Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination
Abstract
1. Introduction
2. Methods
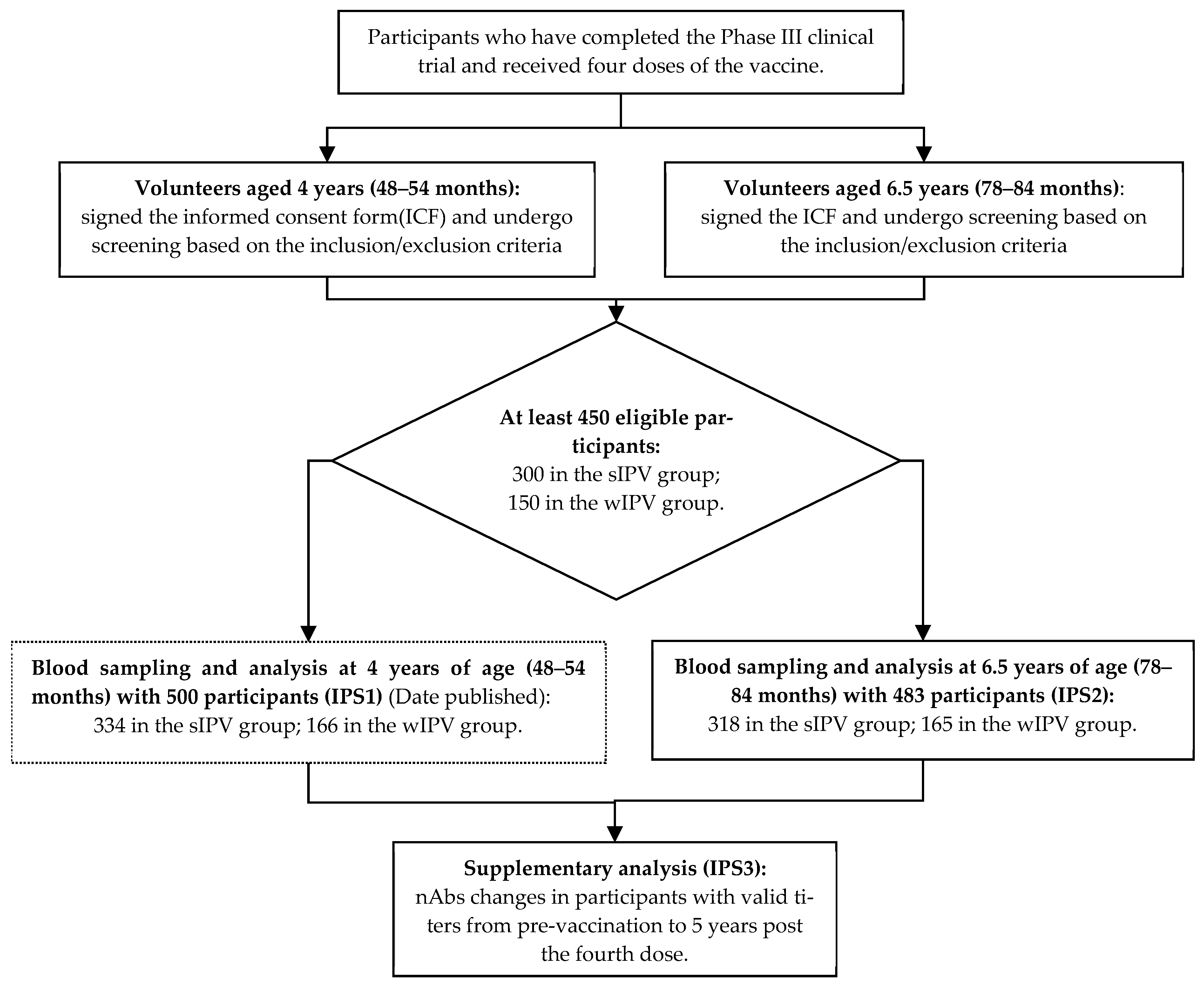
2.1. Study Design
2.2. Sample Sizes
2.3. Participants
2.4. Vaccines
2.5. Assays
2.6. Outcomes
2.7. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Five-Year Immune Persistence Set
3.3. Full Series Immune Persistence Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, Global Polio Eradication Initiative. Polio Eradication Strategy 2022–2026: Delivering on a Promise. 2021. Available online: https://www.who.int/publications/b/57914 (accessed on 25 December 2024).
- World Health Organization. Fact Sheet: Poliomyelitis. Geneva, Switzerland, 2024. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/poliomyelitis (accessed on 26 January 2025).
- Lopez Cavestany, R.; Eisenhawer, M.; Diop, O.M.; Verma, H.; Quddus, A.; Mach, O. The Last Mile in Polio Eradication: Program Challenges and Perseverance. Pathogens 2024, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.C.; Hussain, Z.; Yeoh, S.F.; Koh, D.; Lee, K.S. Circulating vaccine-derived poliovirus: A menace to the end game of polio eradication. Glob. Health 2020, 16, 63. [Google Scholar] [CrossRef]
- mondiale de la Santé, O.; World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, March 2023: Conclusions and recommendations–Réunion du Groupe stratégique consultatif d’experts sur la vaccination, mars 2023: Conclusions et recommandations recommandations recommandations. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2023, 98, 239–255. [Google Scholar]
- World Health Organization. Polio Eradication Strategy: GPEI Response to the Midterm Review; World Health Organization: Geneva, Switzerland, 2023; Available online: https://polioeradication.org/wp-content/uploads/2024/08/Polio-Eradication-Strategy-2024-GPEI-response-to-the-midterm-review.pdf (accessed on 25 December 2024).
- Okayasu, H.; Sein, C.; Hamidi, A.; Bakker, W.A.; Sutter, R.W. Development of inactivated poliovirus vaccine from Sabin strains: A progress report. Biologicals 2016, 44, 581–587. [Google Scholar] [CrossRef]
- Kumar, P.; Bird, C.; Holland, D.; Joshi, S.B.; Volkin, D.B. Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication. Hum. Vacc. Immunother. 2022, 18, 2154100. [Google Scholar] [CrossRef]
- World Health Organization. Polio Vaccines: WHO Position Paper–June 2022–Vaccins; World Health Organization: Geneva, Switzerland, 2022; Volume 97, pp. 277–300. Available online: https://www.who.int/publications/i/item/WHO-WER9725-277-300 (accessed on 25 December 2024).
- Shimizu, H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine 2016, 34, 1975–1985. [Google Scholar] [CrossRef]
- Leung, K.; Pang, C.W.; Lo, T.H.; Vargas-Zambrano, J.C.; Petit, C.; Lam, T.T.; Lau, E.H.; Wu, J.T. Immuno-persistence after the fourth and fifth doses of inactivated polio vaccines in school-aged children. Clin. Microbiol. Infect. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Ying, Z.; Wang, L.; Hu, Y.; Xia, J.; Chen, L.; Wang, J.; Li, C.; Zhang, Q.; Gao, Q.; et al. Safety and immunogenicity of inactivated poliovirus vaccine made from Sabin strains: A phase II, randomized, dose-finding trial. Vaccine 2018, 36, 6782–6789. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, K.; Han, W.; Chu, K.; Jiang, D.; Wang, J.; Tian, X.; Ying, Z.; Zhang, Y.; Li, C.; et al. Safety and immunogenicity of Sabin strain inactivated poliovirus vaccine compared with salk Strain Inactivated poliovirus vaccine, in different sequential schedules with bivalent oral poliovirus vaccine: Randomized controlled noninferiority clinical trials in China. Open Forum. Infect. Dis 2019, 6, ofz380. [Google Scholar]
- Chu, K.; Han, W.; Jiang, D.; Jiang, Z.; Zhu, T.; Xu, W.; Hu, Y.; Zeng, G. Cross-neutralization capacity of immune serum from different dosage of Sabin inactivated poliovirus vaccine immunization against multiple individual polioviruses. Expert. Rev. Vaccines 2021, 20, 761–767. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Zeng, G.; Chu, K.; Jiang, D.; Zhu, F.; Ying, Z.; Chen, L.; Li, C.; Zhu, F.; et al. Immunogenicity and safety of a Sabin strain–based inactivated polio vaccine: A phase 3 clinical trial. J. Infect. Dis. 2019, 220, 1551–1557. [Google Scholar] [CrossRef]
- Zheng, Y.; Ying, Z.; Zou, Y.; Zhu, T.; Qian, D.; Han, W.; Jiang, Y.; Jiang, Z.; Li, X.; Wang, J.; et al. Safety, immunogenicity and lot-to-lot consistency of Sabin-strain inactivated poliovirus vaccine in 2-month-old infants: A double-blind, randomized Phase III trial. Vaccines 2022, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of The People’s Republic of China. Childhood Immunization Schedule for National Immunization Program Vaccines—China (Version 2021). China CDC Wkly. 2021, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Li, Y.; Yu, D.; Song, Y.; Liu, S.; Xue, F.; Shan, Y.; Meng, W.; Pan, H. Immunogenicity and immune persistence in 4-year-old children completing four doses of Sabin strain or wild strain inactivated poliovirus vaccine: A phase IV, open-labeled, parallel-controlled observational study. Vaccine 2023, 41, 3467–3471. [Google Scholar] [CrossRef]
- Verdijk, P.; Rots, N.Y.; Bakker, W.A. Clinical development of a novel inactivated poliomyelitis vaccine based on attenuated Sabin poliovirus strains. Expert. Rev. Vaccines 2011, 10, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Design and Implementation of Clinical Trials for Preventive Vaccines; People’s Medical Publishing House: Beijing, China, 2022; p. 153. [Google Scholar]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://iris.who.int/handle/10665/68762 (accessed on 25 December 2024).
- Resik, S.; Tejeda, A.; Fonseca, M.; Sein, C.; Hung, L.H.; Martinez, Y.; Diaz, M.; Okayasu, H.; Sutter, R.W. Decay of Sabin inactivated poliovirus vaccine (IPV)-boosted poliovirus antibodies. Trials Vaccinol. 2015, 4, 71–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Langue, J.; Matisse, N.; Pacoret, P.; Undreiner, F.; Boisnard, F.; Soubeyrand, B. Persistence of antibodies at 5–6 years of age for children who had received a primary series vaccination with a pentavalent whole-cell pertussis vaccine and a first booster with a pentavalent acellular pertussis vaccine: Immunogenicity and tolerance of second booster with a tetravalent acellular vaccine at 5–6 years of age. Vaccine 2004, 22, 1406–1414. [Google Scholar]
- Mangarule, S.; Palkar, S.; Mitra, M.; Ravi, M.D.; Singh, R.; Moureau, A.; Jayanth, M.V.; Patel, D.M.; Ravinuthala, S.; Patnaik, B.N.; et al. Antibody persistence following administration of a hexavalent DTwP-IPV-HB-PRP∼ T vaccine versus separate DTwP-HB-PRP∼ T and IPV vaccines at 12–24 months of age and safety and immunogenicity of a booster dose of DTwP-IPV-HB-PRP∼ T in healthy infants in India. Vaccine X 2022, 11, 100190. [Google Scholar]
- Stojanov, S.; Liese, J.G.; Bendjenana, H.; Harzer, E.; Barrand, M.; Jow, S.; Dupuy, M.; Belohradsky, B.H. Immunogenicity and safety of a trivalent tetanus, low dose diphtheria, inactivated poliomyelitis booster compared with a standard tetanus, low dose diphtheria booster at six to nine years of age. Pediatr. Infect. Dis. J. 2000, 19, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Li, R.C.; Li, F.X.; Li, Y.P.; Hou, Q.M.; Li, C.G.; Li, Y.N.; Chen, F.S.; Hu, X.Z.; Su, W.B.; Zhang, S.M.; et al. Antibody persistence at 18–20 months of age and safety and immunogenicity of a booster dose of a combined DTaP–IPV//PRP∼ T vaccine compared to separate vaccines (DTaP, PRP∼ T and IPV) following primary vaccination of healthy infants in the People’s Republic of China. Vaccine 2011, 29, 9337–9344. [Google Scholar]
- Nakayama, T.; Suga, S.; Okada, K.; Okabe, N. Persistence of antibodies against diphtheria, tetanus, pertussis, and poliovirus types I, II, and III following immunization with DTaP combined with inactivated wild-type polio vaccine (DTaP-wIPV). Jpn. J. Infect. Dis. 2019, 72, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, V.; Soubeyrand, B.; Vidor, E.; Richard, P.; Boyer, J.; Sadorge, C.; Fiquet, A. Immunogenicity and safety of combined adsorbed low-dose diphtheria, tetanus and inactivated poliovirus vaccine (REVAXIS®) versus combined diphtheria, tetanus and inactivated poliovirus vaccine (DT Polio®) given as a booster dose at 6 years of age. Hum. Vaccines 2011, 7, 549–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gajdos, V.; Vidor, E.; Richard, P.; Tran, C.; Sadorge, C. Diphtheria, tetanus and poliovirus antibody persistence 5 years after vaccination of pre-schoolers with two different diphtheria, tetanus and inactivated poliomyelitis vaccines (Td-IPV or DT-IPV) and immune responses to a booster dose of DTaP-IPV. Vaccine 2015, 33, 3988–3996. [Google Scholar] [CrossRef]
- Vidor, E. The nature and consequences of intra- and inter-vaccine interference. J. Comp. Pathol. 2007, 137, S62–S66. [Google Scholar] [CrossRef]
- Ma, L.; Ying, Z.; Cai, W.; Wang, J.; Zhou, J.; Yang, H.; Gao, J.; Zhao, Z.; Liu, J.; Ouyang, S.; et al. Immune persistence of an inactivated poliovirus vaccine derived from the Sabin strain: A 10-year follow-up of a phase 3 study. EClinicalMedicine 2023, 64, 102151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, H.L.; Zhao, Y.L.; Wang, X.Y. Evaluation of immune persistence of vaccine. Chin. J. Prev. Med. 2022, 56, 212–217. (In Chinese) [Google Scholar]

| Immune Persistence Set | Characteristics | sIPV Group | wIPV Group | p-Value |
|---|---|---|---|---|
| IPS2 | N | 318 | 165 | |
| Age (Months), mean ± (SD) | 80.0 (0.82) | 80.0 (0.75) | 0.6784 | |
| Male n (%) | 180 (56.60) | 84 (50.91) | 0.2332 | |
| Female n (%) | 138 (43.40) | 81 (49.09) | ||
| IPS3 | N | 255 | 132 | |
| Age (Months), mean ± (SD) | 79.9 (0.82) | 80.0 (0.76) | 0.9925 | |
| Male n (%) | 144 (56.47) | 67 (50.76) | 0.2846 | |
| Female n (%) | 111 (43.53) | 65 (49.24) |
| Serotype | Variable | sIPV Group | wIPV Group | p-Value | SPRs Differences or GMT Ratios | Non-Inferiority Test (p-Value) |
|---|---|---|---|---|---|---|
| 1 | N | 318 | 165 | |||
| SPRs (95% CI) | 99.69 (98.26,99.99) | 99.39 (96.67,99.98) | 1.0000 | 0.29 | 1.0000 | |
| GMTs (95% CI) | 543.96 (483.85,611.55) | 190.75 (163.58,222.43) | <0.0001 | 2.85 | <0.0001 | |
| 2 | N | 334 | 166 | |||
| SPRs (95% CI) | 99.37 (97.75,99.92) | 98.79 (95.69,99.85) | 0.6089 | 0.58 | 0.6089 | |
| GMTs (95% CI) | 179.59 (160.80,200.58) | 81.05 (70.66,92.98) | <0.0001 | 2.22 | <0.0001 | |
| 3 | N | 334 | 166 | |||
| SPRs (95% CI) | 100.00 (98.85,100.00) | 99.39 (96.67,99.98) | 0.3416 | 0.61 | 0.3416 | |
| GMTs (95% CI) | 362.72 (328.43,400.58) | 203.95 (169.47,245.45) | <0.0001 | 1.78 | <0.0001 |
| Variable | SPR | GMT | ||||
|---|---|---|---|---|---|---|
| sIPV Group (n = 255) | IPV Group (n = 132) | p-Value | sIPV Group (n = 255) | IPV Group (n = 132) | p-Value | |
| Serotype 1 | ||||||
| 2M | 60.78 (54.50,66.82) | 61.36 (52.50,69.71) | 1.0000 | 13.62 (11.61,15.97) | 13.83 (11.12,17.20) | 0.9101 |
| 5M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 3884.34 (3440.83,4385.01) | 456.24 (393.93,528.41) | <0.0001 |
| 18M | 99.61 (97.83,99.99) | 100.00 (97.24,100.00) | 1.0000 | 696.04 (600.40,806.91) | 186.10 (152.58,226.97) | <0.0001 |
| 19M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 10,954.29 (10230.07,11729.78) | 4389.82 (3854.88,4998.99) | <0.0001 |
| 48M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 1006.42 (884.28,1145.44) | 329.30 (278.62,389.19) | <0.0001 |
| 19M/48M | 10.88 (9.78,12.12) | 13.33 (11.37,15.63) | 0.0343 | |||
| 78M | 100.00 (98.56,100.00) | 99.24 (95.85,99.98) | 0.3411 | 566.01 (499.92,640.84) | 187.41 (158.73,221.27) | <0.0001 |
| 19M/78M | 19.35 (17.42,21.50) | 23.42 (20.09,27.32) | 0.0408 | |||
| Serotype 2 | ||||||
| 2M | 51.76 (45.45,58.04) | 42.42 (33.87,51.32) | 0.0869 | 9.73 (8.55,11.06) | 8.35 (6.92,10.07) | 0.1782 |
| 5M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 363.88 (324.35,408.23) | 147.38 (125.13,173.60) | <0.0001 |
| 18M | 97.65 (94.95,99.13) | 96.97 (92.42,99.17) | 0.7403 | 103.21 (89.75,118.68) | 58.08 (47.52,71.00) | <0.0001 |
| 19M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 4992.32 (4504.67,5532.76) | 1858.72 (1611.97,2143.24) | <0.0001 |
| 48M | 99.61 (97.83,99.99) | 100.00 (97.24,100.00) | 1.0000 | 617.83 (537.32,710.40) | 261.70 (223.70,306.15) | <0.0001 |
| 19M/48M | 8.08 (7.20,9.07) | 7.10 (6.11,8.26) | 0.1892 | |||
| 78M | 99.22 (97.20,99.90) | 98.48 (94.63,99.82) | 0.6082 | 177.55 (156.63,201.27) | 78.01 (66.78,91.14) | <0.0001 |
| 19M/78M | 28.12 (25.31,31.23) | 23.83 (20.50,27.70) | 0.0732 | |||
| Serotype 3 | ||||||
| 2M | 25.88 (20.62,31.72) | 25.00 (17.88,33.28) | 0.9025 | 5.99 (5.41,6.62) | 6.40 (5.45,7.51) | 0.4723 |
| 5M | 100.00 (98.56,100.00 | 100.00 (97.24,100.00) | NA | 1364.22 (1226.61,1517.28) | 523.40 (443.43,617.79) | <0.0001 |
| 18M | 100.00 (98.56,100.00) | 97.73 (93.50,99.53) | 0.0391 | 399.13 (347.01,459.09) | 151.03 (115.27,197.90) | <0.0001 |
| 19M | 100.00 (98.56,100.010) | 100.00 (97.24,100.00) | NA | 11,860.65 (11215.30,12543.14) | 6573.40 (5632.16,7671.93) | <0.0001 |
| 48M | 100.00 (98.56,100.00) | 100.00 (97.24,100.00) | NA | 1604.49 (1425.47,1806.01) | 888.74 (725.34,1088.97) | <0.0001 |
| 19M/48M | 7.39 (326.79,408.73) | 7.40 (155.25,233.30) | 0.9959 | |||
| 78M | 100.00 (98.56,100.00) | 99.24 (95.85,99.98) | 0.3411 | 365.47 (326.79,408.73) | 190.31 (155.25,233.30) | <0.0001 |
| 19M/78M | 32.45 (29.22,36.04) | 34.54 (29.26,40.77) | 0.5140 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kai, C.; Yurong, L.; Sheng, L.; Yongmei, S.; Jianfeng, W.; Xinge, L.; Peng, J.; Hongxing, P. Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination. Vaccines 2025, 13, 253. https://doi.org/10.3390/vaccines13030253
Kai C, Yurong L, Sheng L, Yongmei S, Jianfeng W, Xinge L, Peng J, Hongxing P. Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination. Vaccines. 2025; 13(3):253. https://doi.org/10.3390/vaccines13030253
Chicago/Turabian StyleKai, Chu, Li Yurong, Liu Sheng, Shan Yongmei, Wang Jianfeng, Li Xinge, Jiao Peng, and Pan Hongxing. 2025. "Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination" Vaccines 13, no. 3: 253. https://doi.org/10.3390/vaccines13030253
APA StyleKai, C., Yurong, L., Sheng, L., Yongmei, S., Jianfeng, W., Xinge, L., Peng, J., & Hongxing, P. (2025). Sustained Immune Persistence Five Years Post-Completion of Four-Dose sIPV Vaccination. Vaccines, 13(3), 253. https://doi.org/10.3390/vaccines13030253






