Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Design
2.2. Model Inputs and Calculations
2.3. Analysis
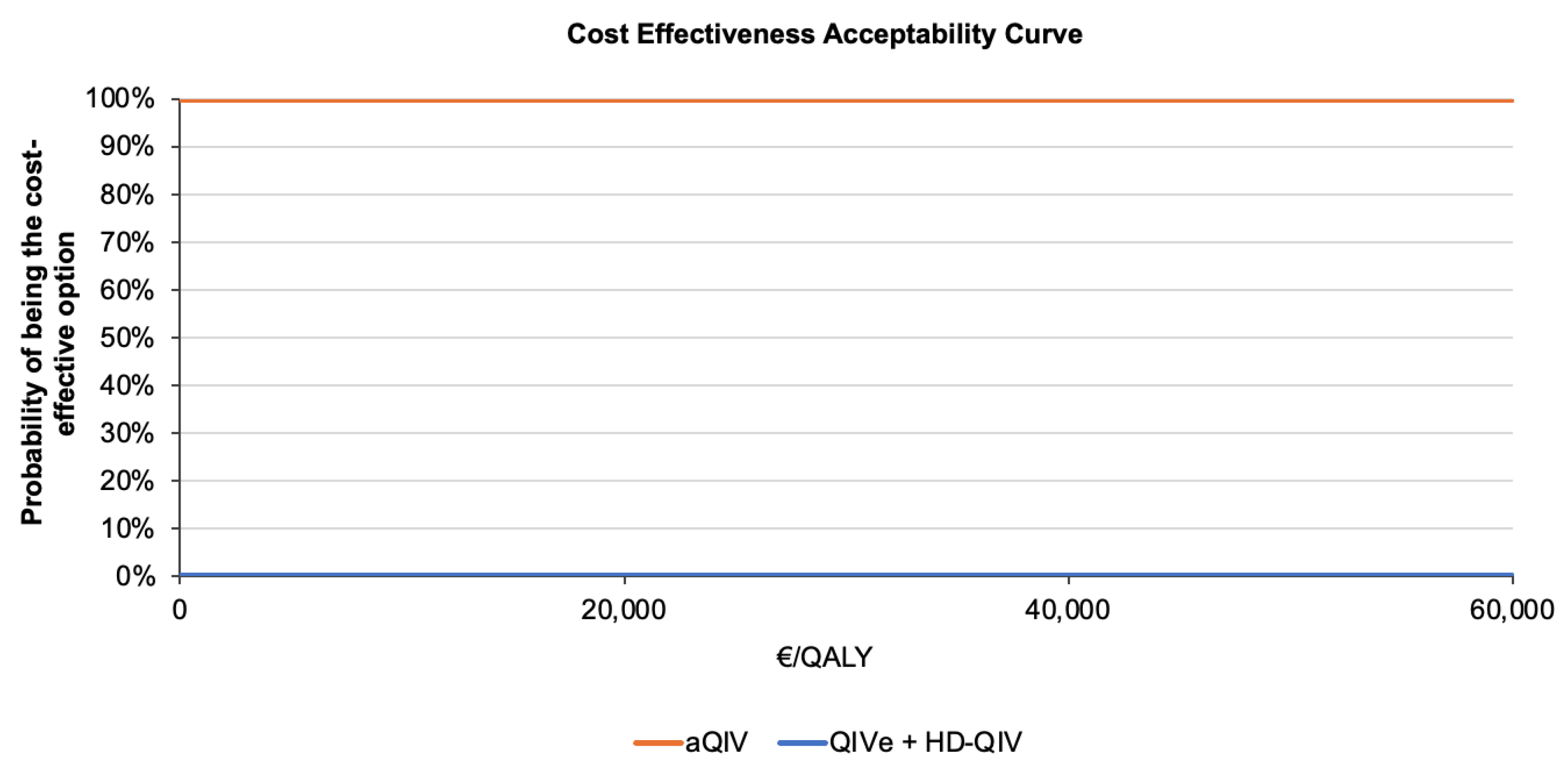
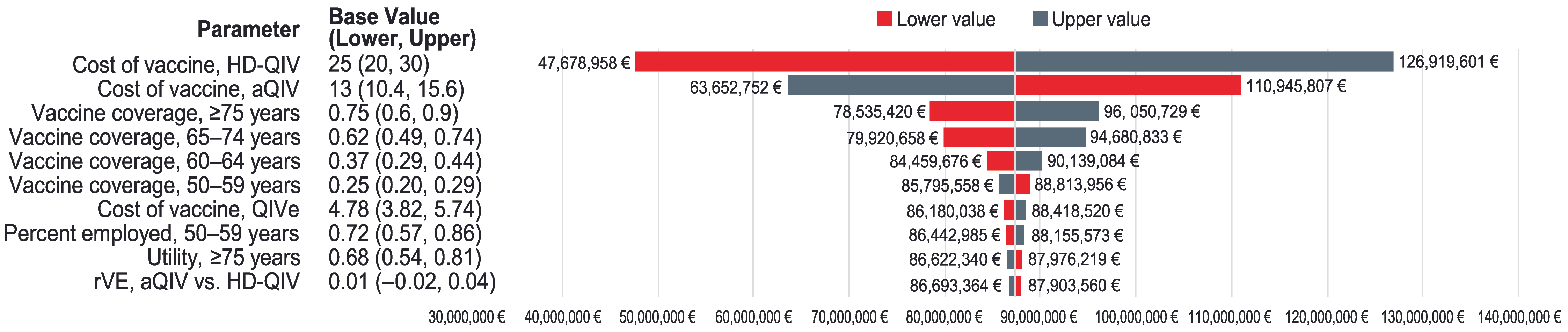
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 13 September 2024).
- Centers for Disease Control and Prevention. Disease Burden of Flu. Available online: https://www.cdc.gov/flu-burden/php/about/index.html (accessed on 27 May 2024).
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rubio, A.; Platero, L.; Eiros Bouza, J.M. Seasonal influenza in Spain: Clinical and economic burden and vaccination programmes. Med. Clin. 2019, 153, 16–27. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States—2022–2023 Influenza Season. Available online: https://www.cdc.gov/flu-burden/php/data-vis/2022-2023.html (accessed on 29 August 2024).
- Mertz, D.; Kim, T.H.; Johnstone, J.; Lam, P.P.; Science, M.; Kuster, S.P.; Fadel, S.A.; Tran, D.; Fernandez, E.; Bhatnagar, N.; et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ 2013, 347, f5061. [Google Scholar] [CrossRef]
- Instituto de Salud Carlos III. Sistema de Vigilancia de la Gripe en España. Available online: https://vgripe.isciii.es/inicio.do (accessed on 22 May 2023).
- Oliva, J.; Delgado-Sanz, C.; Larrauri, A. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010–2016. Influenza Other Respir. Viruses 2018, 12, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Tokars, J.I.; Olsen, S.J.; Reed, C. Seasonal Incidence of Symptomatic Influenza in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 1511–1518. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadistica. Ocupados por Sexo y Grupo de Edad. Valores Absolutos y Porcentajes Respecto del Total de Cada Sexo. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=4076&L=0 (accessed on 9 October 2024).
- de Courville, C.; Cadarette, S.M.; Wissinger, E.; Alvarez, F.P. The economic burden of influenza among adults aged 18 to 64: A systematic literature review. Influenza Other Respir. Viruses 2022, 16, 376–385. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- National Foundation for Infectious Diseases. Call to Action: The Dangers of Influenza and Benefits of Vaccination in Adults with Chronic Health Conditions. Available online: https://www.nfid.org/resource/the-dangers-of-influenza-and-benefits-of-vaccination-in-adults-with-chronic-health-conditions-september-2018/ (accessed on 15 May 2023).
- Near, A.M.; Tse, J.; Young-Xu, Y.; Hong, D.K.; Reyes, C.M. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv. Res. 2022, 22, 1209. [Google Scholar] [CrossRef]
- MacNee, W.; Rabinovich, R.A.; Choudhury, G. Ageing and the border between health and disease. Eur. Respir. J. 2014, 44, 1332–1352. [Google Scholar] [CrossRef]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad Consumo y Bienestar Social. Recomendaciones de Vacunación Frente a Gripe y COVID-19 en la Temporada 2024–2025 en España. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/gripe_covid19/docs/RecomendacionesVacunacion_Gripe-Covid19.pdf (accessed on 11 October 2024).
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert. Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- Frey, S.E.; Reyes, M.R.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Trombetta, C.S.; Fallani, E.; Salvatore, M. Immunogenicity and safety of the MF59-adjuvanted seasonal influenza vaccine in non-elderly adults: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0310677. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- López-Bastida, J.; Oliva, J.; Antoñanzas, F.; García-Altés, A.; Gisbert, R.; Mar, J.; Puig-Junoy, J. Spanish recommendations on economic evaluation of health technologies. Eur. J. Health Econ. 2010, 11, 513–520. [Google Scholar] [CrossRef]
- Ruiz-Aragón, J.; Gani, R.; Márquez, S.; Alvarez, P. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum. Vaccines Immunother. 2020, 16, 2238–2244. [Google Scholar] [CrossRef]
- Jamotte, A.; Chong, C.F.; Manton, A.; Macabeo, B.; Toumi, M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health 2016, 16, 630. [Google Scholar] [CrossRef]
- Uhart, M.; Bricout, H.; Clay, E.; Largeron, N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum. Vaccines Immunother. 2016, 12, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.; Meltzer, M.I.; Finelli, L.; Fiore, A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012, 30, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Chit, A.; Roiz, J.; Aballea, S. An Assessment of the Expected Cost-Effectiveness of Quadrivalent Influenza Vaccines in Ontario, Canada Using a Static Model. PLoS ONE 2015, 10, e0133606. [Google Scholar] [CrossRef]
- Mennini, F.S.; Bini, C.; Marcellusi, A.; Rinaldi, A.; Franco, E. Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum. Vaccines Immunother. 2018, 14, 1867–1873. [Google Scholar] [CrossRef]
- Ruiz-Aragón, J.; Márquez-Peláez, S.; Gani, R.; Alvarez, P.; Guerrero-Luduena, R. Cost-Effectiveness and Burden of Disease for Adjuvanted Quadrivalent Influenza Vaccines Compared to High-Dose Quadrivalent Influenza Vaccines in Elderly Patients in Spain. Vaccines 2022, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Márquez-Peláez, S. An Economic Comparison in the Elderly of Adjuvanted Quadrivalent Influenza Vaccine with Recombinant Quadrivalent Influenza Vaccine in Spain. Vaccines 2023, 11, 427. [Google Scholar] [CrossRef]
- Fochesato, A.; Sottile, S.; Pugliese, A.; Márquez-Peláez, S.; Toro-Diaz, H.; Gani, R.; Alvarez, P.; Ruiz-Aragón, J. An Economic Evaluation of the Adjuvanted Quadrivalent Influenza Vaccine Compared with Standard-Dose Quadrivalent Influenza Vaccine in the Spanish Older Adult Population. Vaccines 2022, 10, 1360. [Google Scholar] [CrossRef]
- Redondo, E.; Drago, G.; López-Belmonte, J.L.; Guillén, J.M.; Bricout, H.; Alvarez, F.P.; Callejo, D.; Gil de Miguel, Á. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine 2021, 39, 5138–5145. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadistica. Población Residente por Fecha, Sexo y Edad. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=59583&L=0 (accessed on 9 October 2024).
- Ministerio de Sanidad. Sistema de Información de Vacunaciones (SIVAMIN). Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/S/sivamin (accessed on 21 November 2024).
- Junta de Castilla de León. Seguimiento de las Coberturas de la Campaña de Vacunación Gripe y COVID-19 2023–2024. Available online: https://www.saludcastillayleon.es/profesionales/en/vacunaciones/campana-vacunacion-frente-gripe-covid-19-temporada-2023-202/seguimiento-coberturas-campana-vacunacion-gripe-covid-19-20 (accessed on 21 November 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2019–2020 (Desde la Semana 40/2019 Hasta la Semana 20/2020). Available online: https://vgripe.isciii.es/documentos/20192020/InformesAnuales/Informe_Vigilancia_GRIPE_2019-2020_03092020.pdf (accessed on 4 October 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2018–2019 (desde la Semana 40/2018 Hasta la Semana 20/2019). Available online: https://vgripe.isciii.es/documentos/20182019/InformesAnuales/Informe_Vigilancia_GRIPE_2018-2019_22julio2019.pdf (accessed on 4 October 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2016–2017 (Desde la Semana 40/2016 Hasta la Semana 20/2017). Available online: https://cne.isciii.es/documents/d/cne/vigilancia-de-la-gripe-en-espana-informe-temporada-2016-2017 (accessed on 4 October 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2017–2018 (Desde la Semana 40/2017 Hasta la Semana 20/2018). Available online: https://cne.isciii.es/documents/d/cne/vigilancia-de-la-gripe-en-espana-informe-temporada-2017-2018 (accessed on 4 October 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2015–2016 (Desde la Semana 40/2015 Hasta la Semana 20/2016). Available online: https://cne.isciii.es/documents/d/cne/vigilancia-de-la-gripe-en-espana-informe-temporada-2015-2016-pdf (accessed on 4 October 2024).
- Instituto de Salud Carlos III. Informe de Vigilancia de la Gripe en España. Temporada 2014–2015 (Desde la Semana 40/2014 Hasta la Semana 20/2015). Available online: https://cne.isciii.es/documents/d/cne/vigilancia-de-la-gripe-en-espana-informe-temporada-2014-2015-pdf (accessed on 4 October 2024).
- Delgado-Sanz, C.; Jiménez-Jorge, S.; Pozo, F.; Gómez-Barroso, D.; León-Gómez, I.; de Mateo, S.; Larrauri, A. Vigilancia de la gripe en Españ;a. Temporada 2013–2014 (Desde la Semana 40/2013 Hasta la Semana 20/2014). Boletín Epidemiol. Sem. 2014, 22, 146–166. [Google Scholar]
- León-Gómez, I.; Delgado-Sanz, C.; Jiménez-Jorge, S.; Flores, V.; Simón, F.; Gómez-Barroso, D.; Larrauri, A.; de Mateo Ontañón, S. Excess mortality associated with influenza in Spain in winter 2012. Gac Sanit 2015, 29, 258–265. [Google Scholar] [CrossRef][Green Version]
- Ministerio de Sanidad Consumo y Bienestar Social. Acuerdo Marco para la Seleccion de Suministradores de Vacunas Frente a la Gripe Estacional (INGESA) y Ciudades de Ceuta y Melilla y Varias Comunidades Autonomas. Available online: https://contrataciondelestado.es/wps/wcm/connect/7c41cd41-00c8-4c07-be3d-272d29585268/DOC20210419131140PCAP+Gripe+2021-2025.pdf?MOD=AJPERES (accessed on 9 October 2024).
- Gobierno de La Rioja. Consulta Expedientes: Expediente nº.06-3-7.07-0019/2023—Adjudicación. Available online: https://www.larioja.org/contratacion-publica/es/licitaciones/consulta-expedientes?homepage=%3Fp_colateral%3D06-3-7.07-0019%2F2023%26p_dia_ini%3D28%26p_mes_ini%3D07%26p_ano_ini%3D2023%26p_dia_fin%3D28%26p_mes_fin%3D07%26p_ano_fin%3D2023%26p_todas%3DN (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: CS/9999/1101105289/23/PNSP. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=kHqM2iNSHV2ExvMJXBMHHQ%3D%3D (accessed on 4 December 2024).
- Gobierno del Principado de Asturias. Documentos Electrónicos Emitidos por el Principado de Asturias, Número de Referencia 14612415324201513343 [Login Required]. Available online: https://tramita.asturias.es/sta/CarpetaPublic/doEvent?APP_CODE=STA&PAGE_CODE=VALDOCS (accessed on 15 January 2025).
- Gobierno Vasco. Suministro de Vacunas Frente a la Gripe Estacional—Perfil de Contratante. Available online: https://www.contratacion.euskadi.eus/webkpe00-kpeperfi/es/contenidos/anuncio_contratacion/exposakisap2021000431/es_doc/index.html?ruta=informacionAmpliadaAnuncios&busquedaAvanzada (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 181/2021/CMA. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=Q0aw7etnXBGmq21uxhbaVQ%3D%3D (accessed on 4 December 2024).
- Xunta de Galicia; Consellería de Sanidade. Datos Xerais. Available online: https://www.contratosdegalicia.gal/licitacion?OP=50&N=819632&lang=gl (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: CS/99/1123044902/23/PNSP. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=HopD%2BsAA%2FDk4NavIWzMcHA%3D%3D (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 725/2021 Tercer Basado Lote1. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=LDBtSC1j%2B%2BHua%2Fi14w%2FPLA%3D%3D (accessed on 4 December 2024).
- Gobierno de Navarra. Comprobación de Documentos Marcados con un Código Seguro de Verificación (CSV) [Verification of Documents Marked with a Secure Verification Code]. Available online: https://www.navarra.es/es/tramites/on/-/line/Comprobacion-de-documentos-marcados-con-un-codigo-seguro-de-verificacion-CSV (accessed on 4 December 2024).
- Comunidad de Madrid. Contratos Basados en el Acuerdo Marco 202101AM0001, Relativo al Suministro de Vacunas Frente a la Gripe Estacional, 2 Lotes (lote 1 y lote 3) Para la Campaña de Vacunación Antigripal de la Temporada 2023–2024 Para la Comunidad de Madrid. Available online: https://contratos-publicos.comunidad.madrid/contrato-publico/contratos-basados-acuerdo-marco-202101am0001-relativo-suministro-vacunas-frente (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 19251/23. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=EjOGS%2BYgL0rzAq95uGTrDQ%3D%3D (accessed on 4 December 2024).
- Catalunya, G.D. Plataforma de Serveis de Contractació Pública: Adquisició de Vacunes per a L’any 2023 (SA-2023-39). Available online: https://contractaciopublica.cat/es/detall-publicacio/200156331 (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 182/2023. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=pmcEYn4kqsVPpzdqOdhuWg%3D%3D (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: SAS-SGE-2023-42. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink:detalle_licitacion&idEvl=st4PYafAE6geC9GJQOEBkQ%3D%3D (accessed on 2 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 2021/ETSAE0287/00000463E. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=i%2Bv38mJHKLnnSoTX3z%2F7wA%3D%3D (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: PRO11 2023 12880. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=OBymDxcgIdKopEMYCmrbmw%3D%3D (accessed on 2 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 02/CONS/DGSP/2023. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=Uh9akaL3pAY%2Bk2oCbDosIw%3D%3D (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 10.2.10/23. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=%2FWwhozokA5q5HQrHoP3G5A%3D%3D (accessed on 4 December 2024).
- Gobierno de España. Plataforma de Contratacion del Sector Publico: Expediente: 2023/007980. Available online: https://contrataciondelestado.es/wps/poc?uri=deeplink%3Adetalle_licitacion&idEvl=Iu2C4QH969fyoM4us5k4vw%3D%3D (accessed on 4 December 2024).
- Junta de Andalucía. CCA:+6.+PJ7JD7 Acuerdo Marco Con Una Única Empresa por Lote, por el que se Fijan las Condiciones Para el Suministro de Tracto Sucesivo y Precio Unitario de Vacuna Antigripal Destinada al Programa de Vacunaciones de Andalucía, Campaña 2023–2024. Available online: https://www.juntadeandalucia.es/haciendayadministracionpublica/apl/pdc_sirec/perfiles-licitaciones/detalle-licitacion.jsf?idExpediente=531651 (accessed on 4 December 2024).
- Osakidetza. Tarifas Para Facturacion de Servicios Sanitarios y Docentes de Osakidetza 2024. Available online: https://www.osakidetza.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO-DE-TARIFAS-2024-CAS_V2.pdf (accessed on 11 March 2025).
- Ministerio de Sanidad. Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud. CMBD. Norma Estatal. Resultados Según la Versión 38 de los APR-GRD. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/cmbd.htm (accessed on 9 October 2024).
- Region de Murcia; Consejeria de Hacienda y Administracion Publica. Boletin Oficial de la Region de Murcia. Available online: https://www.borm.es/services/boletin/ano/2022/numero/94/pdf (accessed on 9 October 2024).
- Ehlken, B.; Anastassopoulou, A.; Hain, J.; Schröder, C.; Wahle, K. Cost for physician-diagnosed influenza and influenza-like illnesses on primary care level in Germany—Results of a database analysis from May 2010 to April 2012. BMC Public Health 2015, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rincón, J.M.; Pinargote-Celorio, H.; González-de-la-Aleja, P.; Sánchez-Payá, J.; Reus, S.; Rodríguez-Díaz, J.C.; Merino, E. Impact of influenza related hospitalization in Spain: Characteristics and risk factor of mortality during five influenza seasons (2016 to 2021). Front. Public Health 2024, 12, 1360372. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadistica. Encuesta de Discapacidad, Autonomía Personal y Situaciones de Dependencia 2008: Discapacidades, Deficiencias y Estado de Salud. Resultados Nacionales: Cifras Relativas. Tasa de Población con Alguna Discapacidad o Limitación por Edad y Sexo. Available online: https://www.ine.es/jaxi/Tabla.htm?path=/t15/p418/a2008/hogares/p01/modulo1/l0/&file=02001.px&L=0 (accessed on 9 October 2024).
- Instituto Nacional de Estadistica. Resultado por Comunidades Autónomas (Desde el Trimestre 1/2008). Componentes del Coste Laboral Total. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=6062 (accessed on 12 November 2024).
- García, A.; Ortiz de Lejarazu, R.; Reina, J.; Callejo, D.; Cuervo, J.; Morano Larragueta, R. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum. Vaccines Immunother. 2016, 12, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Dolk, C.; Eichner, M.; Welte, R.; Anastassopoulou, A.; Van Bellinghen, L.A.; Poulsen Nautrup, B.; Van Vlaenderen, I.; Schmidt-Ott, R.; Schwehm, M.; Postma, M. Cost-Utility of Quadrivalent Versus Trivalent Influenza Vaccine in Germany, Using an Individual-Based Dynamic Transmission Model. PharmacoEconomics 2016, 34, 1299–1308. [Google Scholar] [CrossRef]
- Hollmann, M.; Garin, O.; Galante, M.; Ferrer, M.; Dominguez, A.; Alonso, J. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS ONE 2013, 8, e60477. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; de Waure, C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 855–863. [Google Scholar] [CrossRef]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, e665–e671. [Google Scholar] [CrossRef]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of the Cell-derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-related Medical Encounters During the 2018–2019 Influenza Season in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e692–e698. [Google Scholar] [CrossRef]
- Imran, M.; Ortiz, J.R.; McLean, H.Q.; Fisher, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of Cell-based Versus Egg-based Quadrivalent Influenza Vaccines in Children and Adolescents in the United States During the 2019-2020 Influenza Season. Pediatr. Infect. Dis. J. 2022, 41, 769–774. [Google Scholar] [CrossRef]
- Divino, V.; Ruthwik Anupindi, V.; DeKoven, M.; Mould-Quevedo, J.; Pelton, S.I.; Postma, M.J.; Levin, M.J. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines During the 2019–2020 Influenza Season in the United States. Open Forum Infect. Dis. 2022, 9, ofab604. [Google Scholar] [CrossRef]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef]
- Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ortiz, J.R.; McLean, H.Q.; Fisher, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of Cell-Based Versus Egg-Based Quadrivalent Influenza Vaccines in Adults During the 2019-2020 Influenza Season in the United States. Open Forum Infect. Dis. 2022, 9, ofac532. [Google Scholar] [CrossRef]
- Stein, A.N.; Mills, C.W.; McGovern, I.; McDermott, K.W.; Dean, A.; Bogdanov, A.N.; Sullivan, S.G.; Haag, M.D.M. Relative Vaccine Effectiveness of Cell- vs Egg-Based Quadrivalent Influenza Vaccine Against Test-Confirmed Influenza Over 3 Seasons Between 2017 and 2020 in the United States. Open Forum Infect. Dis. 2024, 11, ofae175. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.; Yee, A.; Leong, T.; Zerbo, O.; Fireman, B.; Jacobson, K.; Hansen, J.; Layefsky, E.; Haag, M.; McGovern, I.; et al. Effectiveness of adjuvanted inactivated influenza vaccine versus high-dose inactivated influenza vaccine against PCR-confirmed influenza among adults ≥ 65 years: A pragmatic randomized study. In Proceedings of the ID Week 2024, Los Angeles, CA, USA, 16–19 October 2024. [Google Scholar]
- McConeghy, K.W.; Davidson, H.E.; Canaday, D.H.; Han, L.; Saade, E.; Mor, V.; Gravenstein, S. Cluster-randomized Trial of Adjuvanted Versus Nonadjuvanted Trivalent Influenza Vaccine in 823 US Nursing Homes. Clin. Infect. Dis. 2021, 73, e4237–e4243. [Google Scholar] [CrossRef]
- Vallejo-Torres, L.; García-Lorenzo, B.; Serrano-Aguilar, P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018, 27, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Sacristán, J.A.; Oliva, J.; Campillo-Artero, C.; Puig-Junoy, J.; Pinto-Prades, J.L.; Dilla, T.; Rubio-Terrés, C.; Ortún, V. What is an efficient health intervention in Spain in 2020? Gac Sanit 2020, 34, 189–193. [Google Scholar] [CrossRef]
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- Calabrò, G.E.; Boccalini, S.; Bonanni, P.; Bechini, A.; Panatto, D.; Lai, P.L.; Amicizia, D.; Rizzo, C.; Ajelli, M.; Trentini, F. Valutazione di Health Technology Assessment (HTA) del Vaccino Antinfluenzale Quadrivalente Adiuvato: Fluad Tetra. Available online: https://www.ijph.it/hta-vaccino-antinfluenzale-quadrivalente-adiuvato-fluad-tetra (accessed on 23 May 2023).
- Aballéa, S.; De Juanes, J.R.; Barbieri, M.; Martin, M.; Chancellor, J.; Oyagüez, I.; Verwee, B.; Largeron, N. The cost effectiveness of influenza vaccination for adults aged 50 to 64 years: A model-based analysis for Spain. Vaccine 2007, 25, 6900–6910. [Google Scholar] [CrossRef]
- Maciosek, M.V.; Solberg, L.I.; Coffield, A.B.; Edwards, N.M.; Goodman, M.J. Influenza vaccination health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am. J. Prev. Med. 2006, 31, 72–79. [Google Scholar] [CrossRef]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Ashraf, M.; Drummond, M.F.; Weinstein, M.C. The Cost-Effectiveness of Expanding Vaccination with a Cell-Based Influenza Vaccine to Low Risk Adults Aged 50 to 64 Years in the United Kingdom. Vaccines 2021, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Ashraf, M.; Mould-Quevedo, J.F. Estimating the impact of influenza vaccination of low-risk 50–64-year-olds on acute and ICU hospital bed usage in an influenza season under endemic COVID-19 in the UK. Hum. Vaccines Immunother. 2023, 19, 2187592. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Fallani, E.; Salvatore, M.; Montomoli, E.; Trombetta, C.M. The burden of influenza and the role of influenza vaccination in adults aged 50–64 years: A summary of available evidence. Hum. Vaccines Immunother. 2023, 19, 2257048. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Grande Tejada, A.M.; Márquez-Peláez, S.; García-Cenoz, M. Estimación del impacto de la vacunación antigripal con adyuvante MF59 en población mayor de 64 años para el Sistema Nacional de Salud: Efectos y costes. Vacunas 2015, 16, 6–11. [Google Scholar] [CrossRef]
- Pérez-Rubio, A.; Eiros, J.M. Economic and Health impact of influenza vaccination with adjuvant MF59 in population over 64 years in Spain. Rev. Esp. Quim. 2018, 31, 43–52. [Google Scholar]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Kim, J.; Krishnan, A.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert. Rev. Vaccines 2018, 17, 435–443. [Google Scholar] [CrossRef]
- Puig-Barberà, J.; Natividad-Sancho, A.; Calabuig-Pérez, J.; Lluch-Rodrigo, J.A.; Pastor-Villalba, E.; Martínez-Úbeda, S.; Pérez-Vilar, S.; Díez-Domingo, J. MF59-adjuvanted and virosomal influenza vaccines for preventing influenza hospitalization in older people: Comparative effectiveness using the Valencia health care information system. Vaccine 2013, 31, 3995–4002. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Blanton, L.H.; Alyanak, E.; Chung, J.R.; Trujillo, L.; Taliano, J.; Morgan, R.L.; Fry, A.M.; Grohskopf, L.A. Protection against influenza hospitalizations from enhanced influenza vaccines among older adults: A systematic review and network meta-analysis. J. Am. Geriatr. Soc. 2024, 72, 3875–3889. [Google Scholar]
- Paget, J.; Caini, S.; Del Riccio, M.; van Waarden, W.; Meijer, A. Has influenza B/Yamagata become extinct and what implications might this have for quadrivalent influenza vaccines? Euro Surveill. Bull. Eur. Sur Les. Mal. Transm. Eur. Commun. Dis. Bull. 2022, 27, 29. [Google Scholar] [CrossRef]


| Age Group | Total Population N | Life Expectancy Years | High Risk n (%) | Low Risk n (%) | Influenza Vaccine Coverage, High Risk % |
|---|---|---|---|---|---|
| 50–59 years | 7,475,397 | 30.6 | 4,770,798 (63.8) | 2,704,599 (36.2) | 24.54% |
| 60–64 years | 3,214,012 | 23.6 | 3,214,012 (100) | 0 | 36.72% |
| 65–74 years | 4,974,175 | 17.9 | 4,974,175 (100) | 0 | 61.57% |
| ≥75 years | 4,890,766 | 6.4 | 4,890,766 (100) | 0 | 75.27% |
| Parameter | Value | Reference | ||
|---|---|---|---|---|
| aQIV tender price | EUR 13 | [47] | ||
| QIVe tender price a | EUR 4.78 | [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] | ||
| HD-QIV tender price | EUR 25 | [47] | ||
| Primary care physician visit costs (per visit) | EUR 65.00 | [68] | ||
| ED visit costs (per visit) | EUR 257.00 | [68] | ||
| Hospitalization costs (per event) | EUR 5809.61 | [69] | ||
| Nurse consultation cost | EUR 25.94 | [70] | ||
| No. business days of disability | Primary care physician visit | 5 | [71] | |
| ED visit | 5 | [71] | ||
| Hospitalization | 15 b | [72] | ||
| Employment rate | 0–50 years | 65.8% | [11] | |
| 50–59 years | 71.6% | [11] | ||
| 60–64 years | 48.9% | [11] | ||
| 65–74 years | 10.0% | [11] | ||
| ≥75 years | 0.8% | [11] | ||
| Proportion requiring care at home | 65–74 years | 18.4% | [73] | |
| ≥75 years | 45.1% | [73] | ||
| Proportion aged 18–64 who care for a family member and are also employed | 35.15% | [73] | ||
| Productivity loss per day c | 0–50 years | EUR 106.58 | [74] | |
| 50–59 years | EUR 126.86 | [74] | ||
| 60–64 years | EUR 129.78 | [74] | ||
| 65–74 years | EUR 129.78 | [74] | ||
| ≥75 years | EUR 129.78 | [74] | ||
| Baseline utility | High risk | Low risk | ||
| 0–50 years | 0.940 | 0.975 | [75] | |
| 50–59 years | 0.870 | 0.960 | [75] | |
| 60–64 years | 0.870 | 0.960 | [75] | |
| 65–74 years | 0.836 | 0.955 | [75] | |
| ≥75 years | 0.676 | 0.836 | [75] | |
| Disutility value for symptomatic patients (all ages) | 0.32 | [76] | ||
| Disutility value for outpatient and ED settings | High risk | Low risk | ||
| 0–50 years | 0.42 | 0.40 | [77] | |
| 50–59 years | 0.42 | 0.40 | [77] | |
| 60–64 years | 0.46 | 0.44 | [77] | |
| 65–74 years | 0.37 | 0.35 | [77] | |
| ≥75 years | 0.33 | — | [77] | |
| Disutility value for an inpatient setting (all ages) | 0.42 | 0.40 | [77] | |
| Disutility duration for symptomatic patients | 7 days | [76] | ||
| Disutility duration in an outpatient setting | 7 days | [77] | ||
| Disutility duration in an inpatient setting | 7 days | [77] | ||
| Discount rate for costs and outcomes d | 3% | [25] | ||
| Current Scenario a | Alternative Scenario a | |||||
|---|---|---|---|---|---|---|
| Costs | Age Group | Number Vaccinated | QIVe | HD-QIV | (aQIV) | Incremental Difference |
| Influenza vaccines | 50–59 years | 1,170,754 | EUR 5,596,204 | EUR 15,219,801 | EUR 9,623,597 | |
| 60–64 years | 1,180,185 | EUR 29,504,630 | EUR 15,342,408 | EUR −14,162,222 | ||
| 65–74 years | 3,062,600 | EUR 76,564,989 | EUR 39,813,794 | EUR −36,751,195 | ||
| ≥75 years | 3,681,280 | EUR 92,031,989 | EUR 47,856,634 | EUR −44,175,355 | ||
| Total b | 9,094,819 | EUR 203,697,812 | EUR 118,232,637 | EUR −85,465,175 | ||
| Vaccine administration | 50–59 years | 1,170,754 | EUR 30,369,357 | EUR 30,369,357 | EUR 0 | |
| 60–64 years | 1,180,185 | EUR 30,614,004 | EUR 30,614,004 | EUR 0 | ||
| 65–74 years | 3,062,600 | EUR 79,443,832 | EUR 79,443,832 | EUR 0 | ||
| ≥75 years | 3,681,280 | EUR 95,492,392 | EUR 95,492,392 | EUR 0 | ||
| Total b | 9,094,819 | EUR 235,919,585 | EUR 235,919,585 | EUR 0 | ||
| Parameter | Current Scenario a (QIVe or HD-QIV) | Alternative Scenario a (aQIV) | Difference | Current Scenario a (QIVe or HD-QIV) | Alternative Scenario a (aQIV) | Difference | |
|---|---|---|---|---|---|---|---|
| Clinical outcomes | QALY per vaccination | ||||||
| QALY loss | 21,191 | 20,950 | −241 | 0.00233 | 0.00230 | −0.00003 | |
| Life years lost | 31,060 | 30,742 | −318 | 0.00342 | 0.00338 | −0.00003 | |
| Events | Events per vaccination | ||||||
| Symptomatic influenza cases | 165,050 | 162,151 | −2899 | 1.815% | 1.783% | −0.00032 | |
| Primary care visits | 107,884 | 106,171 | −1713 | 1.186% | 1.167% | −0.00019 | |
| ED visits | 24,213 | 23,829 | −384 | 0.266% | 0.262% | −0.00004 | |
| Hospitalizations | 15,064 | 14,907 | −157 | 0.166% | 0.164% | −0.00002 | |
| Deaths | 3895 | 3860 | −35 | 0.043% | 0.042% | 0.00000 | |
| Costs | Costs per vaccination | ||||||
| Primary care visits | EUR 7,358,761 | EUR 7,241,929 | EUR −116,832 | EUR 0.8091 | EUR 0.7963 | EUR −0.013 | |
| ED visits | EUR 6,222,855 | EUR 6,124,057 | EUR −98,798 | EUR 0.6842 | EUR 0.6734 | EUR −0.011 | |
| Hospitalizations | EUR 87,514,956 | EUR 86,603,793 | EUR −911,163 | EUR 9.6225 | EUR 9.5223 | EUR −0.100 | |
| Vaccination | EUR 439,617,397 | EUR 354,152,222 | EUR −85,465,175 | EUR 48.3371 | EUR 38.9400 | EUR −9.397 | |
| Total | EUR 540,713,969 | EUR 454,122,002 | EUR −86,591,968 | EUR 59.4530 | EUR 49.9319 | EUR −9.521 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Rubio, A.; Flores, R.; Aragon, J.R.; Sanchez, J.; Marquez-Peláez, S.; Alvarez, P.; Muriel, A.O.; Mould-Quevedo, J. Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain. Vaccines 2025, 13, 323. https://doi.org/10.3390/vaccines13030323
Perez-Rubio A, Flores R, Aragon JR, Sanchez J, Marquez-Peláez S, Alvarez P, Muriel AO, Mould-Quevedo J. Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain. Vaccines. 2025; 13(3):323. https://doi.org/10.3390/vaccines13030323
Chicago/Turabian StylePerez-Rubio, Alberto, Roberto Flores, Jesus Ruiz Aragon, Javier Sanchez, Sergio Marquez-Peláez, Piedad Alvarez, Andres Osorio Muriel, and Joaquin Mould-Quevedo. 2025. "Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain" Vaccines 13, no. 3: 323. https://doi.org/10.3390/vaccines13030323
APA StylePerez-Rubio, A., Flores, R., Aragon, J. R., Sanchez, J., Marquez-Peláez, S., Alvarez, P., Muriel, A. O., & Mould-Quevedo, J. (2025). Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain. Vaccines, 13(3), 323. https://doi.org/10.3390/vaccines13030323






