Meningococcal Vaccination in High-Risk Patients: A Systematic Approach to Evaluating Coverage and Patient Catch-Up Through Healthcare Databases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Sources and Study Variables
- •
- Status: active or passive
- •
- Reason for passive status: dead at the end of follow-up (15 March 2024), not having NUHSA (unique health identifier for Andalusia), living in another autonomous community, and other administrative problems with the Andalusian Health Service. Date of discharge from the first intervention or diagnosis that led to the inclusion in the HR-IMD group.
- •
- Time where the risk condition for vaccine recommendation first appeared: before or after the regional immunization guidelines for meningococcal vaccination.
- •
- Sex: male or female
- •
- Age at the time of data matching.
- •
- Risk group (Table 1).
- •
- ICD-9 and ICD-10 codes.
- •
- Name of the vaccine required: Bexsero®, Trumenba®, Nimenrix®, MenQuadfi®, or Menveo®.
- •
- Date of vaccine administration.
- •
- Number of doses received of each meningococcal vaccine.
2.3. Statistical Analysis of Data
2.4. Ethical Considerations
3. Results
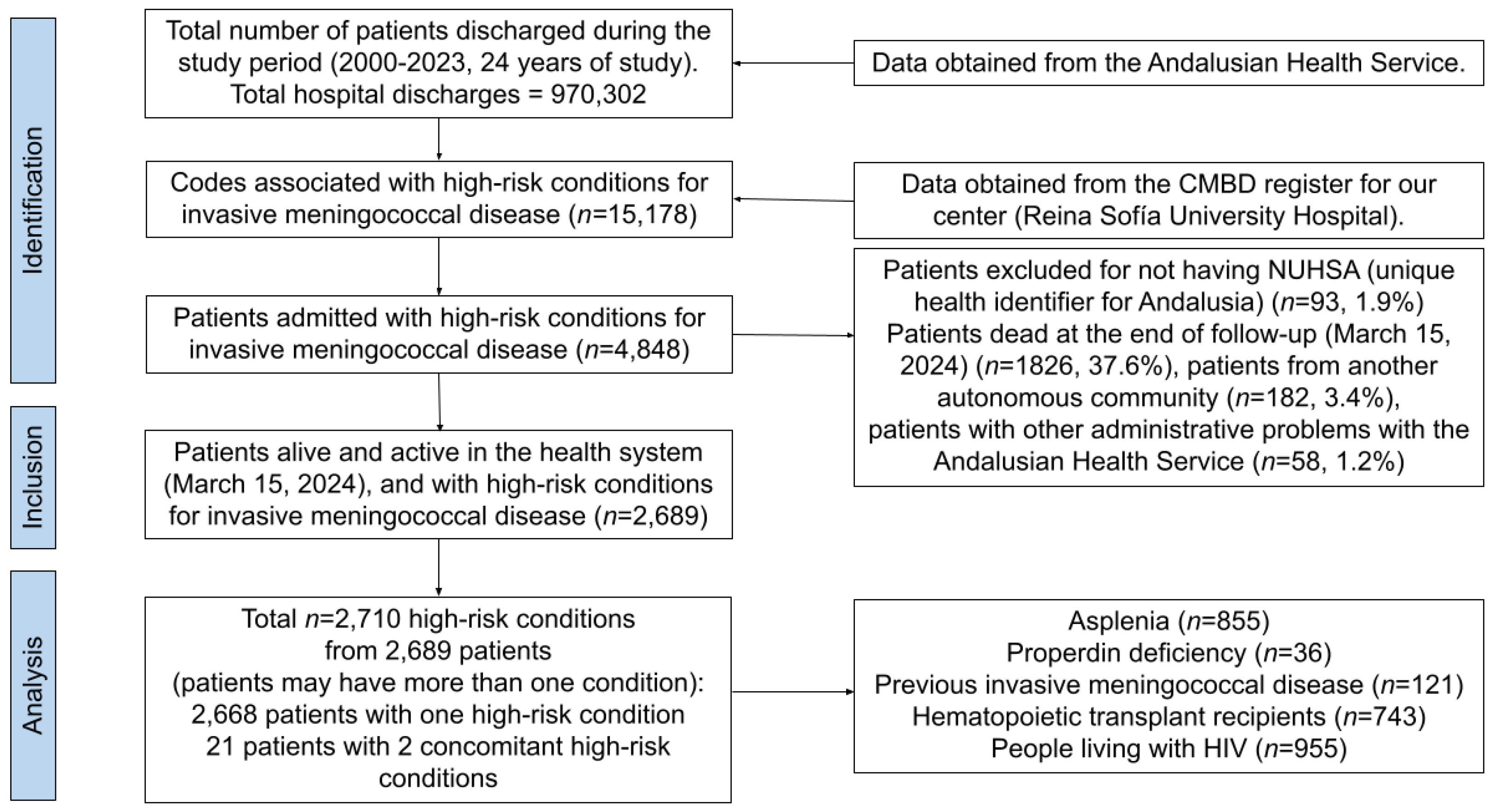
3.1. Selection of the Sample
3.2. Characteristics of the Sample
3.3. Number of MenB and MenACWY Doses Received by Each HR-IMD Condition
3.4. Vaccination Status at the Time of the Analysis
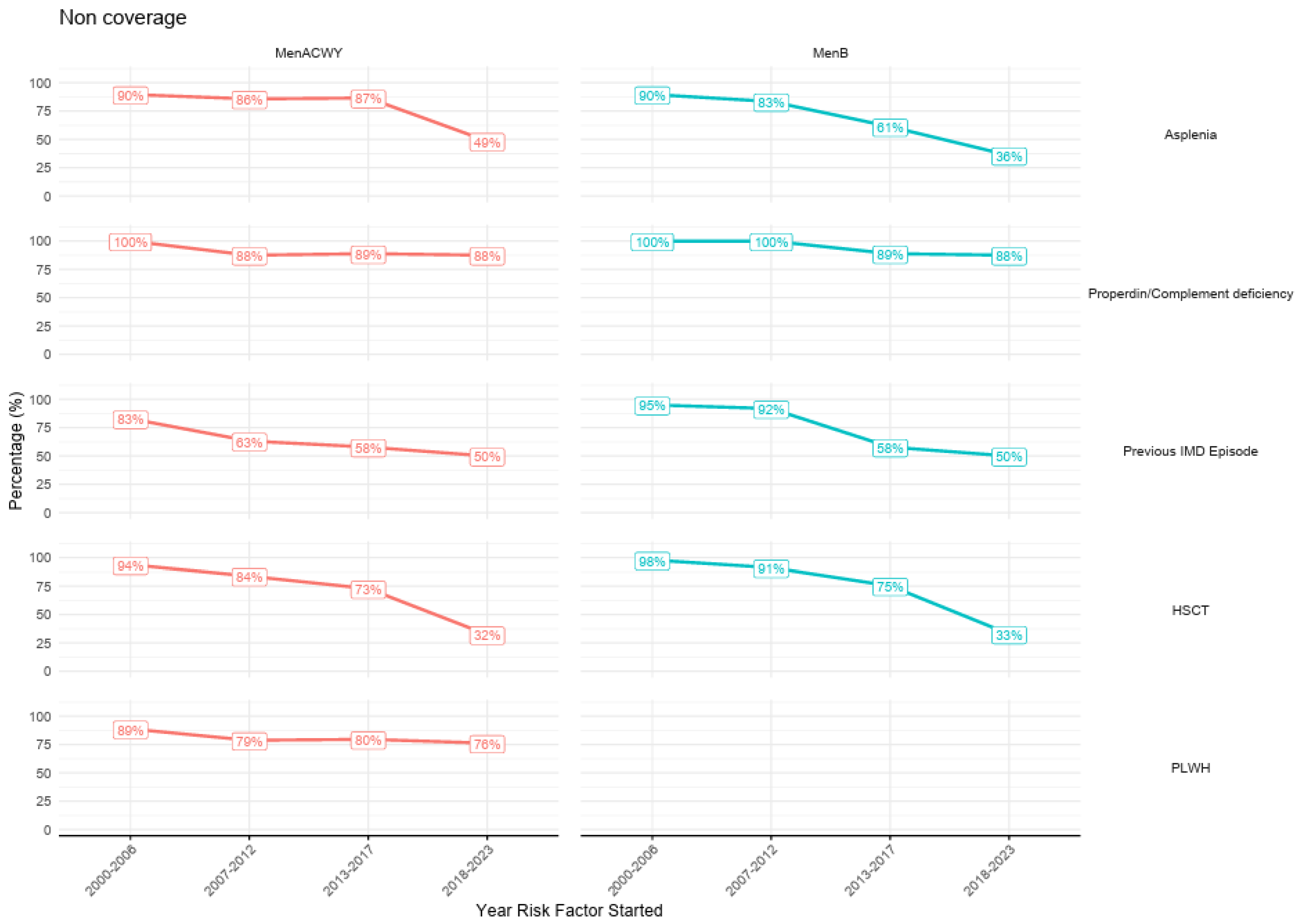
3.5. Vaccination Coverage by Start of the HR-IMD Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | Anatomical Therapeutic Chemical Classification System |
| BDU | User Database (from the Andalusian Health Service) |
| CMBD | Minimum Basic Data Set |
| ICD | International Classification of Diseases |
| IMD | Invasive meningococcal disease |
| MenACWY | Vaccines against meningococcal serogroups A, C, W, and Y |
| MenB | Vaccines against meningococcal serogroup B |
| HSCT | Hematopoietic stem cell transplantation |
| NUHSA | Unique Health Record Number (from the Andalusian Health Service) |
| PLWH | People living with HIV |
Appendix A
| Vaccine Group | Risk Group | Vaccine | Age 1st Dose | Recommended Specific Schedule |
|---|---|---|---|---|
| MenB |
| 4MenB (Bexsero®) | 2 to 5 months | Primary vaccination schedule: Two doses in the primary series. The first dose should not be administered before 2 months of age. Minimum interval between doses: 2 months. Booster dose: One booster dose between 12 and 15 months of age, with a minimum interval of 6 months from the primary series. |
| 6 to 11 months | Primary vaccination schedule: Two doses in the primary series, with a minimum interval of 2 months between doses. Booster dose: One booster dose in the second year of life, with a minimum interval of 2 months from the primary series. | |||
| 12 to 23 months | Primary vaccination schedule: Two doses in the primary series, with a minimum interval of 2 months between doses. Booster dose: One booster dose with an interval of 12 to 23 months from the primary series. | |||
| Over 2 years | Primary vaccination schedule: Two doses with a minimum interval of 1 month. Booster dose: One booster dose 1 year after completing the primary series. | |||
| FHbp (Trumenba®) | Over 10 years | Primary vaccination schedule: Two doses with a minimum interval of 6 months. | ||
| MenACWY |
| MenACWY-TT (Nimenrix®) | 1.5 to 5 months | Primary vaccination schedule: Two doses in the primary series. Minimum interval between doses: 2 months. Booster dose: One booster dose at 12 months of age, with a minimum interval of 6 months from the primary series. |
| 6 to 11 months | Primary vaccination schedule: Two doses in the primary series. Minimum interval between doses: 2 months. Second dose at 12 months of age. | |||
| Over 1 year | Primary vaccination schedule: Two doses with a minimum interval of 2 months. | |||
| MenACWY-TT (MenQuadfi®) | Over 1 year | Primary vaccination schedule: Two doses with a minimum interval of 2 months. | ||
| MenACWY-CRM (Menveo®) | Over 2 years | Primary vaccination schedule: Two doses with a minimum interval of 2 months. |
References
- Morello, B.R.; Milazzo, A.; Marshall, H.S.; Giles, L.C. Public health management of invasive meningococcal disease outbreaks: Worldwide 1973–2018, a systematic review. BMC Public Health 2024, 24, 2254. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.; Pollard, A.J. Meningococcal disease: Clinical presentation and sequelae. Vaccine 2012, 30 (Suppl. S2), B3–B9. [Google Scholar] [CrossRef]
- Bille, E.; Schubert-Unkmeir, A.; Coureuil, M. Neisseria meningitidis. Trends Microbiol. 2025, 33, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Dwilow, R.; Fanella, S. Invasive meningococcal disease in the 21st century—An update for the clinician. Curr. Neurol. Neurosci. Rep. 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Salud Carlos III. Boletín Epidemiológico Semanal. Enfermedad Meningocócica Invasiva en España en 2023 [Invasive Meningococcal Disease in Spain in 2023]. Available online: https://revista.isciii.es/index.php/bes/article/view/1411 (accessed on 2 February 2025).
- Instituto de Salud Carlos III y Centro Nacional de Epidemiología. Informe Semanal de Vigilancia Epidemiológica en España, nº2, Año 2025 [Weekly Report on Epidemiological Surveillance in Spain, nº2, Year 2025]. Available online: https://cne.isciii.es/documents/d/cne/is_n-2-20250107_web (accessed on 2 February 2025).
- Silverii, G.A.; Gabutti, G.; Tafuri, S.; Tereziu, J.; Clerico, A.; Fornengo, R.; Greco, C.; Irace, C.; Sordi, V.; Sorice, G.P.; et al. Diabetes as a risk factor for invasive meningococcal disease. A meta-analysis of observational studies. Acta Diabetol. 2025, 62, 131–134. [Google Scholar] [CrossRef]
- Parikh, S.R.; Campbell, H.; Bettinger, J.A.; Harrison, L.H.; Marshall, H.S.; Martinon-Torres, F.; Safadi, M.A.; Shao, Z.; Zhu, B.; von Gottberg, A.; et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J. Infect. 2020, 81, 483–498. [Google Scholar] [CrossRef]
- Ponencia de Programa y Registro de Vacunaciones, Ministerio de Sanidad, Gobierno de España. Vacunación en Grupos de Riesgo de Todas las Edades y en Determinadas Situaciones [Vaccination in at-Risk Groups of All Ages and in Certain Situations]. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/programasDeVacunacion/riesgo/docs/VacGruposRiesgo_todas_las_edades.pdf (accessed on 2 February 2025).
- Consejería de Salud y Consumo, Junta de Andalucía. Instrucción DGSPyOF-2/2023. Programa de Vacunación Frente a la Enfermedad Meningocócica en Andalucía 2023 [Meningococcal Disease Vaccination Programme in Andalusia 2023]. Available online: https://www.andavac.es/wp-content/uploads/instrucciones/Instruccion_Vacunacion_Meningococo_Andalucia.pdf (accessed on 2 February 2025).
- Ballalai, I.; Dawson, R.; Horn, M.; Smith, V.; Bekkat-Berkani, R.; Soumahoro, L.; Vicic, N. Understanding barriers to vaccination against invasive meningococcal disease: A survey of the knowledge gap and potential solutions. Expert Rev. Vaccines 2023, 22, 457–467. [Google Scholar] [CrossRef]
- National Health Service of England. Coding Classifications Used. Available online: https://digital.nhs.uk/services/cohorting-as-a-service-caas/covid-19-spring-2024-campaign-cohort-identification-methodology/coding-classifications-used (accessed on 2 February 2025).
- Secretaría General de Salud Digital, Información e Innovación del Sistema Nacional de Salud, Subdirección General de Información Sanitaria. Ecie-Maps. Edición Electrónica de la Clasificación Internacional de Enfermedades [Electronic Edition of the International Classification of Diseases]. Available online: https://www.eciemaps.sanidad.gob.es/documentation (accessed on 2 February 2025).
- Bonanni, P.; Villani, A.; Scotti, S.; Biasci, P.; Russo, R.; Maio, T.; Vitali Rosati, G.; Moscadelli, A.; Conforti, G.; Azzari, C.; et al. The recommended lifetime immunization schedule from the board of vaccination calendar for life in Italy: A continuing example of impact on public health policies. Vaccine 2021, 39, 1183–1186. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; Di Lorenzo, A.; Cuscianna, E.; Tafuri, S.; Germinario, C.A. Vaccine coverage for recommended vaccines among splenectomised patients in Apulia, South Italy: A retrospective cohort study. BMJ Open 2023, 13, e069316. [Google Scholar] [CrossRef]
- Orangzeb, S.; Watle, S.V.; Caugant, D.A. Adherence to vaccination guidelines of patients with complete splenectomy in Norway, 2008–2020. Vaccine 2023, 41, 4579–4585. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, G.; Mazzocca, R.; Masci, T.; Berghella, L.; Del Papa, J.; D’Aloisio, F.; Messinese, M.; Cedrone, F.; Marani Toro, P.; Soldato, G. Vaccination Coverages Among Splenectomized Patients: A Retrospective Study from an Italian Southern Province. Vaccines 2025, 13, 138. [Google Scholar] [CrossRef]
- Ghaswalla, P.K.; Bengtson, L.G.S.; Marshall, G.S.; Buikema, A.R.; Bancroft, T.; Schladweiler, K.M.; Koep, E.; Novy, P.; Hogea, C.S. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine 2021, 39, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Boeddha, C.; de Graaf, W.; Overbosch, D.; van Genderen, P.J. Travel health preparation and travel-related morbidity of splenectomised individuals. Travel Med. Infect. Dis. 2012, 10, 197–200. [Google Scholar] [CrossRef]
- Martino, C.; Gallone, M.S.; Quarto, M.; Germinario, C.; Tafuri, S. Immunization coverage among splenectomized patients: Results of an ad hoc survey in Puglia Region (South of Italy). Hum. Vaccines Immunother. 2016, 12, 1277–1279. [Google Scholar] [CrossRef][Green Version]
- Kuchar, E.; Nitsch-Osuch, A.; Stolarczyk, C.; Kurpas, D.; Zycinska, K.; Wardyn, K.; Szenborn, L. Immunization coverage against capsular bacteria in splenectomized patients. Adv. Exp. Med. Biol. 2013, 788, 139–145. [Google Scholar]
- Nived, P.; Jørgensen, C.S.; Settergren, B. Vaccination status and immune response to 13-valent pneumococcal conjugate vaccine in asplenic individuals. Vaccine 2015, 33, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Ghaswalla, P.K.; Bengtson, L.G.S.; Buikema, A.R.; Bancroft, T.; Koep, E.; Novy, P.; Hogea, C.S. Low Meningococcal Vaccination Rates Among Patients with Newly Diagnosed Complement Component Deficiencies in the United States. Clin. Infect. Dis. 2022, 75, 155–158. [Google Scholar] [CrossRef]
- MacDonald, S.E.; Palichuk, A.; Slater, L.; Tripp, H.; Reifferscheid, L.; Burton, C. Gaps in knowledge about the vaccine coverage of immunocompromised children: A scoping review. Hum. Vaccines Immunother. 2022, 18, 1–16. [Google Scholar] [CrossRef]
- Khemani, K.; Steele, M.K.; Bakshi, N.; Krishnamurti, L.; Yildrim, I. Vaccination Adherence in Pediatric Patients Post-Hematopoietic Stem Cell Transplant. Blood 2018, 132, 3406. [Google Scholar] [CrossRef]
- Breitschwerdt, S.; Schwarze-Zander, C.; Al Tayy, A.; Mutevelli, J.; Wasmuth, J.C.; Rockstroh, J.K.; Boesecke, C. Implementation of EACS vaccination recommendations among people living with HIV. Infection 2022, 50, 1491–1497. [Google Scholar] [CrossRef]
- Ghaswalla, P.K.; Marshall, G.S.; Bengtson, L.G.S.; Buikema, A.R.; Bancroft, T.; Koep, E.; Novy, P.; Hogea, C.S. Meningococcal Vaccination Rates Among People with a New Diagnosis of HIV Infection in the US. JAMA Netw. Open. 2022, 5, e228573. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sánchez, M.; Fairley, C.K.; Bradshaw, C.S.; Chen, M.Y. Chow EPF. Meningococcal vaccine uptake among men who have sex with men in response to an invasive meningococcal C disease outbreak in Melbourne, Australia. Sex Transm. Infect. 2020, 96, 246–250. [Google Scholar] [CrossRef]
- Lammers, A.J.; Hoekstra, J.B.; Speelman, P.; Lombarts, K.M. Physicians report barriers to deliver best practice care for asplenic patients: A cross-sectional survey. PLoS ONE. 2011, 6, e17302. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, N.E.; Baker, J.; Ward, R.; Johnson, C.; Taggart, L.; Sholzberg, M. The development of a quality improvement project to improve infection prevention and management in patients with asplenia or hyposplenia. BMJ Open Qual. 2020, 9, e000770. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.P.; Boggan, J.C.; Lau, K.; Simel, D.L. Splenectomy as a Destination: Improving Quality of Care Among Asplenic Veterans Through a Travel Clinic. Am. J. Med. 2017, 130, 856–861. [Google Scholar] [CrossRef]
- Arzt, N.H. Clinical Decision Support for Immunizations (CDSi): A Comprehensive, Collaborative Strategy. Biomed. Inform. Insights 2016, 8, S40204. [Google Scholar]
- Martinelli, D.; Fortunato, F.; Iannazzo, S.; Cappelli, M.G.; Prato, R. Using Routine Data Sources to Feed an Immunization Information System for High-Risk Patients—A Pilot Study. Front. Public Health 2018, 6, 37. [Google Scholar] [CrossRef]
- Felzer, J.R.; Finney Rutten, L.J.; Wi, C.I.; LeMahieu, A.M.; Beam, E.; Juhn, Y.J.; Jacobson, R.M.; Kennedy, C.C. Disparities in vaccination rates in solid organ transplant patients. Transpl. Infect Dis. 2023, 25, e14010. [Google Scholar] [CrossRef]
- François, G.; Hallauer, J.; Van Damme, P. Hepatitis B vaccination: How to reach risk groups. Vaccine 2002, 21, 1–4. [Google Scholar] [CrossRef]
- Hernando, V.; Suárez, L.; Gutiérrez, G.; López, J.C.; Navarro-Soler, R.; Cabello, A.; Sanz, J.; Suarez-García, I.; Fernández, M.T.; Losa, J.E.; et al. Vaccination trends in people with HIV infection participanting in the hospital-based survey of patients infected with HIV, 2006–2021. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2024, 42, 339–346. [Google Scholar] [CrossRef] [PubMed]

| Risk Group Label | Risk Group Description | ICD-9 Diagnose | ICD-9 Procedure | ICD-10 Diagnose | ICD-10 Procedure | Total |
|---|---|---|---|---|---|---|
| Count | Count | Count | Count | Count | ||
| Asplenia | Individuals with anatomical asplenia or severe splenic dysfunction (e.g., sickle cell anemia) and those scheduled for surgical splenectomy. | 13 | 2 | 45 | 5 | 65 |
| Properdin-complement deficiency | Individuals with properdin deficiency or complement deficiencies. | 2 | 0 | 2 | 0 | 4 |
| Previous IMD | Individuals who have experienced an episode of IMD, regardless of vaccination status prior to the episode. | 14 | 0 | 18 | 0 | 32 |
| Hematopoietic stem cell transplantation | Individuals who have undergone hematopoietic stem cell transplantation | 8 | 9 | 13 | 68 | 98 |
| Outbreak | In outbreak situations where the health authority determines the need for vaccination | 0 | 0 | 0 | 0 | 0 |
| Laboratory personnel | Laboratory personnel (lab technicians and microbiologists) working with samples that may potentially contain Neisseria meningitidis | 0 | 0 | 0 | 0 | 0 |
| PLWH | People living with HIV | 3 | 0 | 11 | 0 | 14 |
| Total | - | 40 | 11 | 89 | 73 | 214 |
| High-Risk Group | No. of Conditions (%) | Males | Females | p-Value 1 | ||
|---|---|---|---|---|---|---|
| n (%) | Median Age (IRQ) | n (%) | Median Age (IRQ) | |||
| Asplenia | 855 (31.4%) | 432 (50.5%) | 58.5 (46.4–69.9) | 423 (49.5%) | 63.2 (49.2–74.0) | 0.006 |
| Properdin-complement deficiency | 36 (1.3%) | 16 (44.4%) | 55.5 (46.0–64.7) | 20 (55.5%) | 47.7 (40.0–57.2) | 0.275 |
| Previous IMD | 121 (4.4%) | 65 (53.7%) | 25.5 (19.9–36.2) | 56 (46.3%) | 26.0 (18.3–43.6) | 0.541 |
| HSCT | 743 (27.3%) | 406 (54.6%) | 56.5 (41.7–68.3) | 337 (45.4%) | 56.3 (39.3–66.4) | 0.117 |
| PLWH | 955 (35.0%) | 711 (74.5%) | 55.6 (45.0–62.3) | 243 (25.4%) | 53.7 (44.7–60.1) | 0.090 |
| Total | 2710 (100.0%) | 1630 (60.1%) | 56.1 (43.1–64.7) | 1079 (39.8%) | 56.7 (42.8–67.1) | - |
| Risk Group | n Total | Vaccine | 0 Doses | 1 Dose | 2 Doses | 3 Doses | 4 Doses | Males ≥ 1 Dose | Females ≥ 1 Dose | p-Value 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Asplenia | 855 | MenB | 516 (60.4%) | 22 (2.6%) | 300 (35.1%) | 13 (1.5%) | 4 (0.5%) | 150 (34.7%) | 189 (44.7%) | <0.001 |
| MenACWY | 615 (71.9%) | 63 (7.4%) | 174 (20.4%) | 1 (0.1%) | 2 (0.2%) | 98 (22.7%) | 142 (33.6%) | <0.001 | ||
| Properdin-complement deficiency | 36 | MenB | 34 (94.4%) | 1 (2.8%) | 0 (0.0%) | 1 (2.8%) | 0 (0.0%) | 2 (12.5%) | 0 (0.0%) | 0.190 1 |
| MenACWY | 33 (91.7%) | 2 (5.6%) | 1 (2.8%) | 0 (0.0%) | 0 (0.0%) | 2 (12.5%) | 1 (5.0%) | 0.574 1 | ||
| Previous IMD | 121 | MenB | 101 (83.5%) | 0 (0.0%) | 19 (15.7%) | 1 (0.8%) | 0 (0.0%) | 12 (18.5%) | 8 (14.3%) | 0.537 |
| MenACWY | 82 (67.8%) | 34 (28.1%) | 4 (3.3%) | 1 (0.8%) | 0 (0.0%) | 21 (32.3%) | 18 (32.1%) | 0.984 | ||
| HSCT | 743 | MenB | 480 (64.6%) | 43 (5.8%) | 210 (28.3%) | 6 (0.8%) | 4 (0.5%) | 147 (36.2%) | 116 (34.4%) | 0.612 |
| MenACWY | 451 (60.7%) | 88 (11.8%) | 200 (26.9%) | 2 (0.3%) | 2 (0.3%) | 161 (39.7%) | 131 (38.9%) | 0.827 | ||
| PLWH | 955 | MenACWY | 745 (78.0%) | 77 (8.1%) | 132 (13.8%) | 0 (0.0%) | 1 (0.1%) | 172 (24.2%) | 38 (15.6%) | <0.001 |
| Total | 1755 | MenB | 1131 (64.4%) | 66 (3.8%) | 529 (30.1%) | 21 (1.2%) | 8 (0.5%) | 311 (33.8%) | 313 (37.4%) | 0.116 |
| 2710 | MenACWY | 1926 (71.1%) | 264 (9.7%) | 511 (18.9%) | 4 (0.1%) | 5 (0.2%) | 454 (27.9%) | 330 (30.6%) | 0.125 |
| Assessment | Vaccine Status | MenB, n (%) | MenACWY, n (%) | Required Recommendations |
|---|---|---|---|---|
| Full schedule | Adequately vaccinated | 18 (1.0%) | 378 (13.9%) | None. Correct vaccination status at the time of the analysis |
| Recently initiated schedule | Adequately vaccinated | 8 (0.5%) | 0 (0.0%) | |
| Time interval between primary vaccination schedule and booster dose not exceeded | Adequately vaccinated | 0 (0.0% | 176 (6.5%) | |
| Time interval between booster dose and periodic booster dose not exceeded | Adequately vaccinated | 96 (5.4%) | 0 (0.0%) | |
| Time interval between primary vaccination doses not exceeded | Adequately vaccinated | 10 (0.6%) | 62 (2.3%) | |
| Exceeded recommended interval between schedule and booster dose | Not adequately vaccinated | 435 (24.6%) | 1 (0.0%) | Book an appointment to continue with vaccination schedule |
| Exceeded recommended interval between booster dose and periodic booster dose | Not adequately vaccinated | 2 (0.1%) | 0 (0.0%) | |
| Exceeded recommended interval between primary vaccination doses | Not adequately vaccinated | 56 (3.2%) | 171 (6.3%) | |
| Vaccination not initiated or not documented | Not adequately vaccinated | 1145 (64.7%) | 1937 (71.1%) | Book an appointment to initiate vaccination schedule |
| High-Risk Group | n Total | Vaccine | Before Immunization Guideline | After Immunization Guideline | p-Value 1 | ||
|---|---|---|---|---|---|---|---|
| n | Non-Coverage, n (%) | n | Non-Coverage, n (%) | ||||
| Asplenia | 855 | MenB | 386 | 327 (84.7%) | 469 | 189 (40.3%) | <0.001 |
| MenACWY | 542 | 473 (87.3%) | 313 | 142 (45.4%) | <0.001 | ||
| Properdin-complement deficiency | 36 | MenB | 23 | 22 (95.7%) | 13 | 12 (92.3%) | 1.000 |
| MenACWY | 28 | 26 (92.9%) | 8 | 7 (87.5%) | 0.541 | ||
| Previous IMD | 121 | MenB | 101 | 91 (90.1%) | 20 | 10 (50.0%) | <0.001 |
| MenACWY | 111 | 78 (70.3%) | 10 | 4 (40.0%) | 0.107 | ||
| HSCT | 743 | MenB | 460 | 381 (82.8%) | 283 | 99 (35.0%) | <0.001 |
| MenACWY | 460 | 359 (78.0%) | 283 | 92 (32.5%) | <0.001 | ||
| PLWH | 955 | MenACWY | 322 | 266 (82.6%) | 633 | 479 (75.7%) | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Montero, R.; Serrano-Ortiz, Á.; Rivera-Izquierdo, M.; Galvache Murillo-Rico, P.; Moñiz-Díez, A.; Onieva-García, M.Á.; Girela-López, E.; Salcedo-Leal, I. Meningococcal Vaccination in High-Risk Patients: A Systematic Approach to Evaluating Coverage and Patient Catch-Up Through Healthcare Databases. Vaccines 2025, 13, 287. https://doi.org/10.3390/vaccines13030287
Ruiz-Montero R, Serrano-Ortiz Á, Rivera-Izquierdo M, Galvache Murillo-Rico P, Moñiz-Díez A, Onieva-García MÁ, Girela-López E, Salcedo-Leal I. Meningococcal Vaccination in High-Risk Patients: A Systematic Approach to Evaluating Coverage and Patient Catch-Up Through Healthcare Databases. Vaccines. 2025; 13(3):287. https://doi.org/10.3390/vaccines13030287
Chicago/Turabian StyleRuiz-Montero, Rafael, Álvaro Serrano-Ortiz, Mario Rivera-Izquierdo, Piedad Galvache Murillo-Rico, Ana Moñiz-Díez, María Ángeles Onieva-García, Eloy Girela-López, and Inmaculada Salcedo-Leal. 2025. "Meningococcal Vaccination in High-Risk Patients: A Systematic Approach to Evaluating Coverage and Patient Catch-Up Through Healthcare Databases" Vaccines 13, no. 3: 287. https://doi.org/10.3390/vaccines13030287
APA StyleRuiz-Montero, R., Serrano-Ortiz, Á., Rivera-Izquierdo, M., Galvache Murillo-Rico, P., Moñiz-Díez, A., Onieva-García, M. Á., Girela-López, E., & Salcedo-Leal, I. (2025). Meningococcal Vaccination in High-Risk Patients: A Systematic Approach to Evaluating Coverage and Patient Catch-Up Through Healthcare Databases. Vaccines, 13(3), 287. https://doi.org/10.3390/vaccines13030287






