Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anti SARS-CoV-2 Antibodies
2.3. SARS-CoV-2 Specific T Cell Response
2.4. SARS-CoV-2 Infections
2.5. Statistical Analysis
3. Results
3.1. Study Population
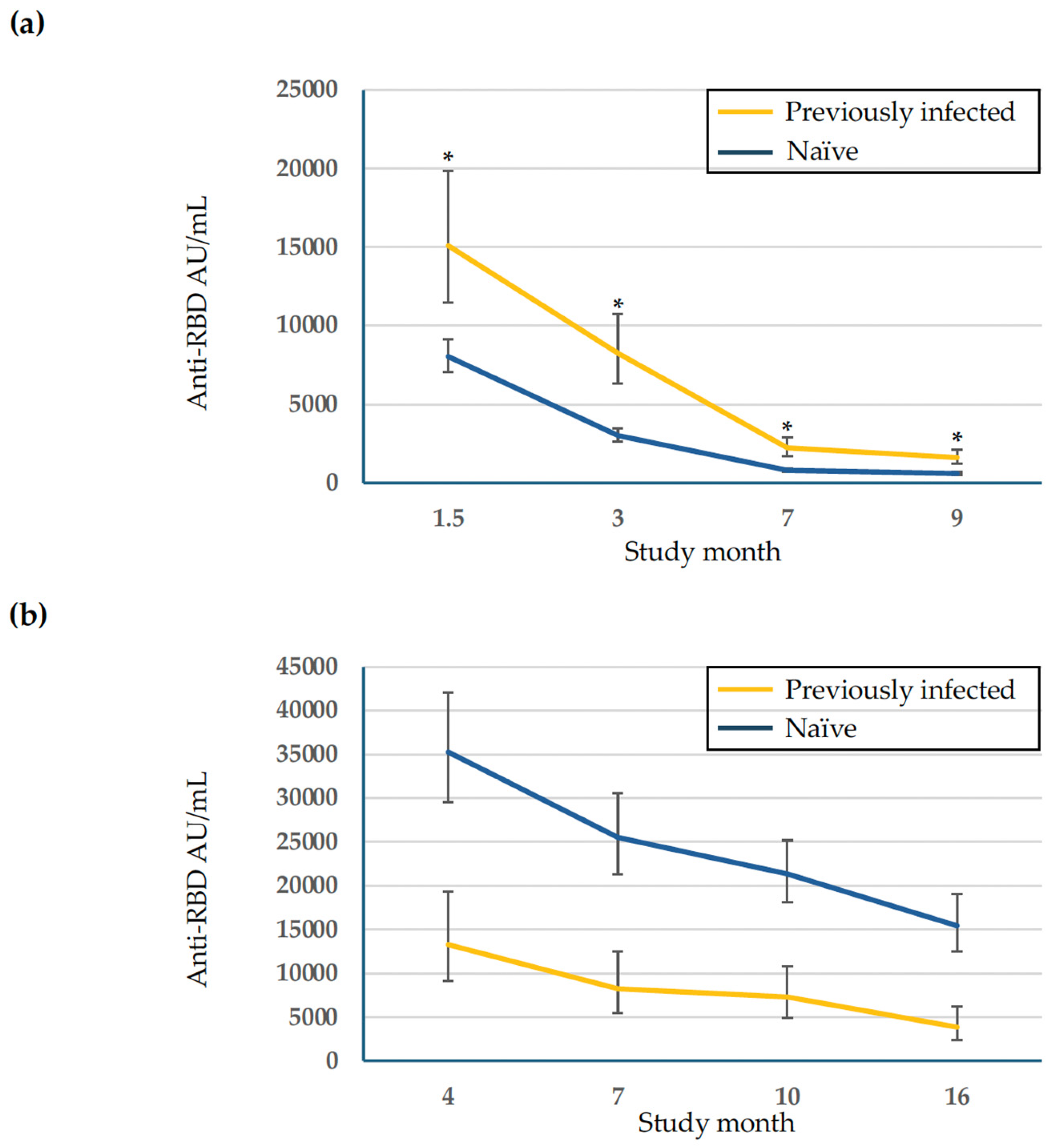
3.2. Kinetics of SARS-CoV-2 Anti-RBD Antibodies
3.3. SARS-CoV-2 Breakthrough Infections During Follow-Up
3.4. SARS-CoV-2-Specific T Cell Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, S.F.; Frey, S.; Novack, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Brosh-Nissimov, T.; Orenbuch-Harroch, E.; Chowers, M.; Elbaz, M.; Nesher, L.; Stein, M.; Maor, Y.; Cohen, R.; Hussein, K.; Weinberger, M.; et al. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-129 patients in Israel. Clin. Microbiol. Infect. 2021, 27, 1652–1657. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappel, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of responses after SARSCoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W., Jr.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Lockhart, S.; Swanson, K.A.; Xu, X.; Koury, K.; Warren, K.; et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef]
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I.; et al. Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years. N. Engl. J. Med. 2023, 388, 214–227. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Saiag, E.; Gamzu, R.; Padova, H.; Paran, Y.; Goldiner, I.; Cohen, N.; Bomze, D. Antibody Response After a Fifth Dose (Third Booster) of BNT162b2 mRNA COVID-19 Vaccine in Healthcare Workers. J. Clin. Med. 2024, 13, 6538. [Google Scholar] [CrossRef]
- Barouch, D.H. Covid-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streek, H.; Saschenbrecker, S.; Steinhagenet, S.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Damodara, R.M.; Mueller, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldbaltt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S. Evidence for antibody as a protective correlate for COVID-19 vaccine. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Sendi, P.; Widmer, N.; Branca, M.; Thierstein, M.; Büchi, A.E.; Güntensperger, D.; Blum, M.R.; Baldan, R.; Tinguely, C.; Hek, D.; et al. Do quantitative levels of antispike-IgG antibodies aid in predicting protection from SARS-CoV-2 infection? Results from a longitudinal study in a police cohort. J. Med. Virol. 2023, 95, e28904. [Google Scholar] [CrossRef]
- Erice, A.; Prieto, L.; Caballero, C. Long-term analyses of SARS-CoV-2 humoral and T cell responses and breakthrough SARS-CoV-2 infections after two doses of BNT162b2 followed by mRNA-1273 and bivalent Omicron-adapted BNT162b2 vaccines: A prospective study over 2 years in non-immunocompromised individuals. Vaccines 2023, 11, 1835. [Google Scholar] [CrossRef]
- Actualizaciones de la Situación de las Variantes SARS-CoV-2 en España. Available online: https://www.sanidad.gob.es/areas/alertasEmergenciasSanitarias/alertasActuales/nCov/variantesSARS-COV-2/informesPrevios/situacionVariantes.htm (accessed on 1 December 2024).
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, T.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2 infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e4. [Google Scholar] [CrossRef]
- Ciccone, E.J.; Zhu, D.R.; Gunderson, A.K.; Hawke, S.; Ajeen, R.; Lodge, E.K.; Shook-Sa, B.E.; Abernathy, H.; Garrett, H.E.; King, E.; et al. Magnitude and Durability of the Antibody Response to mRNA-Based Vaccination Among SARS-CoV-2 Seronegative and Seropositive Health Care Personnel. Open Forum Inf. Dis. 2024, 11, ofae009. [Google Scholar] [CrossRef]
- Lasrado, N.; Barouch, D.H. SARS-CoV-2 Hybrid Immunity: The Best of Both Worlds. J. Inf. Dis. 2023, 228, 1311–1313. [Google Scholar] [CrossRef]
- Tsagkly, P.; Geropeppa, M.; Papadatou, I.; Spolou, V. Hybrid Immunity against SARS-CoV-2 Variants: A Narrative Review of the Literature. Vaccines 2024, 12, 1051. [Google Scholar] [CrossRef]
- Blom, K.; Marking, U.; Havervall, S.; Gordon, M.; García, M.; Tecleab, T.; Christ, W.; Forsell, M.; Phillipson, M.; Nilsson, P.; et al. Immune responses after omicron infection in triple-vaccinated health-care workers with and without previous SARS-CoV-2 infection. Lancet Infect. Dis. 2022, 22, 943–945. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Otter, A.D.; Lin, K.-M.; Muñoz, D.; Pieper, F.; Butler, D.K.; Liu, S.; Joy, G.; et al. Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science 2022, 377, 275. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.J.; Gibbons, J.M.; Pade, C.; Lin, K.-M.; Muñoz, D.; Pieper, F.; Butler, D.K.; Liu, S.; Otter, A.D.; Joy, G.; et al. Heterologous infection and vaccination shapes immunity against SARS-CoV-2 variants. Science 2022, 375, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Erice, A.; Varillas-Delgado, D.; Caballero, C. Decline of antibody titers 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin. Microbiol. Infect. 2022, 28, 139e1–139e4. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.; Dhar, M.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Aguilar-Bretones, M.; Fouchier, R.A.; Koopmans, M.P.; van Nierop, G.P. Impact of antigenic evolution and original antigenic sin on SARS-CoV-2 immunity. J. Clin. Investig. 2023, 133, e162192. [Google Scholar] [CrossRef]
- Gartlan, C.; Tipton, T.; Salguero, F.J.; Sattentau, Q.; Gorringe, A.; Carroll, M.W. Vaccine-associated enhanced disease and pathogenic human coronaviruses. Front. Immunol. 2022, 13, 882972. [Google Scholar] [CrossRef]
- Shimizu, J.; Sasaki, T.; Koketsu, R.; Morita, R.; Yoshimura, Y.; Murakami, A.; Saito, Y.; Kusunoki, T.; Samune, Y.; Nakayama, E.E.; et al. Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells. Sci. Rep. 2022, 12, 15612. [Google Scholar] [CrossRef]
- Sawant, J.; Patil, A.; Kurle, S. A review: Understanding molecular mechanisms of antibody-dependent enhancement in viral infections. Vaccines 2023, 11, 1240. [Google Scholar] [CrossRef]
- Yaugel-Novoa, M.; Noailly, B.; Jospin, F.; Berger, A.-E.; Waeckel, L.; Botelho-Nevers, E.; Longet, S.; Bourlet, T.; Paul, S. Prior COVID-19 Immunization Does Not Cause IgA- or IgG-Dependent Enhancement of SARS-CoV-2 Infection. Vaccines 2023, 11, 773. [Google Scholar] [CrossRef]
- Gan, L.; Chen, Y.; Tan, J.; Wang, X.; Zhang, D. Does potential antibody-dependent enhancement occur during SARS-CoV-2 infection after natural infection or vaccination? A meta-analysis. BMC Infect. Dis. 2022, 22, 742. [Google Scholar] [CrossRef]
- Tehrani, Z.R.; Habibzadeh, P.; Flinko, R.; Chen, H.; Abbasi, A.; Yared, J.A.; Ciupe, S.M.; Lewis, G.K.; Sajadi, M.M. Deficient Generation of Spike-Specific Long-Lived Plasma Cells in the Bone Marrow After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2024, 230, e30–e33. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillo, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat. Med. 2024, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, S. T-Cell Immune Responses to SARS-CoV-2 Infection and Vaccination. Vaccines 2024, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
| Months [median (IQR) days] from primary BNT162b2 vaccination | |||||
N. (%) | 1.5 [40 (38–42)] | 3 [89 (88–91)] | 7 [215 (214–220)] | 9 [279 (276–280)] | |
| All subjects | 258 (100) | 258 (100) | 253 (98) | 252 (98) | 236 (91) |
| Estimated Anti-RBD Antibody Titers (AU/mL), GMT (95% CI) a | |||||
| Previously infected subjects b | 50 (19) | 15,095 * (11,486–19,840) | 8259 * (6347–10,746) | 2227 * (1707–2905) | 1613 * (1218–2136) |
| Naïve subjects c | 208 (81) | 8038 (7061–9149) | 3023 (2652–3466) | 792 (695–903) | 597 (522–682) |
| Months [median (IQR) days] from mRNA-1273 booster | |||||
N. (%) | 4 [97 (96–97)] | 7 [188 (183–189)] | 10 [286 (285–289)] | 16 [503 (502–505)] | |
| All subjects | 130 (100) | 130 (100) | 112 (86) | 126 (97) | 83 (64) |
| Estimated Anti-RBD Antibody Titers (AU/mL), GMT (95% CI) a | |||||
| Previously infected subjects b | 21 (16) | 18,484 (12,668–26,970) | 14,874 (9888–22,376) | 15,701 (10,759–22,913) | 9230 (5808–14,669) |
| Naïve subjects c | 109 (84) | 24,360 (20,639–28,751) | 22,188 (18,541–26,552) | 20,082 (16,947–23,798) | 18,783 (15,245–23,141) |
| Months from Primary BNT162b2 Vaccination | |||||
|---|---|---|---|---|---|
| 1.5 | 3 | 7 | 9 | N. Breakthrough Infections | |
| Previously infected subjects a | 50 | 50 | 49 | 44 | |
| N. breakthrough infections | 13 c | 0 | 3 | 4 | 20 |
| Infection rate (%) | 26.0 | 0.0 | 6.1 | 9.1 | |
| Naïve subjects b | 208 | 203 | 203 | 192 | |
| N. breakthrough infections | 0 | 1 | 11 | 4 | 16 |
| Infection rate (%) | 0.0 | 0.5 | 5.4 | 2.1 | |
| Total breakthrough infections | 36 | ||||
| Months from mRNA-1273 Booster | |||||
| 4 | 7 | 10 | 16 | ||
| Previously infected subjects a | 21 | 18 | 21 | 14 | |
| N. breakthrough infections | 9 | 6 | 7 | 4 | 26 |
| Infection rate (%) | 42.9 | 33.3 | 33.3 | 26.0 | |
| Naïve subjects b | 109 | 94 | 105 | 69 | |
| N. breakthrough infections | 35 | 22 | 47 | 30 | 134 |
| Infection rate (%) | 32.1 | 23.4 | 44.8 | 43.5 | |
| Total breakthrough infections | 160 | ||||
| 1st Measurement a | 2nd Measurement b | |
|---|---|---|
| N. tested | 107 | 83 |
| T-cell response | ||
| N. (%) positive | 102 (95) | 76 (92) |
| N. (%) negative | 5 (5) | 7 (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erice, A.; Nuño, N.; Prieto, L.; Caballero, C. Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273. Vaccines 2025, 13, 310. https://doi.org/10.3390/vaccines13030310
Erice A, Nuño N, Prieto L, Caballero C. Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273. Vaccines. 2025; 13(3):310. https://doi.org/10.3390/vaccines13030310
Chicago/Turabian StyleErice, Alejo, Néstor Nuño, Lola Prieto, and Cristina Caballero. 2025. "Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273" Vaccines 13, no. 3: 310. https://doi.org/10.3390/vaccines13030310
APA StyleErice, A., Nuño, N., Prieto, L., & Caballero, C. (2025). Immune Imprinting, Non-Durable Hybrid Immunity, and Hybrid Immune Damping Following SARS-CoV-2 Primary Vaccination with BNT162b2 and Boosting with mRNA-1273. Vaccines, 13(3), 310. https://doi.org/10.3390/vaccines13030310






