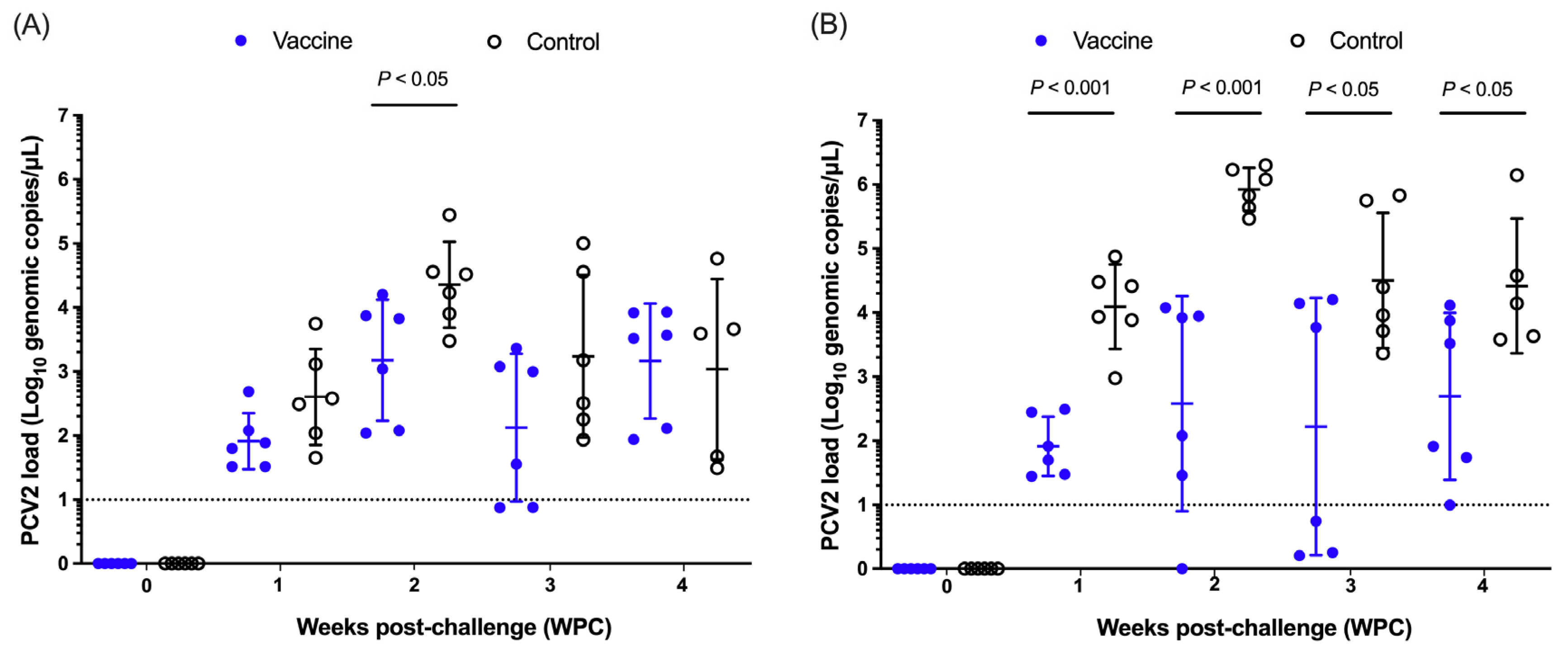
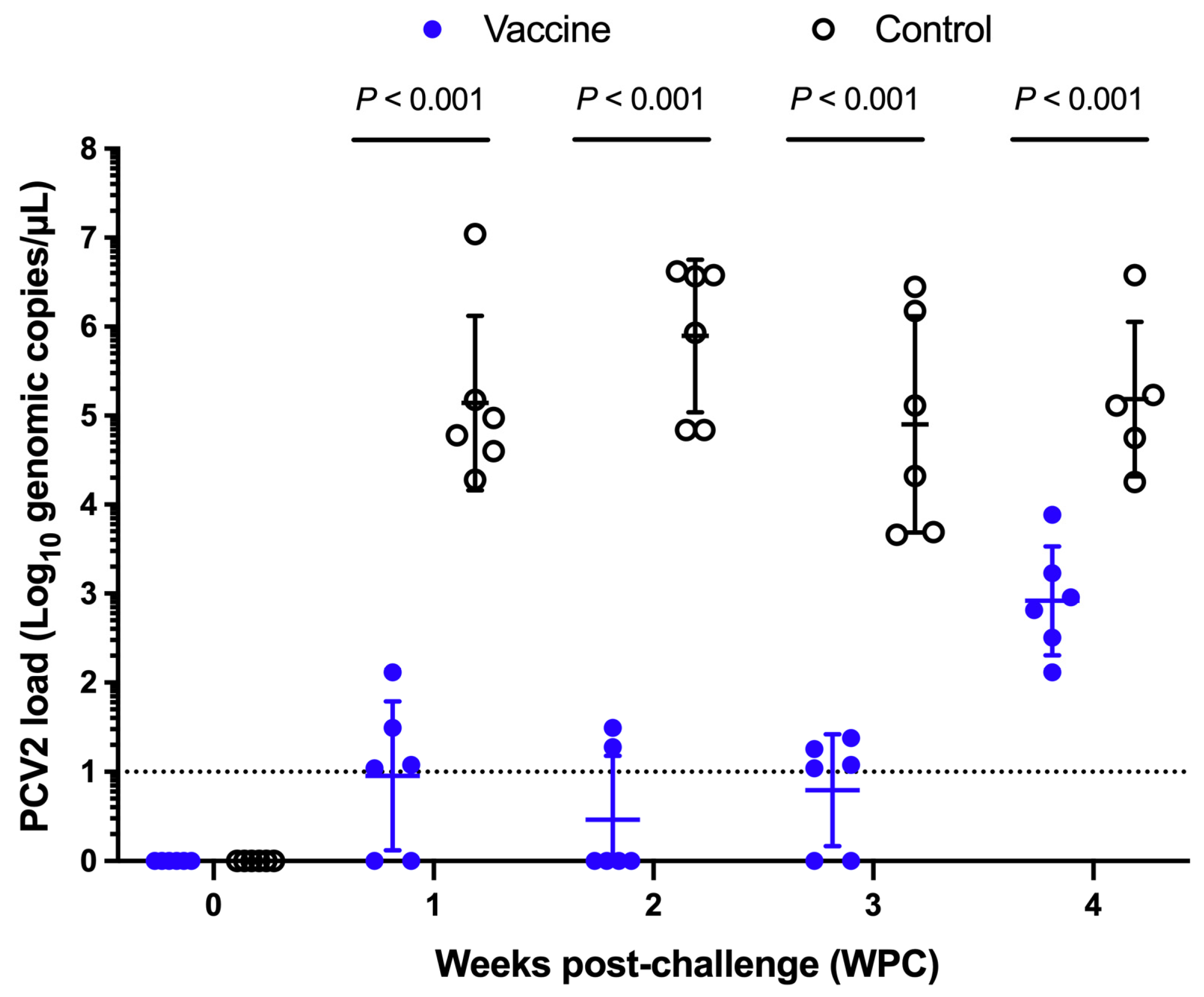
3.2.4. Nasal and Fecal Shedding of Porcine Circovirus Type 2 (PCV2)
3.2.5. Porcine Circovirus Type 2 (PCV2) Load in Serum
3.2.6. Pathological Examination
3.2.7. Immunohistochemical Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A. ICTV virus taxonomy profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997–1998. [Google Scholar] [CrossRef]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef]
- Xiao, C.T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Yu, C.; Cao, M.; Wei, Y.; Liu, J.; Zhang, H.; Liu, C.; Feng, L.; Huang, L. Evaluation of cross-immunity among major porcine circovirus type 2 genotypes by infection with PCV2b and PCV2d circulating strains. Vet. Microbiol. 2023, 283, 109796. [Google Scholar] [CrossRef]
- Kolyvushko, O.; Rakibuzzaman, A.; Pillatzki, A.; Webb, B.; Ramamoorthy, S. Efficacy of a commercial PCV2a vaccine with a two-dose regimen against PCV2d. Vet. Sci. 2019, 6, 61. [Google Scholar] [CrossRef]
- Fort, M.; Sibila, M.; Pérez-Martín, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef]
- Martelli, P.; Ferrari, L.; Morganti, M.; De Angelis, E.; Bonilauri, P.; Guazzetti, S.; Caleffi, A.; Borghetti, P. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 2011, 149, 339–351. [Google Scholar] [CrossRef]
- Martelli, P.; Saleri, R.; Ferrarini, G.; De Angelis, E.; Cavalli, V.; Benetti, M.; Ferrari, L.; Canelli, E.; Bonilauri, P.; Arioli, E. Impact of maternally derived immunity on piglets’ immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCV2 at different age. BMC Vet. Res. 2016, 12, 77. [Google Scholar] [CrossRef]
- Oh, Y.; Seo, H.W.; Park, C.; Chae, C. Comparison of sow and/or piglet vaccination of 3 commercial porcine circovirus type 2 (PCV2) single-dose vaccines on pigs under experimental PCV2 challenge. Vet. Microbiol. 2014, 172, 371–380. [Google Scholar] [CrossRef]
- Kristensen, C.S.; Baadsgaard, N.P.; Toft, N. A meta-analysis comparing the effect of PCV2 vaccines on average daily weight gain and mortality rate in pigs from weaning to slaughter. Prev. Vet. Med. 2011, 98, 250–258. [Google Scholar] [CrossRef]
- Afghah, Z.; Webb, B.; Meng, X.-J.; Ramamoorthy, S. Ten years of PCV2 vaccines and vaccination: Is eradication a possibility? Vet. Microbiol. 2017, 206, 21–28. [Google Scholar] [CrossRef]
- Dvorak, C.M.; Yang, Y.; Haley, C.; Sharma, N.; Murtaugh, M.P. National reduction in porcine circovirus type 2 prevalence following introduction of vaccination. Vet. Microbiol. 2016, 189, 86–90. [Google Scholar] [CrossRef]
- Có-Rives, I.; Chen, Y.A.; Moore, A.C. Skin-based vaccination: A systematic mapping review of the types of vaccines and methods used and immunity and protection elicited in pigs. Vaccines 2023, 11, 450. [Google Scholar] [CrossRef]
- Owen, K.; Blackie, N.; Gibson, T.J. The effect of needle reuse on piglet skin puncture force. Vet. Sci. 2022, 9, 90. [Google Scholar] [CrossRef]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Safety of PRRSV-2 MLV vaccines administrated via the intramuscular or intradermal route and evaluation of PRRSV transmission upon needle-free and needle delivery. Sci. Rep. 2021, 11, 23107. [Google Scholar] [CrossRef]
- Salman, M.; Lin, H.; Suntisukwattana, R.; Watcharavongtip, P.; Jermsutjarit, P.; Tantituvanont, A.; Nilubol, D. Intradermal needle-free injection prevents African Swine Fever transmission, while intramuscular needle injection does not. Sci. Rep. 2023, 13, 4600. [Google Scholar]
- Patterson, A.R.; Ramamoorthy, S.; Madson, D.M.; Meng, X.J.; Halbur, P.G.; Opriessnig, T. Shedding and infection dynamics of porcine circovirus type 2 (PCV2) after experimental infection. Vet. Microbiol. 2011, 149, 91–98. [Google Scholar] [CrossRef]
- Imeah, B.; Penz, E.; Rana, M.; Trask, C. Needle-less Injector Study Team. Economic analysis of new workplace technology including productivity and injury: The case of needle-less injection in swine. PLoS ONE 2020, 15, e0233599. [Google Scholar] [CrossRef]
- Daniels, C.S.; Funk, J.A. Prevalence of carcass defects in market swine at harvest. In Proceedings of the International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Québec, QC, Canada, 30 September–2 October 2009; pp. 281–282. [Google Scholar] [CrossRef]
- King, D.; Painter, T.; Holtkamp, D.; DuBois, P.; Wang, C. Effect of injection tool on incidence of head and neck abscesses at slaughter. J. Swine Health Prod. 2010, 18, 290–293. [Google Scholar] [CrossRef]
- Blaha, T.G. Pre-harvest food safety as integral part of quality assurance systems in the pork chain from “stable to table”. In Proceedings of the International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork, Maastricht, The Netherlands, 19–22 June 2001. [Google Scholar] [CrossRef]
- Weniger, B.G.; Glenn, G.M. Cutaneous vaccination: Antigen delivery into or onto the skin. Vaccine 2013, 31, 3389–3391. [Google Scholar] [CrossRef]
- Le Luduec, J.B.; Debeer, S.; Piras, F.; Andreoni, C.; Boudet, F.; Laurent, P.; Kaiserlian, D.; Dubois, B. Intradermal vaccination with un-adjuvanted sub-unit vaccines triggers skin innate immunity and confers protective respiratory immunity in domestic swine. Vaccine 2016, 34, 914–922. [Google Scholar] [CrossRef]
- Martelli, P.; Gozio, S.; Ferrari, L.; Rosina, S.; De Angelis, E.; Quintavalla, C.; Bottarelli, E.; Borghetti, P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine 2009, 27, 3788–3799. [Google Scholar] [CrossRef]
- Ferrari, L.; Martelli, P.; Saleri, R.; De Angelis, E.; Cavalli, V.; Bresaola, M.; Benetti, M.; Borghetti, P. Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection. Vet. Immunol. Immunopathol. 2013, 151, 193–206. [Google Scholar]
- Chiu, H.J.; Chang, S.W.; Lin, H.; Chuang, Y.C.; Kuo, K.L.; Lin, C.H.; Chiou, M.T.; Lin, C.N. Lingeage 7 porcine reproductive and respiratory syndrome vaccine demonstrates cross-protection against lineage 1 and lineage 3 strains. Vaccines 2025, 13, 102. [Google Scholar] [CrossRef]
- Chen, C.C.; Hung, Y.C.; Lin, C.Y.; Chi, T.Y.; Chen, H.Y.; Chiu, C.C.; Huang, P.M.; Wu, T.H.; Lin, J.S.; Lin, P.H.; et al. A viral challenge pig model with porcine circovirus disease. Austin J. Vet. Sci. Anim. Husb. 2020, 7, 1070. [Google Scholar]
- Tsai, G.T.; Lin, Y.C.; Lin, W.H.; Lin, J.H.; Chiou, M.T.; Liu, H.F.; Lin, C.N. Phylogeographic and genetic characterization of porcine circovirus type 2 in Taiwan from 2001–2017. Sci. Rep. 2019, 9, 10782. [Google Scholar] [CrossRef]
- Opriessnig, T.; Thacker, E.L.; Yu, S.; Fenaux, M.; Meng, X.J.; Halbur, P.G. Expdrimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 2004, 41, 624–640. [Google Scholar] [CrossRef]
- Horsington, J.; Witvliet, M.; Jacobs, A.A.C.; Segers, R.P.A.M. Efficacy of simultaneous intradermal vaccination of swine against Porcine Circovirus 2, Porcine Reproductive and Respiratory Syndrome Virus, Mycoplasma hyopneumoniae and Lawsonia intracellularis. Animals 2021, 11, 2225. [Google Scholar] [CrossRef]
- Hernandez-Franco, J.F.; Yadagiri, G.; Patil, V.; Bugybayeva, D.; Dolatyabi, S.; Dumkliang, E.; Singh, M.; Suresh, R.; Akter, F.; Schrock, J.; et al. Intradermal vaccination against influenza with a STING-Targeted manoparticle combination adjuvant induces superior cross-protective humoral immunity in swine compared with intranasal and intramuscular immunization. Vaccines 2023, 11, 1699. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Q.L.; Nie, L.B.; Wang, Q.; Ge, G.Y.; Li, D.L.; Ma, B.Y.; Sheng, C.Y.; Su, N.; Zong, Y.; et al. Prevalence of porcine circovirus 2 throughout China in 2015–2019: A systematic review and meta-analysis. Microb. Pathog. 2020, 149, 104490. [Google Scholar] [CrossRef]
- De Lange, F.M.; Dewey, C. Chapter 6—Management of growing-finishing pigs. In Disease of Swine, 9th ed.; Straw, B.E., Zimmerman, J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Publishing: Ames, IA, USA, 2009. [Google Scholar]
- Guo, J.; Hou, L.; Zhou, J.; Wang, D.; Cui, Y.; Feng, X.; Liu, J. Porcine circovirus type 2 vaccines: Commercial application and research advances. Viruses 2022, 14, 2005. [Google Scholar] [CrossRef]
- Engle, T.B.; Jobman, E.E.; Moural, T.W.; McKnite, A.M.; Bundy, J.W.; Barnes, S.Y.; Davis, E.H.; Galeota, J.A.; Burkey, T.E.; Plastow, G.S.; et al. Variation in time and magnitude of immune response and viremia in experimental challenges with Porcine circovirus 2b. BMC Vet. Res. 2014, 10, 286. [Google Scholar] [CrossRef]
- López-Soria, S.; Sibila, M.; Nofrarías, M.; Calsamiglia, M.; Manzanilla, E.G.; Ramírez-Mendoza, H.; Mínguez, A.; Serrano, J.M.; Marín, O.; Joisel, F.; et al. Effect of porcine circovirus type 2 (PCV2) load in serum on average daily weight gain during the postweaning period. Vet. Microbiol. 2014, 174, 296–301. [Google Scholar] [CrossRef]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 93. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Pacheco, J.M.; Brito, B.P.; Moreno-Torres, K.I.; Branan, M.A.; Delgado, A.H.; Rodriguez, L.L.; Arzt, J. Transmission of Foot-and-Mouth Disease Virus during the incubation period in pigs. Front. Vet. Sci. 2016, 3, 105. [Google Scholar] [CrossRef]
- Feng, H.; Blanco, G.; Segalés, J.; Sibila, M. Can Porcine circovirus type 2 (PCV2) infection be eradicated by mass vaccination? Vet. Microbiol. 2014, 172, 92–99. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-infection of swine with porcine circovirus type 2 and other swine viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Szczotka, A.; Stadejek, T.; Pejsak, Z. A comparison of immunohistochemistry and in situ hybridization for the detection of porcine circovirus type 2 in pigs. Pol. J. Vet. Sci. 2011, 14, 565–571. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Weeks of Age | Vaccinated Group | Control Group | p Value |
|---|---|---|---|---|
| Body weight (kg) | 3 | 4.04 ± 0.21 | 3.93 ± 0.26 | 0.416 |
| 7 | 10.91 ± 2.19 | 11.82 ± 1.53 | 0.424 | |
| 11 | 21.46 ± 2.80 | 18.87 ± 4.75 | 0.276 | |
| Average daily weight gain (kg/day) | 3–7 | 0.245 | 0.282 | 0.3939 |
| 7–11 | 0.377 | 0.250 | 0.0043 | |
| 3–11 | 0.311 | 0.267 | 0.6991 | |
| Mortality rate (%) | 3–11 | 0 | 16.7 | |
| Incidence of poor growth pigs (%) | 3–11 | 0 | 16.7 |
| Groups | Ear Tags | Lung | Lymph Node | Kidney | ||
|---|---|---|---|---|---|---|
| Hilar | Inguinal | Mesenteric | ||||
| Control | 605 | 0.5 | 1.5 | 1.5 | 2 | 1 |
| 607 | 1.5 | 0.5 | 0 | 1.5 | 0 | |
| 608 | 1 | 1 | 0.5 | 1.5 | 0 | |
| 609 | 2.5 | 0.5 | 3 | 1 | 0 | |
| 610 | 2.5 | 0.5 | 0 | 0.5 | 0.5 | |
| 611 | 2 | 2 | 0.5 | 2.5 | 0 | |
| Total | 7.5 | 6 | 5.5 | 9 | 1.5 | |
| Vaccine | 612 | 0 | 0 | 1 | 0.5 | 0 |
| 613 | 1 | 1.5 | 0 | 1 | 0 | |
| 615 | 1 | 0.5 | 1 | 0.5 | 0 | |
| 618 | 0.5 | 0.5 | 0.5 | 2 | 0 | |
| 619 | 1 | 1.5 | 0.5 | 2 | 0 | |
| 620 | 1.5 | 1 | 0 | 1.5 | 0 | |
| Total | 5 | 5 | 3 | 7.5 | 0 | |
| p value a | 0.092 | 0.688 | 0.748 | 0.575 | 0.336 | |
| Groups | Ear Tags | Lung | Lymph Node | Kidney | ||
|---|---|---|---|---|---|---|
| Hilar | Inguinal | Mesenteric | ||||
| Control | 605 | 1 | 1.5 | 1 | 3.5 | 4 |
| 607 | 1 | 1 | 2 | 2 | 0.5 | |
| 608 | 1.5 | 1.5 | 2.5 | 1.5 | 0.5 | |
| 609 | 3 | 1.5 | 2 | 2 | 3.5 | |
| 610 | 0 | 1 | 1 | 1.5 | 0 | |
| 611 | 1 | 1 | 1.5 | 1 | 1 | |
| Total | 7.5 | 7.5 | 10 | 11.5 | 9.5 | |
| Vaccine | 612 | 0.5 | 1 | 0.5 | 1 | 0 |
| 613 | 0 | 0.5 | 0 | 2 | 1 | |
| 615 | 0 | 0 | 1.5 | 1.5 | 0 | |
| 618 | 0 | 0 | 0 | 2 | 0 | |
| 619 | 0.5 | 0 | 0.5 | 2 | 1 | |
| 620 | 0 | 0 | 0.5 | 0 | 0 | |
| Total | 1 | 1.5 | 3 | 8.5 | 2 | |
| p value a | 0.024 | 0.008 | 0.013 | 0.575 | 0.149 | |
| Groups | Ear Tags | Lymph Node | ||
|---|---|---|---|---|
| Hilar | Inguinal | Mesenteric | ||
| Control | 605 | 1.5 | 1.5 | 2 |
| 607 | 0 | 0 | 1 | |
| 608 | 0 | 2 | 0.5 | |
| 609 | 3.5 | 2 | 3.5 | |
| 610 | 2.5 | 2 | 2.5 | |
| 611 | 0 | 1 | 0.5 | |
| Total | 7.5 | 8.5 | 10 | |
| Vaccine | 612 | 0 | 0.5 | 0.5 |
| 613 | 0 | 0 | 0 | |
| 615 | 0 | 0 | 0 | |
| 618 | 0 | 0 | 0 | |
| 619 | 0 | 0 | 0 | |
| 620 | 0 | 1 | 0 | |
| Total | 0 | 1.5 | 0.5 | |
| p value a | 0.149 | 0.030 | 0.006 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-K.; Chien, C.-Y.; Liu, C.-W.; Chang, S.-W.; Lin, H.; Ellerma, L.; Chiou, M.-T.; Lin, C.-N. The Evaluation of a Porcine Circovirus Type 2 (PCV2) Intradermal Vaccine Against a PCV2 Field Strain. Vaccines 2025, 13, 343. https://doi.org/10.3390/vaccines13040343
Hsieh C-K, Chien C-Y, Liu C-W, Chang S-W, Lin H, Ellerma L, Chiou M-T, Lin C-N. The Evaluation of a Porcine Circovirus Type 2 (PCV2) Intradermal Vaccine Against a PCV2 Field Strain. Vaccines. 2025; 13(4):343. https://doi.org/10.3390/vaccines13040343
Chicago/Turabian StyleHsieh, Cheng-Kai, Chia-Yi Chien, Chun-Wei Liu, Shu-Wei Chang, Hongyao Lin, Leonardo Ellerma, Ming-Tang Chiou, and Chao-Nan Lin. 2025. "The Evaluation of a Porcine Circovirus Type 2 (PCV2) Intradermal Vaccine Against a PCV2 Field Strain" Vaccines 13, no. 4: 343. https://doi.org/10.3390/vaccines13040343
APA StyleHsieh, C.-K., Chien, C.-Y., Liu, C.-W., Chang, S.-W., Lin, H., Ellerma, L., Chiou, M.-T., & Lin, C.-N. (2025). The Evaluation of a Porcine Circovirus Type 2 (PCV2) Intradermal Vaccine Against a PCV2 Field Strain. Vaccines, 13(4), 343. https://doi.org/10.3390/vaccines13040343







