Computationally Selected Multivalent HIV-1 Subtype C Vaccine Protects Against Heterologous SHIV Challenge
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Vaccine Design and Production
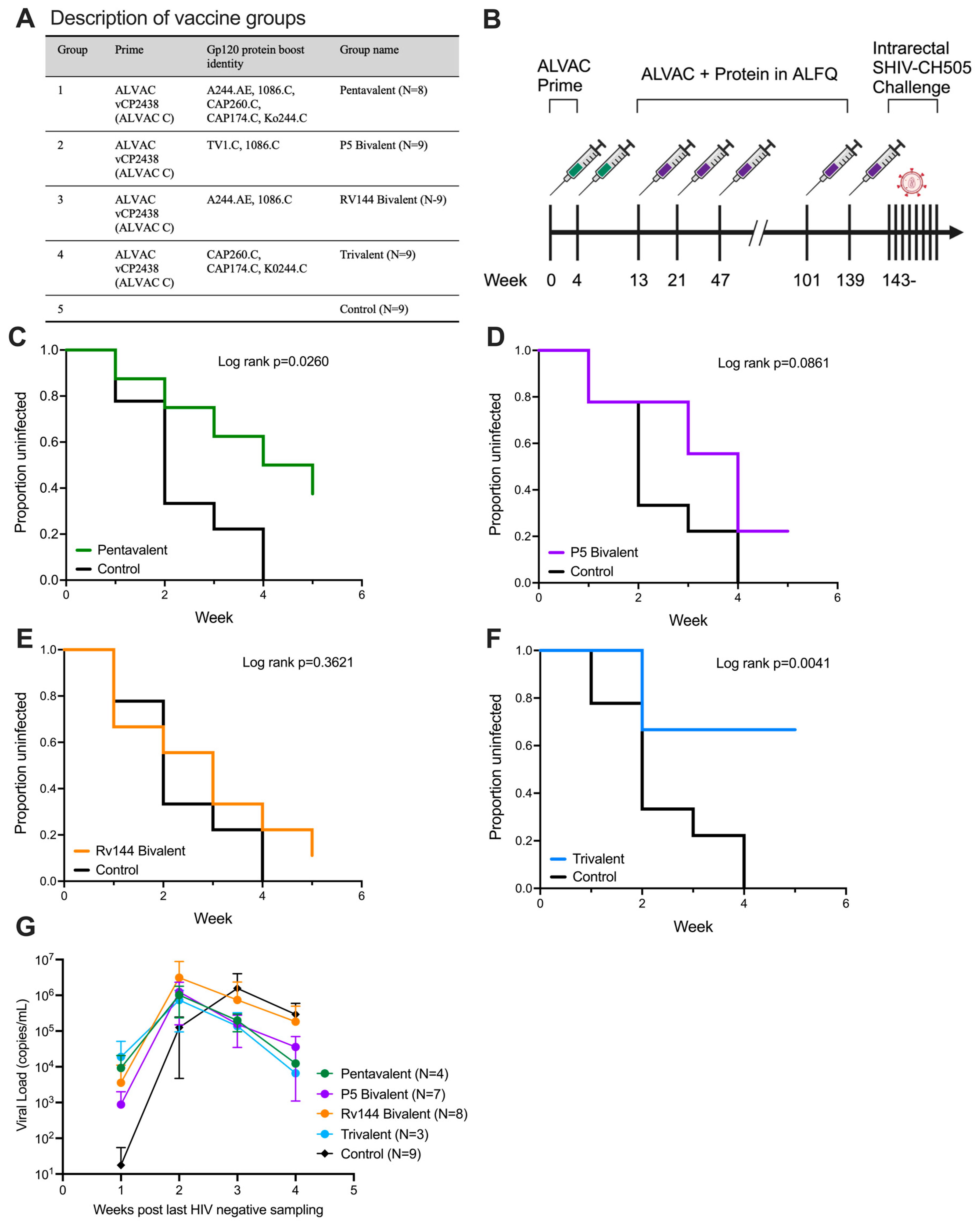
2.3. Immunization and SHIV Challenge of Rhesus Macaques
2.4. HIV-1-Specific Binding Antibody Multiplex Assay (BAMA)
2.5. Magnitude-Breadth Score Calculation
2.6. ADCC Against gp120-Coated Target Cells
2.7. Antibody-Dependent Cellular Phagocytosis Assay
2.8. Monoclonal Antibody Competition ELISAs
2.9. Neutralization
2.10. Machine Learning
2.10.1. Missing Data
2.10.2. Dimensionality Reduction
2.11. Group Classification
2.12. Classification of Animals According to Susceptibility to Infection
2.13. Survival Analysis Using Predictive Models
2.14. Model Training and Risk Prediction
2.15. Representative Model Predicted Risk of Infection for Each Animal
2.16. ‘Risk Group’ Model to Compare Survival Probability of the Predicted and Susceptible Groups
2.17. Statistical Analyses
2.18. Systems Serology Analysis and Code Availability
3. Results
3.1. ALVAC Prime with Subtype C gp120 Trivalent Boost Protects from Repeated Intrarectal SHIV Challenge
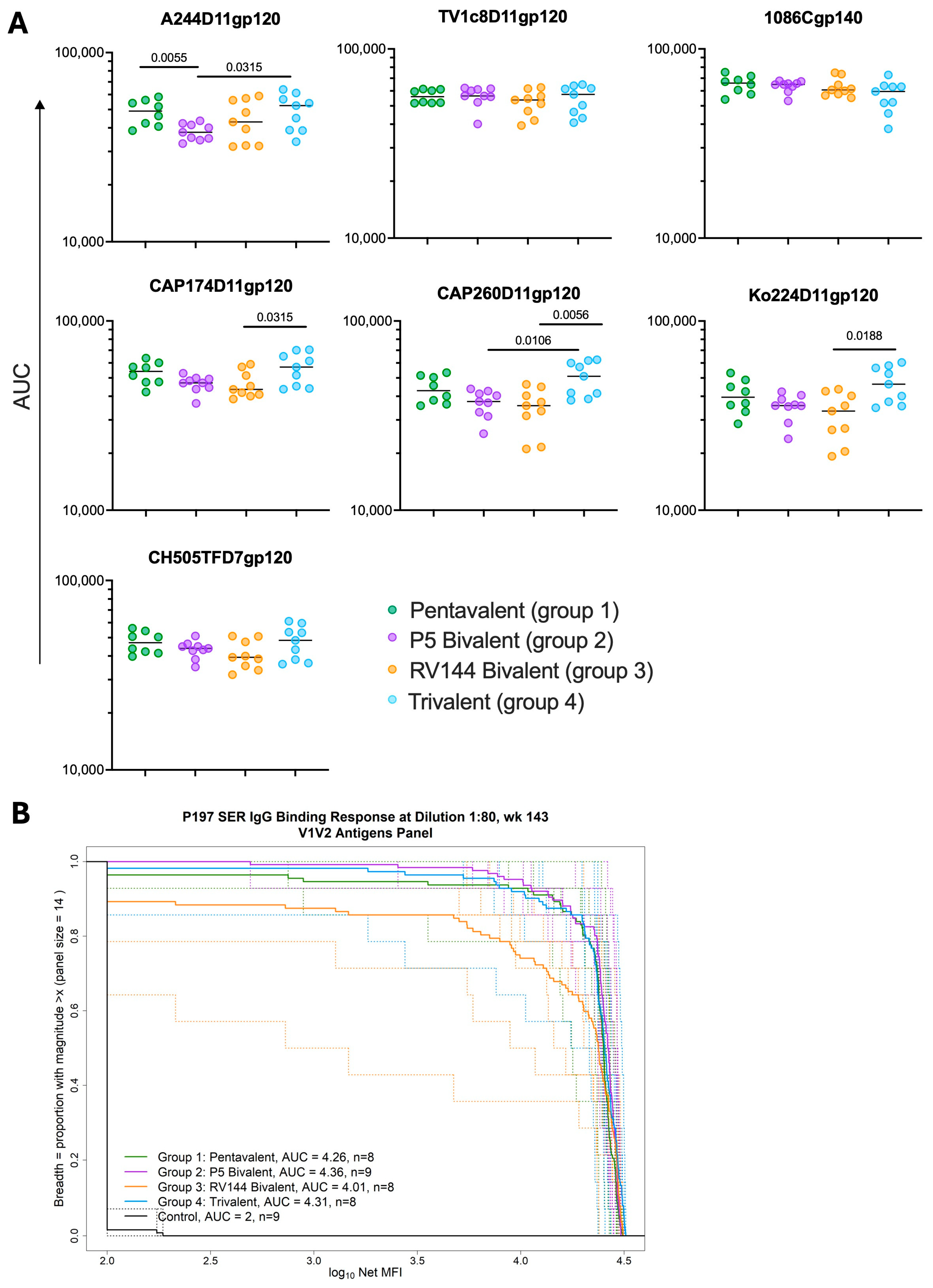
3.2. Different gp120 Combination Boosts Elicit Similar Magnitudes of gp120 and Breadth of V1V2-Binding Antibodies
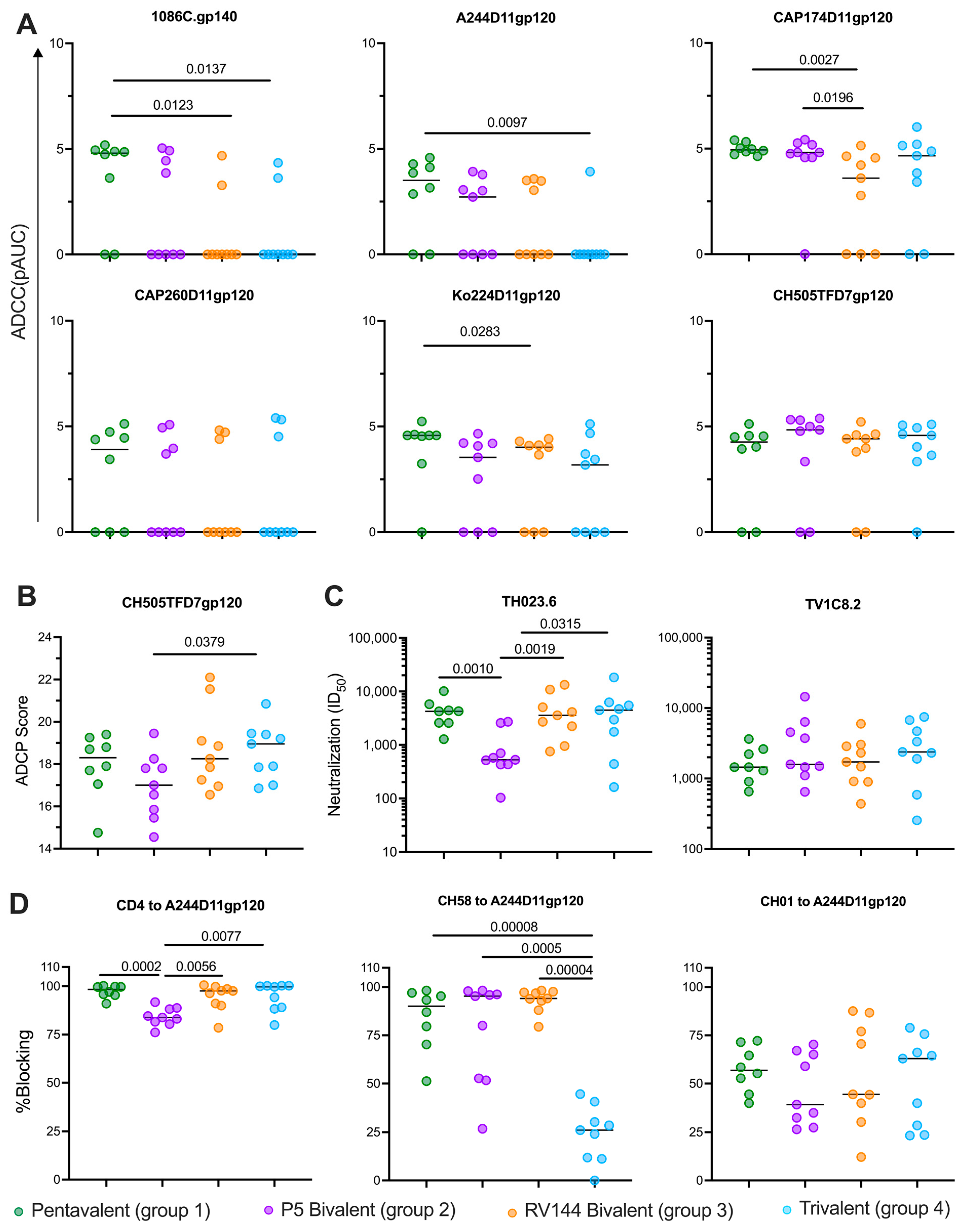
3.3. Vaccines Elicit Strong Functional Antibody Responses
3.4. ADCC Responses Against CH505 at the Time of Challenge Correlated with Protection from Infection
3.5. Immune Responses Induced by the Different Vaccine Arms
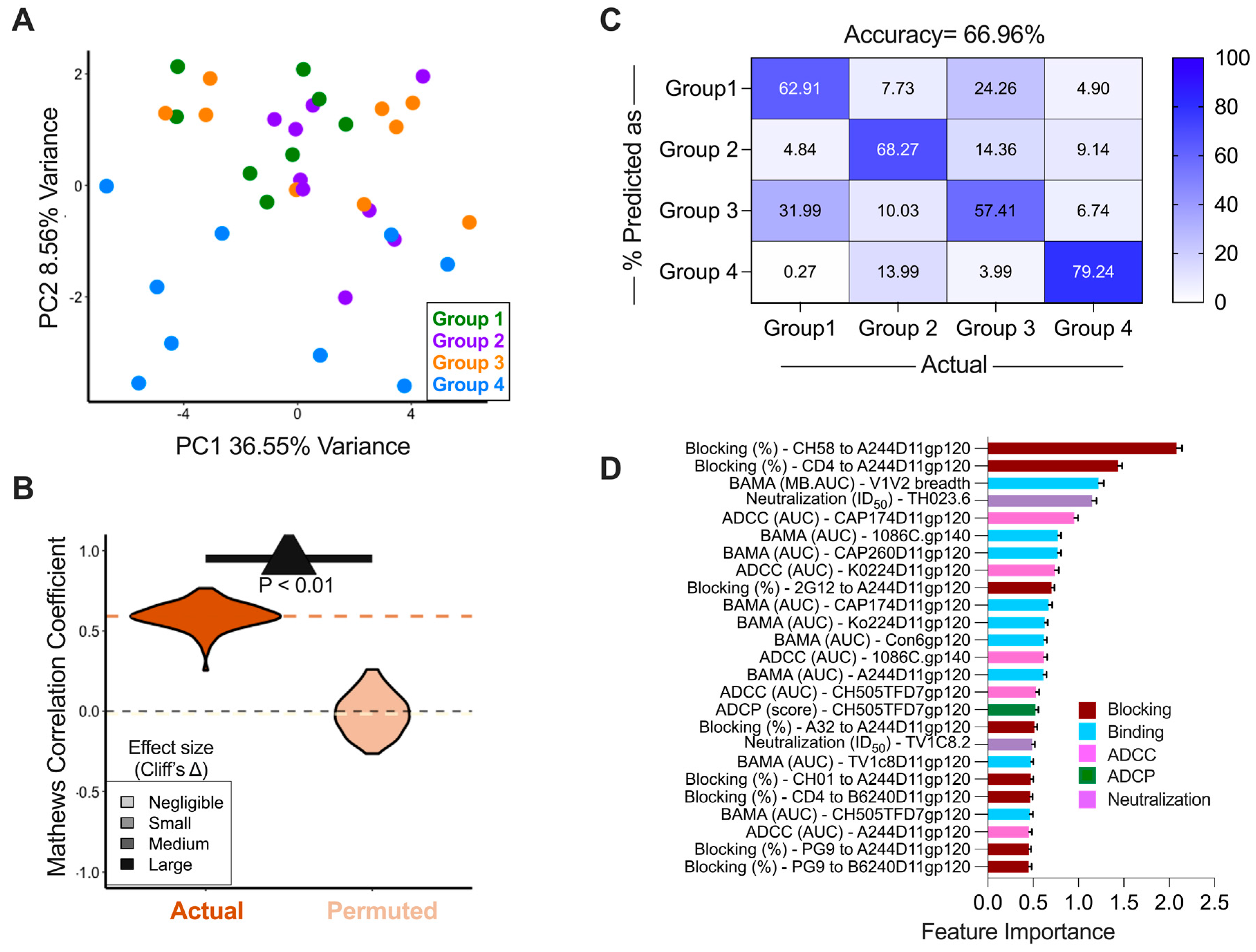
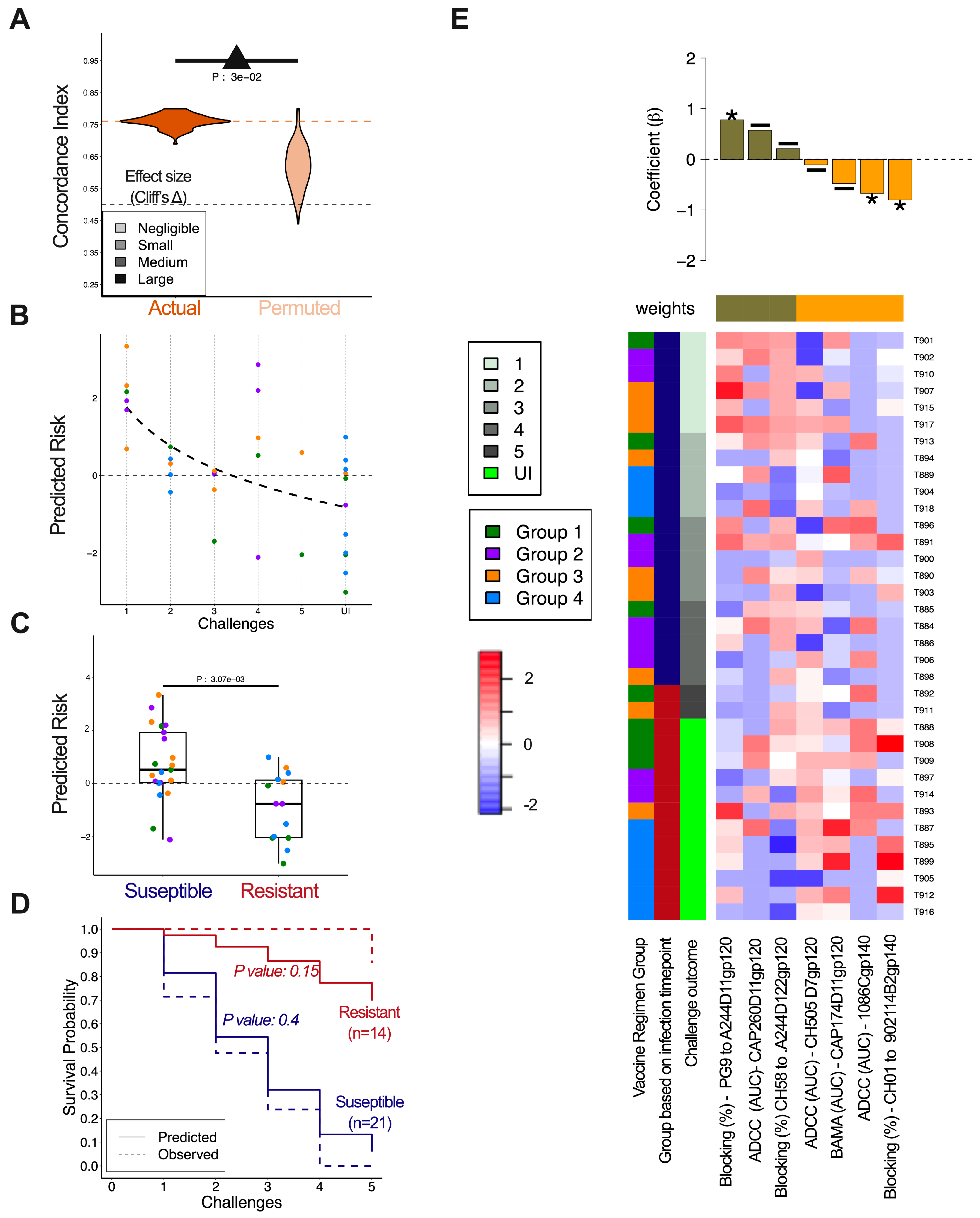
3.6. Immune Correlates of Decreased Infection Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.L.; Liao, H.-X.; Fong, Y.; deCamp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.; Seaton, K.E.; et al. Vaccine-Induced Env V1-V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon after Vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. 2024 Global AIDS Report—The Urgency of Now: AIDS at a Crossroads. Available online: https://www.unaids.org/en/resources/documents/2024/global-aids-update-2024 (accessed on 12 December 2024).
- Gray, G.E.; Bekker, L.-G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C Gp120-MF59 in Adults. N. Engl. J. Med. 2021, 384, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.E.; Mngadi, K.; Lavreys, L.; Nijs, S.; Gilbert, P.B.; Hural, J.; Hyrien, O.; Juraska, M.; Luedtke, A.; Mann, P.; et al. Mosaic HIV-1 Vaccine Regimen in Southern African Women (Imbokodo/HVTN 705/HPX2008): A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet Infect. Dis. 2024, 24, 1201–1212. [Google Scholar] [CrossRef]
- Moodie, Z.; Dintwe, O.; Sawant, S.; Grove, D.; Huang, Y.; Janes, H.; Heptinstall, J.; Omar, F.L.; Cohen, K.; De Rosa, S.C.; et al. Analysis of the HIV Vaccine Trials Network 702 Phase 2b-3 HIV-1 Vaccine Trial in South Africa Assessing RV144 Antibody and T-Cell Correlates of HIV-1 Acquisition Risk. J. Infect. Dis. 2022, 226, 246–257. [Google Scholar] [CrossRef]
- Kenny, A.; van Duijn, J.; Dintwe, O.; Heptinstall, J.; Burnham, R.; Sawant, S.; Zhang, L.; Mielke, D.; Khuzwayo, S.; Omar, F.L.; et al. Immune Correlates Analysis of the Imbokodo (HVTN 705/HPX2008) Efficacy Trial of a Mosaic HIV-1 Vaccine Regimen Evaluated in Southern African People Assigned Female Sex at Birth: A Two-Phase Case-Control Study. EBioMedicine 2024, 108, 105320. [Google Scholar] [CrossRef]
- Bradley, T.; Pollara, J.; Santra, S.; Vandergrift, N.; Pittala, S.; Bailey-Kellogg, C.; Shen, X.; Parks, R.; Goodman, D.; Eaton, A.; et al. Pentavalent HIV-1 Vaccine Protects against Simian-Human Immunodeficiency Virus Challenge. Nat. Commun. 2017, 8, 15711. [Google Scholar] [CrossRef]
- Shen, X.; Korber, B.; Spreng, R.L.; Sawant, S.S.; deCamp, A.; McMillan, A.S.; Mathura, R.; Zolla-Pazner, S.; Pinter, A.; Parks, R.; et al. A Pentavalent HIV-1 Subtype C Vaccine Containing Computationally Selected gp120 Strains Improves the Breadth of V1V2 Region Responses. Vaccines 2025, 13, 133. [Google Scholar] [CrossRef]
- Bekker, L.-G.; Moodie, Z.; Grunenberg, N.; Laher, F.; Tomaras, G.D.; Cohen, K.W.; Allen, M.; Malahleha, M.; Mngadi, K.; Daniels, B.; et al. Subtype C ALVAC-HIV and Bivalent Subtype C Gp120/MF59 HIV-1 Vaccine in Low-Risk, HIV-Uninfected, South African Adults: A Phase 1/2 Trial. Lancet HIV 2018, 5, e366–e378. [Google Scholar] [CrossRef]
- Liao, H.-X.; Sutherland, L.L.; Xia, S.-M.; Brock, M.E.; Scearce, R.M.; Vanleeuwen, S.; Alam, S.M.; McAdams, M.; Weaver, E.A.; Camacho, Z.; et al. A Group M Consensus Envelope Glycoprotein Induces Antibodies That Neutralize Subsets of Subtype B and C HIV-1 Primary Viruses. Virology 2006, 353, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Tomaras, G.D.; Warrier, R.; Nicely, N.I.; Liao, H.-X.; Pollara, J.; Liu, P.; Alam, S.M.; Zhang, R.; Cocklin, S.L.; et al. Human Non-Neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS Pathog. 2015, 11, e1005042. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, G.D.; Yates, N.L.; Liu, P.; Qin, L.; Fouda, G.G.; Chavez, L.L.; Decamp, A.C.; Parks, R.J.; Ashley, V.C.; Lucas, J.T.; et al. Initial B-Cell Responses to Transmitted Human Immunodeficiency Virus Type 1: Virion-Binding Immunoglobulin M (IgM) and IgG Antibodies Followed by Plasma Anti-Gp41 Antibodies with Ineffective Control of Initial Viremia. J. Virol. 2008, 82, 12449–12463. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Hart, L.; Brewer, F.; Pickeral, J.; Packard, B.Z.; Hoxie, J.A.; Komoriya, A.; Ochsenbauer, C.; Kappes, J.C.; Roederer, M.; et al. High-Throughput Quantitative Analysis of HIV-1 and SIV-Specific ADCC-Mediating Antibody Responses. Cytometry A 2011, 79, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Koene, H.R.; Kleijer, M.; Algra, J.; Roos, D.; von dem Borne, A.E.; de Haas, M. Fc GammaRIIIa-158V/F Polymorphism Influences the Binding of IgG by Natural Killer Cell Fc GammaRIIIa, Independently of the Fc GammaRIIIa-48L/R/H Phenotype. Blood 1997, 90, 1109–1114. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Moldt, B.; Wyatt, R.T.; Dugast, A.-S.; McAndrew, E.; Tsoukas, S.; Jost, S.; Berger, C.T.; Sciaranghella, G.; Liu, Q.; et al. A Robust, High-Throughput Assay to Determine the Phagocytic Activity of Clinical Antibody Samples. J. Immunol. Methods 2011, 366, 8–19. [Google Scholar] [CrossRef]
- Tay, M.Z.; Liu, P.; Williams, L.D.; McRaven, M.D.; Sawant, S.; Gurley, T.C.; Xu, T.T.; Dennison, S.M.; Liao, H.-X.; Chenine, A.-L.; et al. Antibody-Mediated Internalization of Infectious HIV-1 Virions Differs among Antibody Isotypes and Subclasses. PLoS Pathog. 2016, 12, e1005817. [Google Scholar] [CrossRef]
- Hulot, S.L.; Korber, B.; Giorgi, E.E.; Vandergrift, N.; Saunders, K.O.; Balachandran, H.; Mach, L.V.; Lifton, M.A.; Pantaleo, G.; Tartaglia, J.; et al. Comparison of Immunogenicity in Rhesus Macaques of Transmitted-Founder, HIV-1 Group M Consensus, and Trivalent Mosaic Envelope Vaccines Formulated as a DNA Prime, NYVAC, and Envelope Protein Boost. J. Virol. 2015, 89, 6462–6480. [Google Scholar] [CrossRef]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and Validation of the TZM-Bl Assay for Standardized Assessments of Neutralizing Antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef]
- Seaman, M.S.; Janes, H.; Hawkins, N.; Grandpre, L.E.; Devoy, C.; Giri, A.; Coffey, R.T.; Harris, L.; Wood, B.; Daniels, M.G.; et al. Tiered Categorization of a Diverse Panel of HIV-1 Env Pseudoviruses for Assessment of Neutralizing Antibodies. J. Virol. 2010, 84, 1439–1452. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023.
- Wickam, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2/3, 18–22. [Google Scholar]
- Torchiano, M. Effsize: Efficient Effect Size Computation 2020. Available online: https://cran.r-project.org/web/packages/effsize/effsize.pdf (accessed on 12 December 2024).
- Ackerman, M.E.; Das, J.; Pittala, S.; Broge, T.; Linde, C.; Suscovich, T.J.; Brown, E.P.; Bradley, T.; Natarajan, H.; Lin, S.; et al. Route of Immunization Defines Multiple Mechanisms of Vaccine-Mediated Protection against SIV. Nat. Med. 2018, 24, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Pittala, S.; Bagley, K.; Schwartz, J.A.; Brown, E.P.; Weiner, J.A.; Prado, I.J.; Zhang, W.; Xu, R.; Ota-Setlik, A.; Pal, R.; et al. Antibody Fab-Fc Properties Outperform Titer in Predictive Models of SIV Vaccine-Induced Protection. Mol. Syst. Biol. 2019, 15, e8747. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.; Grambsch, P. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2010. [Google Scholar]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Yates, N.L.; deCamp, A.C.; Korber, B.T.; Liao, H.-X.; Irene, C.; Pinter, A.; Peacock, J.; Harris, L.J.; Sawant, S.; Hraber, P.; et al. HIV-1 Envelope Glycoproteins from Diverse Clades Differentiate Antibody Responses and Durability among Vaccinees. J. Virol. 2018, 92, 10–128. [Google Scholar] [CrossRef]
- Rolland, M.; Edlefsen, P.T.; Larsen, B.B.; Tovanabutra, S.; Sanders-Buell, E.; Hertz, T.; deCamp, A.C.; Carrico, C.; Menis, S.; Magaret, C.A.; et al. Increased HIV-1 Vaccine Efficacy against Viruses with Genetic Signatures in Env V2. Nature 2012, 490, 417–420. [Google Scholar] [CrossRef]
- Liao, H.-X.; Bonsignori, M.; Alam, S.M.; McLellan, J.S.; Tomaras, G.D.; Moody, M.A.; Kozink, D.M.; Hwang, K.-K.; Chen, X.; Tsao, C.-Y.; et al. Vaccine Induction of Antibodies against a Structurally Heterogeneous Site of Immune Pressure within HIV-1 Envelope Protein Variable Regions 1 and 2. Immunity 2013, 38, 176–186. [Google Scholar] [CrossRef]
- Felber, B.K.; Lu, Z.; Hu, X.; Valentin, A.; Rosati, M.; Remmel, C.A.L.; Weiner, J.A.; Carpenter, M.C.; Faircloth, K.; Stanfield-Oakley, S.; et al. Co-Immunization of DNA and Protein in the Same Anatomical Sites Induces Superior Protective Immune Responses against SHIV Challenge. Cell Rep. 2020, 31, 107624. [Google Scholar] [CrossRef]
- Bonsignori, M.; Hwang, K.-K.; Chen, X.; Tsao, C.-Y.; Morris, L.; Gray, E.; Marshall, D.J.; Crump, J.A.; Kapiga, S.H.; Sam, N.E.; et al. Analysis of a Clonal Lineage of HIV-1 Envelope V2/V3 Conformational Epitope-Specific Broadly Neutralizing Antibodies and Their Inferred Unmutated Common Ancestors. J. Virol. 2011, 85, 9998–10009. [Google Scholar] [CrossRef]
| Immune Marker | Antigen | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|
| IgG-binding antibodies | CH505TFD7gp120 | 0.5530 (0.060–5.125) | 0.6020 |
| V1V2-binding antibodies | V1V2 breadth (MB-AUC) | 2.91 (0.27–31.69) | 0.3808 |
| ADCC | CH505TFD7gp120 | 0.793 (0.648–0.970) | 0.0241 |
| ADCP | CH505TFD7gp120 | 1.1607 (1.161–1.504) | 0.2595 |
| CD4 Blocking | A244D11gp120 | 0.1547 (0–59569) | 0.7761 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielke, D.; Tuyishime, M.; Kelkar, N.S.; Wang, Y.; Parks, R.; Santra, S.; Rountree, W.; Williams, L.D.; Peters, T.; Eisel, N.; et al. Computationally Selected Multivalent HIV-1 Subtype C Vaccine Protects Against Heterologous SHIV Challenge. Vaccines 2025, 13, 231. https://doi.org/10.3390/vaccines13030231
Mielke D, Tuyishime M, Kelkar NS, Wang Y, Parks R, Santra S, Rountree W, Williams LD, Peters T, Eisel N, et al. Computationally Selected Multivalent HIV-1 Subtype C Vaccine Protects Against Heterologous SHIV Challenge. Vaccines. 2025; 13(3):231. https://doi.org/10.3390/vaccines13030231
Chicago/Turabian StyleMielke, Dieter, Marina Tuyishime, Natasha S. Kelkar, Yunfei Wang, Robert Parks, Sampa Santra, Wes Rountree, LaTonya D. Williams, Tiffany Peters, Nathan Eisel, and et al. 2025. "Computationally Selected Multivalent HIV-1 Subtype C Vaccine Protects Against Heterologous SHIV Challenge" Vaccines 13, no. 3: 231. https://doi.org/10.3390/vaccines13030231
APA StyleMielke, D., Tuyishime, M., Kelkar, N. S., Wang, Y., Parks, R., Santra, S., Rountree, W., Williams, L. D., Peters, T., Eisel, N., Sawant, S., Zhang, L., Goodman, D., Jha, S., Zalaquett, A., Ramasubramanian, P., Stanfield-Oakley, S., Matyas, G., Beck, Z., ... Ferrari, G. (2025). Computationally Selected Multivalent HIV-1 Subtype C Vaccine Protects Against Heterologous SHIV Challenge. Vaccines, 13(3), 231. https://doi.org/10.3390/vaccines13030231






