The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting
Abstract
1. Introduction
2. Methods
2.1. Participants and Study Design
2.2. CMV Antibody Measurements
2.3. Fluorospot Assays
2.4. VZV-gE Antibody Measurements
2.5. Antibody Avidity
2.6. T Cell Phenotypic Characterization by Flow Cytometry
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
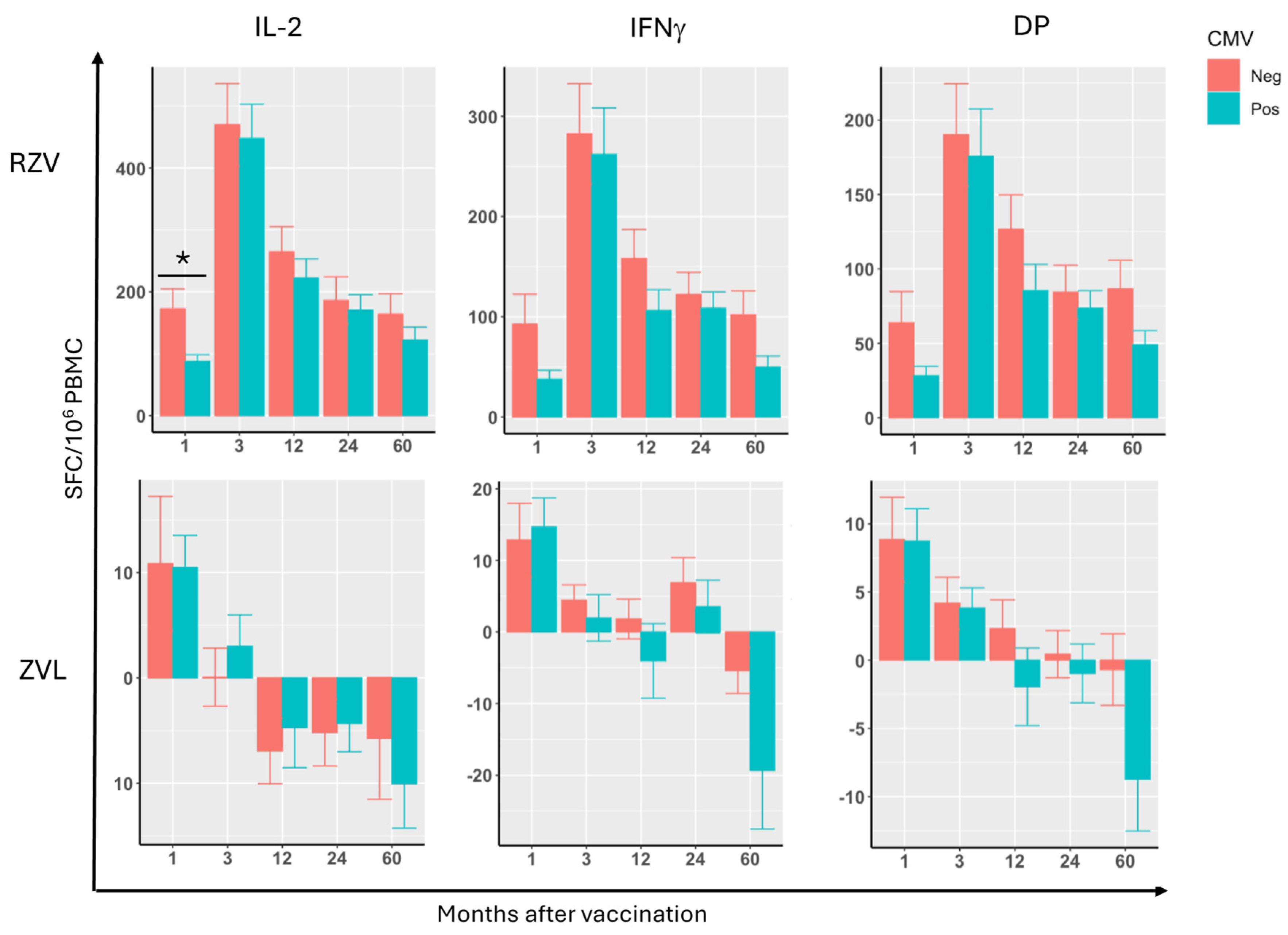
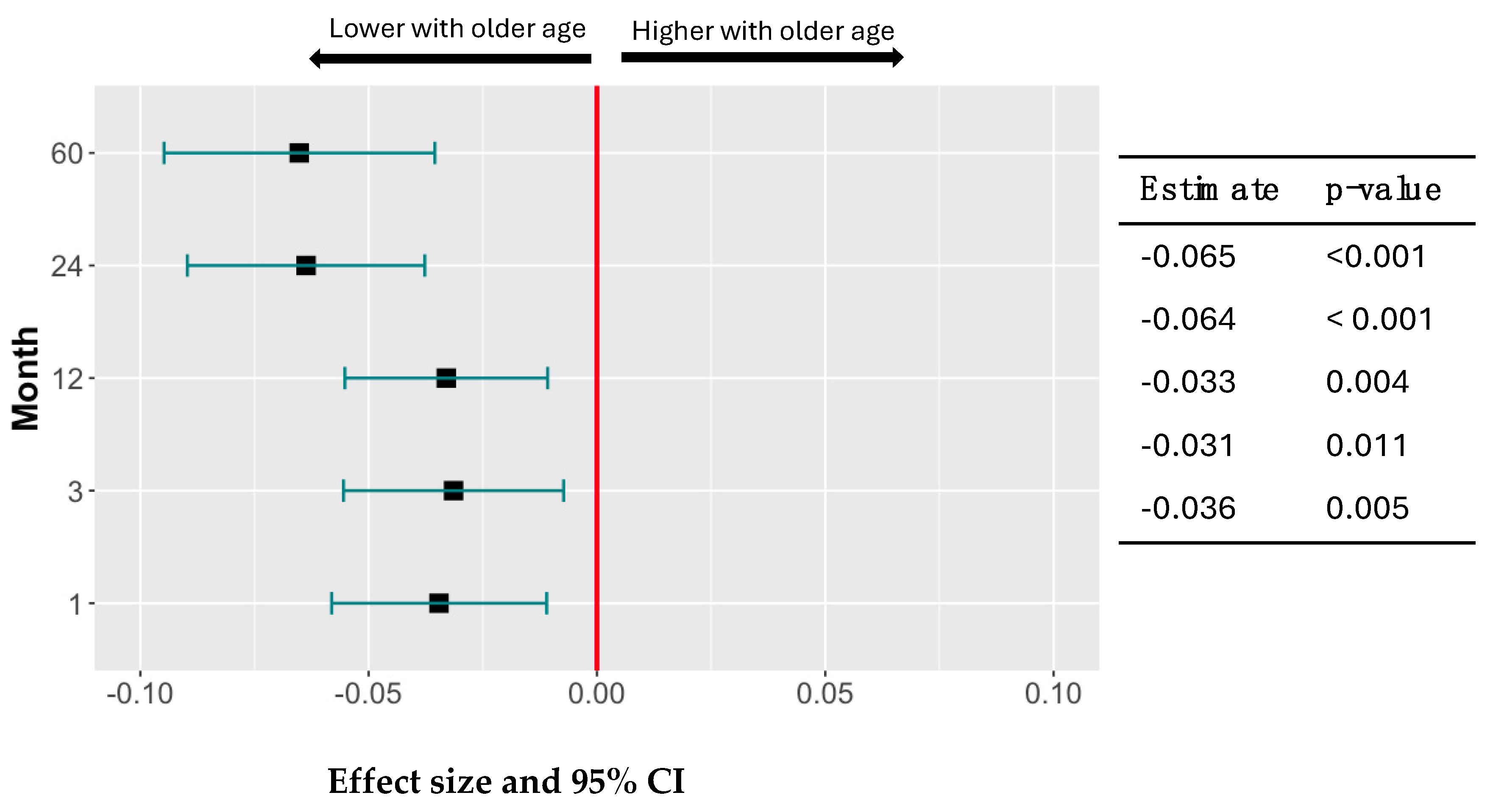
3.2. Effect of CMV Serostatus, Age, and Sex on VZV-gE-Specific CMI Responses After RZV or ZVL Administration
3.3. Effect of CMV Serostatus, Age, and Sex on Antibody Responses to VZV-gE After RZV or ZVL Administration
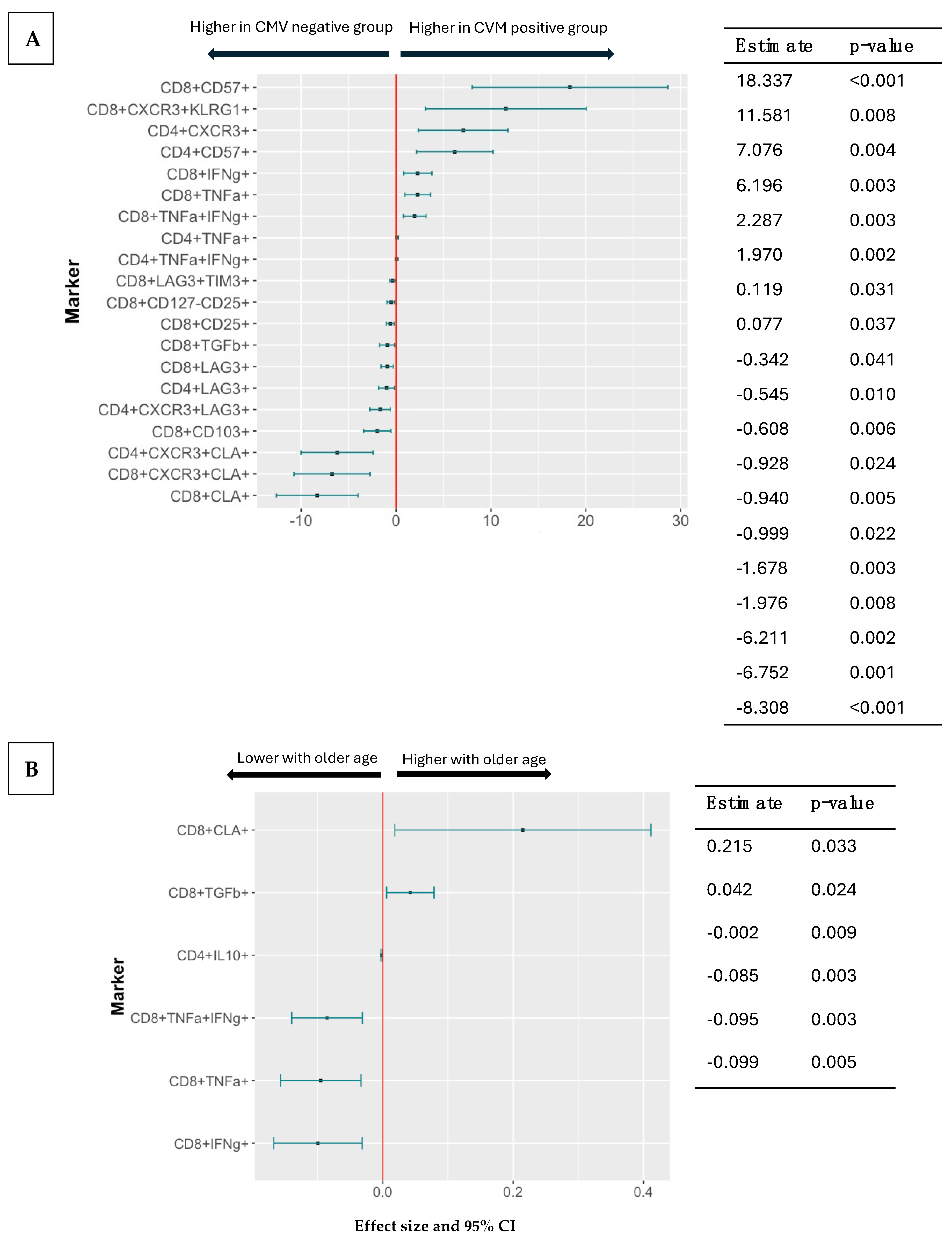
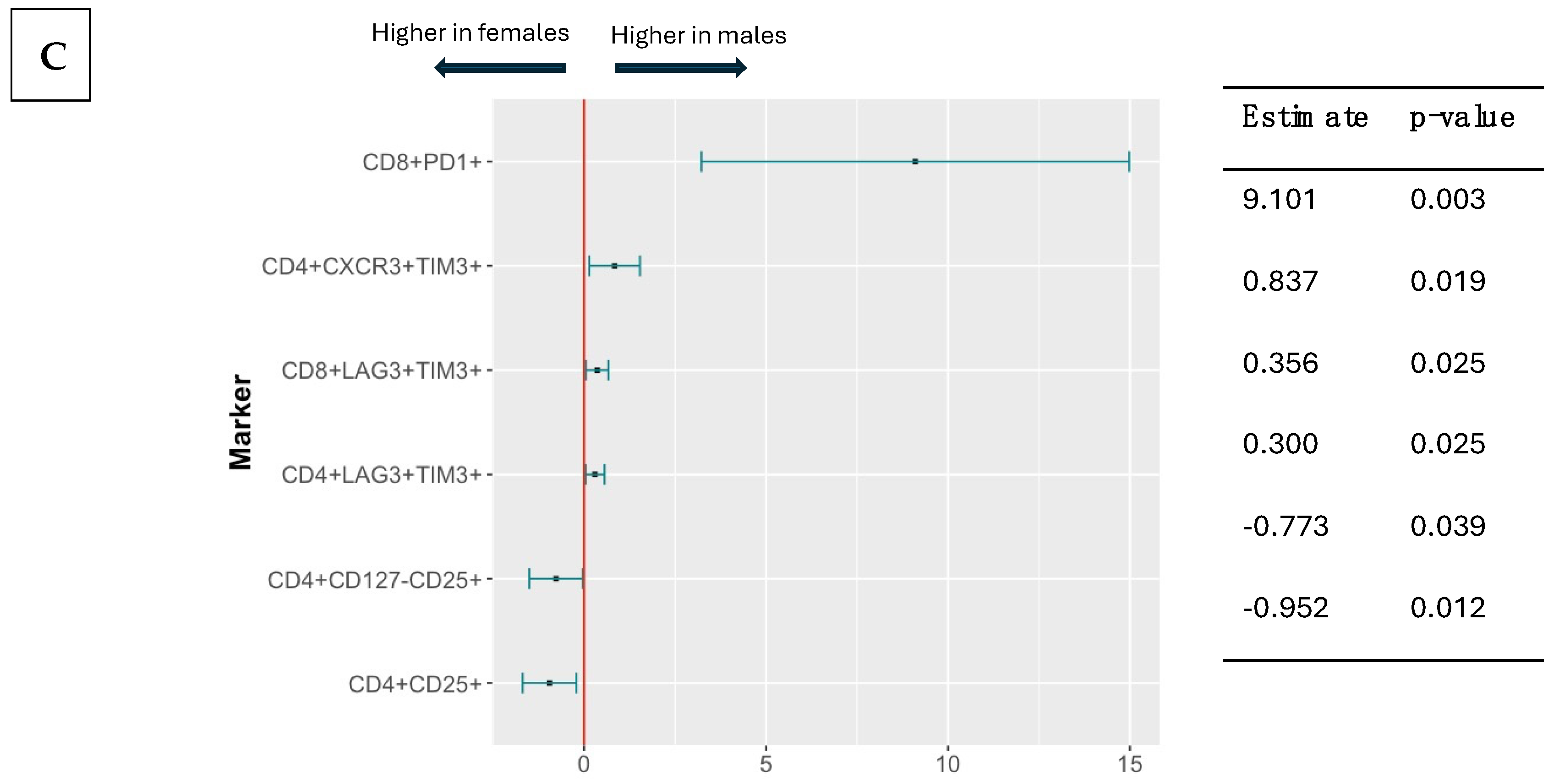
3.4. Effect of CMV Serostatus, Age, and Sex on Pre-Vaccination T Cell Phenotypes
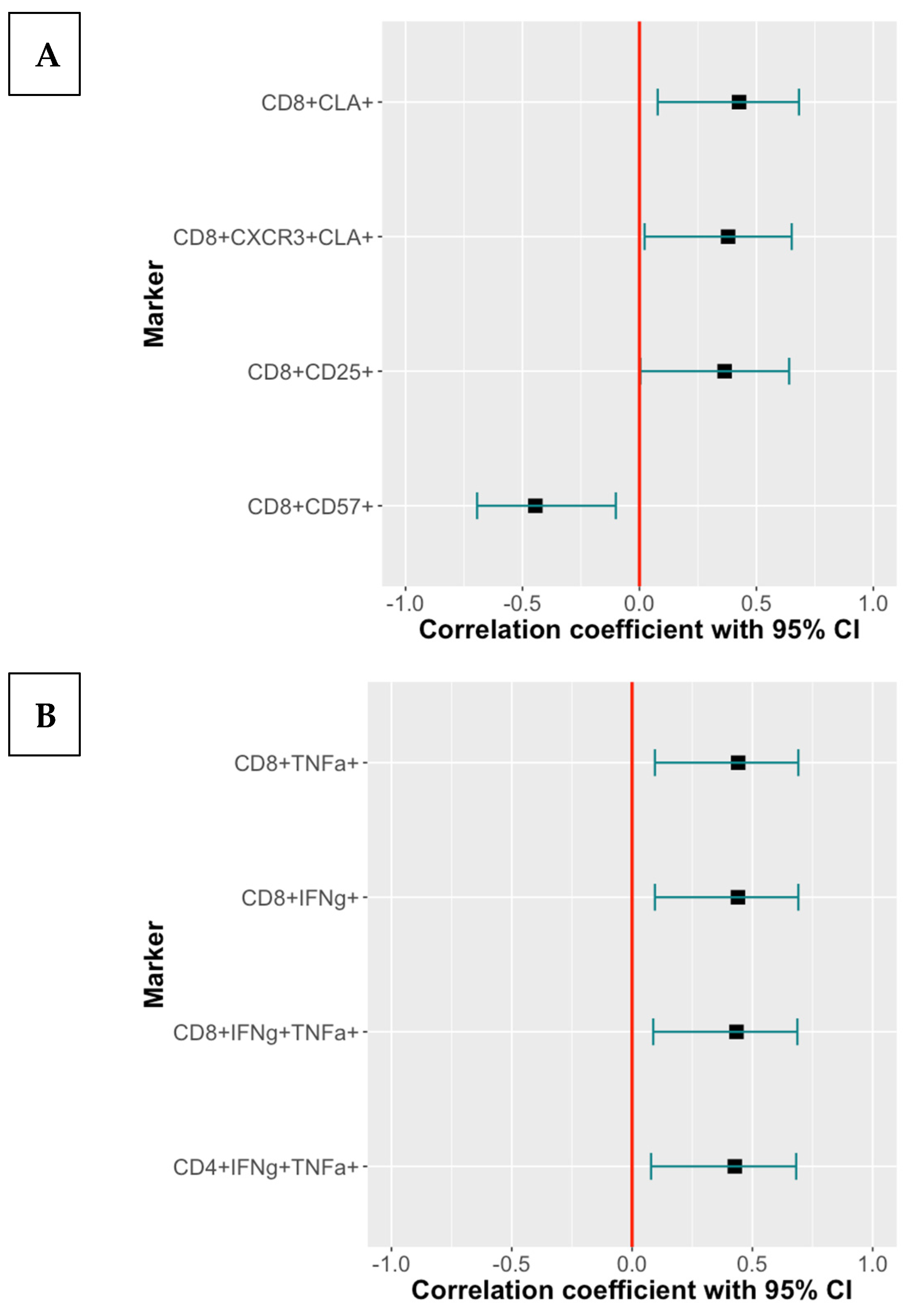
3.5. Effect of T Cell Subsets Associated with CMV-Seropositivity or Age on the Immunogenicity of RZV and ZVL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luz Correa, B.; Ornaghi, A.P.; Cerutti Muller, G.; Engroff, P.; Pestana Lopes, R.; Gomes da Silva Filho, I.; Bosch, J.A.; Bonorino, C.; Bauer, M.E. The inverted CD4:CD8 ratio is associated with cytomegalovirus, poor cognitive and functional states in older adults. Neuroimmunomodulation 2014, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Pourgheysari, B.; Khan, N.; Best, D.; Bruton, R.; Nayak, L.; Moss Paul, A.H. The Cytomegalovirus-Specific CD4+ T-Cell Response Expands with Age and Markedly Alters the CD4+ T-Cell Repertoire. J. Virol. 2007, 81, 7759–7765. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, S.P.H.; Pardieck, I.N.; Lanfermeijer, J.; Sauce, D.; Klenerman, P.; van Baarle, D.; Arens, R. The hallmarks of CMV-specific CD8 T-cell differentiation. Med. Microbiol. Immunol. 2019, 208, 365–373. [Google Scholar] [CrossRef]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Álvarez-Heredia, P.; Reina-Alfonso, I.; Domínguez-del-Castillo, J.J.; Gutiérrez-González, C.; Hassouneh, F.; Batista-Duharte, A.; Pérez, A.-B.; Tarazona, R.; Solana, R.; Pera, A. Accelerated T-Cell Immunosenescence in Cytomegalovirus-Seropositive Individuals After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2023, 228, 576–585. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Immunosenescence and Cytomegalovirus: Exploring Their Connection in the Context of Aging, Health, and Disease. Int. J. Mol. Sci. 2024, 25, 753. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C.; Fornara, O.; Rahbar, A. Cytomegalovirus driven immunosenescence—An immune phenotype with or without clinical impact? Mech. Ageing Dev. 2016, 158, 3–13. [Google Scholar] [CrossRef]
- Hassouneh, F.; Goldeck, D.; Pera, A.; van Heemst, D.; Slagboom, P.E.; Pawelec, G.; Solana, R. Functional Changes of T-Cell Subsets with Age and CMV Infection. Int. J. Mol. Sci. 2021, 22, 9973. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Landin, A.M.; Blomberg, B.B. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 2015, 33, 1433–1439. [Google Scholar] [CrossRef]
- van den Berg, S.P.H.; Lanfermeijer, J.; Jacobi, R.H.J.; Hendriks, M.; Vos, M.; van Schuijlenburg, R.; Nanlohy, N.M.; Borghans, J.A.M.; van Beek, J.; van Baarle, D.; et al. Latent CMV Infection Is Associated with Lower Influenza Virus-Specific Memory T-Cell Frequencies, but Not with an Impaired T-Cell Response to Acute Influenza Virus Infection. Front. Immunol. 2021, 12, 663664. [Google Scholar] [CrossRef]
- Freeman, M.L.; Oyebanji, O.A.; Moisi, D.; Payne, M.; Sheehan, M.L.; Balazs, A.B.; Bosch, J.; King, C.L.; Gravenstein, S.; Lederman, M.M.; et al. Association of Cytomegalovirus Serostatus with Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Responsiveness in Nursing Home Residents and Healthcare Workers. Open Forum Infect. Dis. 2023, 10, ofad063. [Google Scholar] [CrossRef] [PubMed]
- Mukhiya, R.; Fleischmann, W.A.; Loughland, J.R.; Chan, J.-A.; de Labastida Rivera, F.; Andrew, D.; Beeson, J.G.; McCarthy, J.S.; Barber, B.E.; Lopez, J.A.; et al. Heterogeneity of the human immune response to malaria infection and vaccination driven by latent cytomegalovirus infection. eBioMedicine 2024, 109, 105419. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Jojic, V.; Sharma, S.; Shen-Orr, S.S.; Angel, C.J.; Onengut-Gumuscu, S.; Kidd, B.A.; Maecker, H.T.; Concannon, P.; Dekker, C.L.; et al. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 2015, 7, 281ra243. [Google Scholar] [CrossRef]
- Lelic, A.; Verschoor, C.P.; Lau, V.W.; Parsons, R.; Evelegh, C.; Bowdish, D.M.; Bramson, J.L.; Loeb, M.B. Immunogenicity of Varicella Vaccine and Immunologic Predictors of Response in a Cohort of Elderly Nursing Home Residents. J. Infect. Dis. 2016, 214, 1905–1910. [Google Scholar] [CrossRef]
- Pereira, B.; Xu, X.-N.; Akbar, A.N. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Front. Immunol. 2020, 11, 583019. [Google Scholar] [CrossRef]
- Chen, L.; Shao, C.; Li, J.; Zhu, F. Impact of Immunosenescence on Vaccine Immune Responses and Countermeasures. Vaccines 2024, 12, 1289. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. NPJ Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Arnou, R.; Fiquet, A.; Thomas, S.; Sadorge, C. Immunogenicity and safety of ZOSTAVAX® approaching expiry potency in individuals aged ≥50 years. Hum. Vaccin. 2011, 7, 1060–1065. [Google Scholar] [CrossRef]
- Vesikari, T.; Roland, H.; Hans, R.; Giancarlo, I.; Jordi, M.; Stéphane, T.; Christine, S.; Fiquet, A. Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥70 years: A randomized study of a single dose vs. two different two-dose schedules. Human. Vaccines Immunother. 2013, 9, 858–864. [Google Scholar] [CrossRef][Green Version]
- Weinberg, A.; Pang, L.; Johnson Michael, J.; Caldas, Y.; Cho, A.; Tovar-Salazar, A.; Canniff, J.; Schmader Kenneth, E.; Popmihajlov, Z.; Levin Myron, J. The Effect of Age on the Immunogenicity of the Live Attenuated Zoster Vaccine Is Predicted by Baseline Regulatory T Cells and Varicella-Zoster Virus-Specific T Cell Immunity. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Denly, L. The effect of sex on responses to influenza vaccines. Human. Vaccines Immunother. 2021, 17, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Aldakak, L.; Huber, V.M.; Rühli, F.; Bender, N. Sex difference in the immunogenicity of the quadrivalent Human Papilloma Virus vaccine: Systematic review and meta-analysis. Vaccine 2021, 39, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Addo, M.M.; Dahlke, C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front. Immunol. 2020, 11, 601170. [Google Scholar] [CrossRef]
- Tadount, F.; Kiely, M.; Assi, A.; Rafferty, E.; Sadarangani, M.; MacDonald, S.E.; Quach, C. Sex Differences in the Immunogenicity and Efficacy of Seasonal Influenza Vaccines: A Meta-analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2024, 11, ofae222. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
- Strezova, A.; Diez-Domingo, J.; Al Shawafi, K.; Tinoco, J.C.; Shi, M.; Pirrotta, P.; Mwakingwe-Omari, A.; Zoster-049 Study, G. Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination. Open Forum Infect. Dis. 2022, 9, ofac485. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Sun, Y.; Kim, E.; Kong, C.L.; Arnold, B.F.; Porco, T.C.; Acharya, N.R. Effectiveness of the Recombinant Zoster Vaccine in Adults Aged 50 and Older in the United States: A Claims-Based Cohort Study. Clin. Infect. Dis. An. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 949–956. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Heineman, T.C.; Lal, H.; Godeaux, O.; Chlibek, R.; Hwang, S.J.; McElhaney, J.E.; Vesikari, T.; Andrews, C.; Choi, W.S.; et al. Immune Responses to a Recombinant Glycoprotein E Herpes Zoster Vaccine in Adults Aged 50 Years or Older. J. Infect. Dis. 2018, 217, 1750–1760. [Google Scholar] [CrossRef]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hastie, A.; Catteau, G.; Enemuo, A.; Mrkvan, T.; Salaun, B.; Volpe, S.; Smetana, J.; Rombo, L.; Schwarz, T.; Pauksens, K.; et al. Immunogenicity of the Adjuvanted Recombinant Zoster Vaccine: Persistence and Anamnestic Response to Additional Doses Administered 10 Years After Primary Vaccination. J. Infect. Dis. 2021, 224, 2025–2034. [Google Scholar] [CrossRef]
- Staras, S.A.S.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of Cytomegalovirus Infection in the United States, 1988–1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef]
- Levin, M.J.; Kroehl, M.E.; Johnson, M.J.; Hammes, A.; Reinhold, D.; Lang, N.; Weinberg, A. Th1 memory differentiates recombinant from live herpes zoster vaccines. J. Clin. Investig. 2018, 128, 4429–4440. [Google Scholar] [CrossRef]
- Chlibek, R.; Smetana, J.; Pauksens, K.; Rombo, L.; Van den Hoek, J.A.R.; Richardus, J.H.; Plassmann, G.; Schwarz, T.F.; Ledent, E.; Heineman, T.C. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: A phase II, randomized, controlled study. Vaccine 2014, 32, 1745–1753. [Google Scholar] [CrossRef]
- Laing, K.J.; Ford, E.S.; Johnson, M.J.; Levin, M.J.; Koelle, D.M.; Weinberg, A. Recruitment of naive CD4+ T cells by the recombinant zoster vaccine correlates with persistent immunity. J. Clin. Investig. 2023, 133, e172634. [Google Scholar] [CrossRef]
- Vermeulen, J.N.; Lange, J.M.; Tyring, S.K.; Peters, P.H.; Nunez, M.; Poland, G.; Levin, M.J.; Freeman, C.; Chalikonda, I.; Li, J.; et al. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults ≥ 60 years of age. Vaccine 2012, 30, 904–910. [Google Scholar]
- Appay, V.; Dunbar, P.R.; Callan, M.; Klenerman, P.; Gillespie, G.M.A.; Papagno, L.; Ogg, G.S.; King, A.; Lechner, F.; Spina, C.A.; et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002, 8, 379–385. [Google Scholar] [CrossRef]
- Loffredo, L.F.; Savage, T.M.; Ringham, O.R.; Arpaia, N. Treg–tissue cell interactions in repair and regeneration. J. Exp. Med. 2024, 221, e20231244. [Google Scholar] [CrossRef]
- Marchesini Tovar, G.; Gallen, C.; Bergsbaken, T. CD8+ Tissue-Resident Memory T Cells: Versatile Guardians of the Tissue. J. Immunol. 2024, 212, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.L.; Reuter-Monslow, M.A.; Sei, J.; Durr, E.; Davis, C.W.; Chang, C.; McCausland, M.; Wieland, A.; Krah, D.; Rouphael, N.; et al. Breadth and Functionality of Varicella-Zoster Virus Glycoprotein-Specific Antibodies Identified after Zostavax Vaccination in Humans. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Weinberg, A.; Scott Schmid, D.; Leung, J.; Johnson, M.J.; Miao, C.; Levin, M.J. Predictors of 5-Year Persistence of Antibody Responses to Zoster Vaccines. J. Infect. Dis. 2023, 228, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Sieiro-Santos, C.; Herrero, J.G.; Ordas Martinez, J.; Alvarez Castro, C.; Lopez Robles, A.; Colindres, R.; Martin, E.R.; Sahagun, A.M.; Ruiz de Morales, J.G. Immunogenicity to Herpes Zoster recombinant subunit vaccine in immune-mediated rheumatic patients under treatment with JAK inhibitors. Rheumatology, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef]

| Vaccine | RZV (N = 80) | ZVL (N = 79) | |||
|---|---|---|---|---|---|
| CMV-Positive (N = 45) | CMV-Negative (N = 35) | CMV-Positive (N = 46) | CMV-Negative (N = 33) | ||
| Median age (IQR) | 74 [71, 77] | 72 [56, 75] | 73 [58, 76] | 74 [71, 77] | |
| Sex N (%) | F | 28 (62.2) | 14 (40.0) | 32 (69.6) | 16 (62.2) |
| M | 17 (37.8) | 21 (60.0) | 14 (30.4) | 17 (37.8) | |
| Race N (%) | NW | 2 (4.4) | 0 (0.0) | 2 (4.3) | 2 (4.4) |
| W | 43 (95.6) | 35 (100.0) | 44 (95.7) | 43 (95.6) | |
| Ethnicity N (%) | NH | 44 (97.8) | 35 (100.0) | 44 (95.7) | 44 (97.8) |
| H | 1 (2.2) | 0 (0.0) | 2 (4.3) | 1 (2.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinberg, A.; Vu, T.; Johnson, M.J.; Schmid, D.S.; Levin, M.J. The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting. Vaccines 2025, 13, 340. https://doi.org/10.3390/vaccines13040340
Weinberg A, Vu T, Johnson MJ, Schmid DS, Levin MJ. The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting. Vaccines. 2025; 13(4):340. https://doi.org/10.3390/vaccines13040340
Chicago/Turabian StyleWeinberg, Adriana, Thao Vu, Michael J. Johnson, D. Scott Schmid, and Myron J. Levin. 2025. "The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting" Vaccines 13, no. 4: 340. https://doi.org/10.3390/vaccines13040340
APA StyleWeinberg, A., Vu, T., Johnson, M. J., Schmid, D. S., & Levin, M. J. (2025). The Reduced Immunogenicity of Zoster Vaccines in CMV-Seropositive Older Adults Correlates with T Cell Imprinting. Vaccines, 13(4), 340. https://doi.org/10.3390/vaccines13040340






