Abstract
Background/Objectives: COVID-19 vaccines have significantly reduced the mortality and morbidity associated with SARS-CoV-2. In the fall of 2021, the U.S. Food and Drug Administration amended its emergency use authorization guidelines for COVID-19 vaccines to allow the administration of booster vaccine doses based on sound scientific evidence of the increase in effectiveness conferred by boosters. The effectiveness of the Ad26.COV2.S COVID-19 booster vaccine during the periods of Delta and Omicron variant dominance is unknown. This study used real-world data to estimate the effectiveness of booster heterologous or homologous Ad26.COV2.S vaccination compared to that of a primary Ad26.COV2.S or mRNA COVID-19 vaccination series. Methods: A retrospective, observational, longitudinal cohort study design was used with a total eligible sample population consisting of 72,461,026 individuals in the HealthVerity dataset. The study cohort consisted of individuals ≥18 years in the United States with evidence of a COVID-19 primary vaccination series (Ad26.COV2.S or mRNA) administered between 1 January 2021 and 6 July 2022. Two exposure groups were considered based on retrospective database classification: a heterologous Ad26.COV2.S booster and a homologous Ad26.COV2.S booster. Individuals eligible for the referent groups, defined as those with a primary vaccine series alone, were identified through exact matching by age, sex, time since primary series vaccine, location, and Gagne comorbidity score. Propensity score-matched Cox proportional hazards models were used to evaluate outcomes, including COVID-19-related hospitalization and medically attended COVID-19. Results: Depending on the comparison group of interest, the adjusted hazard ratios for COVID-19-related hospitalization ranged from 0.63 (95% CI: 0.56, 0.72) to 0.82 (95% CI: 0.75, 0.90), and 0.93 (95% CI: 0.90, 0.96) to 0.94 (95% CI: 0.91, 0.97) for medically attended COVID-19, both favoring booster vaccination. Conclusions: The results of this study demonstrate the effectiveness of an Ad26.COV2.S booster vaccination compared to primary series vaccination in preventing COVID-19 hospitalization and medically attended COVID-19 for at least 12 months. This study adds to the scientific evidence that demonstrates the importance of COVID-19 booster vaccinations to support public health policy.
1. Introduction
The development and distribution of effective vaccines in the general population was proven to be a successful strategy for reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission and the burden of coronavirus disease 2019 (COVID-19) [1,2]. The Ad26.COV2.S COVID-19 vaccine, a one-dose adenovirus primary vaccination (Johnson & Johnson Innovative Medicine), was given emergency use authorization (EUA) for adults aged 18+ by the Food and Drug Administration (FDA) on 27 February 2021 [3]. Despite the uptake rate of COVID-19 vaccines (of any type) reaching greater than 50% by May 2021 [4], the emergence of more transmissible SARS-CoV-2 variants (Delta and Omicron) in the summer and early fall months led to an increase in breakthrough infections among vaccinated persons and an observed waning vaccine effectiveness [5].
In September and October of 2021, the FDA amended the EUAs for the three COVID-19 vaccines, including Ad26.COV2.S, to allow a one-dose booster vaccination (from any manufacturer) at least 2 months after the completion of the Ad26.COV2.S primary regimen or at least 6 months after the completion of a two-dose mRNA primary series for eligible individuals [6,7]. The FDA also authorized the use of heterologous booster doses, allowing for “mix-and-match” primary and booster COVID-19 vaccine regimens [8]. In preclinical and phase 2 studies, vaccine booster doses demonstrated an increase in binding and neutralizing antibodies, which are thought to increase protection against infection and severe illness [9,10]. FDA decision-making was based on data from clinical trials that demonstrated the efficacy of a homologous Ad26.COV2.S booster dose when administered 2 months after the primary vaccination and a heterologous booster dose when administered 3 months after a primary series [11,12,13].
Several studies, including a recent meta-analysis, have assessed the longer-term effectiveness of booster vaccinations [14,15]. However, evaluations of the Ad26.COV2.S COVID-19 vaccine in these studies were limited by small sample sizes, focusing on mRNA booster vaccinations or comparisons between boosted groups and unvaccinated groups. Few observational studies have reported on the effectiveness of the Ad26.COV2.S booster vaccination in heterologous and homologous combinations during the periods of Delta and Omicron variant dominance [16,17]. Both studies only included hospitalized individuals and thus do not capture the entire spectrum of individuals who have received COVID-19 booster vaccines. Evidence on the real-world relative effectiveness of homologous and heterologous Ad26.COV2.S booster vaccines over periods of high-transmissibility variants is needed to inform evolving COVID-19 public health policy globally.
Here, we report results on the effectiveness of a booster heterologous or homologous Ad26.COV2.S vaccination compared to that of a primary Ad26.COV2.S or mRNA COVID-19 vaccination series in preventing COVID-19-related hospitalization and medically attended COVID-19 using real-world data.
2. Materials and Methods
This was a retrospective, observational, longitudinal cohort study of the real-world effectiveness of an Ad26.COV2.S booster vaccination in preventing COVID-19-related hospitalization and medically attended COVID-19.
2.1. Data
The study sample was identified from the HealthVerity Marketplace, a unified database of open and closed medical and pharmacy claims, hospital transactional records for inpatient and outpatient hospital encounters, and laboratory data for individuals in the U.S. The data encompass all major payer types (commercial, Medicaid, and Medicare), and they include details on service dates, medications, diagnoses, procedures, and laboratory testing activity (including COVID-19 diagnostic and antibody tests and results). Data elements in the dataset include both open and closed claims. The HealthVerity data are nationally representative of the U.S. population across age groups and most regions, with data coverage comparable to that reported by the 2010 Census Bureau’s American Community Survey [18].
2.2. Population and Exposure
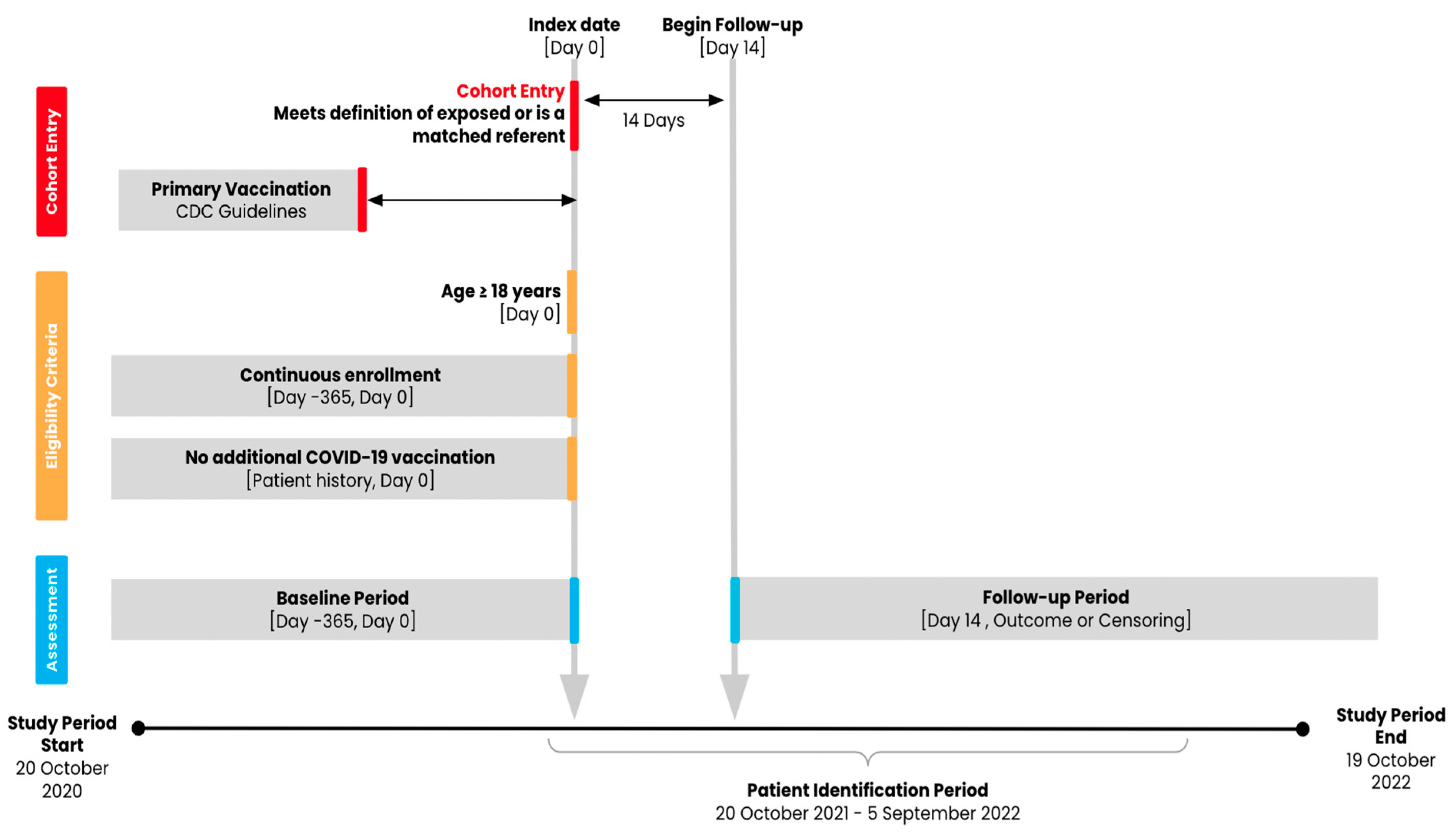
Adults aged 18 years or older with evidence of a COVID-19 primary series vaccination (two mRNA vaccinations from the same manufacturer [Pfizer-BioNTech or Moderna] or one Ad26.COV2.S vaccination) administered between 1 January 2021 and 6 July 2022 and with at least 365 days of medical and pharmacy pre-index enrollment were eligible for the study cohorts (Figure 1). The national drug codes (NDCs) or Current Procedural Terminology (CPT) codes used to identify the vaccines are provided in Supplemental Table S1. We considered two definitions of exposure or receipt of booster vaccination: a heterologous Ad26.COV2.S booster, defined as an Ad26.COV2.S booster administered at least 152 days after the completion of a two-dose mRNA primary series, and a homologous Ad26.COV2.S booster, defined as an Ad26.COV2.S booster administered at least 61 days after a one-dose Ad26.COV2.S primary vaccination [19]. We used 152 days because, at the time of protocol development and submission for IRB approval, the official FDA guidance of at least 6 months after the completion of a two-dose mRNA primary series for eligible individuals had not yet been developed. This 152-day period reflected the most scientifically accurate timeframe based on evolving data from clinical trials and scientific manuscripts in development. We compared groups of individuals with each exposure definition to those with an mRNA primary vaccine series (two doses) alone (i.e., without evidence of a booster vaccine) and to an Ad26.COV2.S vaccine (one dose) alone, for a total of four contrasts of interest (Table 1).
Figure 1.
Study design diagram.

Table 1.
Exposure contrasts of interest.
Separate analytic cohorts were identified for each contrast of interest using a combination of exact matching and propensity score (PS) matching. For each analytic cohort, individuals who received an Ad26.COV2.S booster vaccine (exposed group) were matched on the same calendar day with up to 10 referent individuals who had no evidence of a COVID-19 booster vaccination (referent group). Referent group individuals were matched by age (±5 years), sex, days since completed primary vaccination (±30 days), geographic location of residence (state) at index, and the Gagne combined comorbidity index (assessed over the 365-day baseline period) [20]. Exact matching was conducted without replacement.
After exact matching, exposed patients were then matched with up to four referent patients using parallel propensity score (PS) matching with a 1% caliper based on demographic and clinical characteristics in the baseline period in order to control for potential confounders. The PS model included the following characteristics: age, sex, Gagne comorbidity index score, calendar month of index date, calendar month of primary vaccination, state, insurance type, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, HIV infection status as defined by ICD-10-CM codes, immunocompromised status including from blood or organ transplant, liver disease, malignancies excluding non-melanoma skin cancer, moderate-to-severe asthma, cerebrovascular disease, chronic kidney disease, hypertension, serious heart condition, obesity, sickle cell disease, thalassemia, type 1 diabetes, type 2 diabetes, neurologic condition, COVID-19 history, recent (≤60 days from index) medical claims, and recent pharmacy claims. To assess the balance of measured confounders after PS matching, the absolute standardized difference (ASD) between the exposed and referent groups was examined; any measured covariates with a value <0.1 were considered balanced.
2.3. Follow-Up and Outcomes
COVID-19-related hospitalization and medically attended COVID-19 were assessed as study outcomes. COVID-19-related hospitalization was defined as any inpatient medical claim or inpatient hospital encounter where any medically attended COVID-19 began in the 21 days prior to the start of the hospitalization and lasted until the final day of hospitalization. Medically attended COVID-19 was defined as a positive COVID-19 diagnostic test (NAAT) or any medical claim, inpatient hospital encounter, or outpatient hospital encounter indicating a diagnosis of COVID-19 (ICD-10-CM code: U07.1). Follow-up began 14 days after the index date and ended at the receipt of any additional COVID-19 vaccine after index, death, loss to follow-up/disenrollment from the insurance plan, the end of the study period (19 October 2022), or the occurrence of the outcome of interest during the follow-up period.
2.4. Participant Characteristics
We evaluated the participant characteristics used in the PS matching over a 365-day baseline period using descriptive statistics. Additional baseline patient characteristics included the receipt of a COVID-19 laboratory test, the days since the primary COVID-19 vaccine series, and the number of medical and pharmacy claims. ASDs were reported for differences in characteristics between the exposed and referent groups, both pre- and post-PS matching. Demographic, clinical, and HCRU-related characteristics were described over the baseline period using summary statistics: for binary and categorical variables, counts and percentages (n, %) were used; for continuous variables, mean, standard deviation (SD), median, and interquartile range (IQR) were used.
2.5. Statistical Analysis
Incidence rates per 1000 person-years and 95% confidence intervals (CIs) were calculated for each exposure and referent group. Cox proportional hazard models were used to report hazard ratios (HRs) and 95% CIs for the relative risk of study outcomes [21]. Doubly robust outcome models were used in cases where patient characteristics remained imbalanced after PS matching [22]. The proportional hazards assumption was assessed through a visual inspection of Kaplan–Meier curves. Sensitivity analyses were conducted among individuals from the California state registry to assess the impact of potential vaccine underreporting.
To understand the potential effect of misclassification, we conducted sensitivity analyses in a subgroup of individuals residing in California using comprehensive COVID-19 vaccination data from the California state registry, linked to HealthVerity data (the results are not reported; see Supplementary Materials). No substantial differences in the results were noted between the primary analyses and the sensitivity analyses.
3. Results
Of the 548,788,380 individuals in the HealthVerity dataset as of 25 August 2023, 72,461,026 had evidence of a completed primary COVID-19 vaccination between 1 January 2021 and 6 July 2022. Approximately 90% (n = 65,000,652) had an mRNA primary vaccination between 1 January 2021 and 18 March 2022 (mRNA primary vaccine eligibility period), and 10% (n = 7,158,010) had an Ad26.COV2.S primary vaccination between 1 January 2021 and 6 July 2022 (Ad26.COV2.S primary vaccine eligibility period) (Supplemental Figures S1–S4).
3.1. Descriptive Statistics
3.1.1. Contrast: mRNA + Ad26.COV2.S vs. mRNA + No Boost
After meeting the eligibility criteria, exact matching, and PS matching, the final analytic cohort consisted of 2969 exposed patients (mRNA + Ad26.COV2.S) and 11,492 referent patients (mRNA + no boost) (Table 2). Of the 50 characteristics used for PS matching, two were unbalanced pre-PS matching (Supplemental Table S2a). After PS matching, all characteristics were balanced (Table 2). In the PS-matched cohort, the mean age was 47 years in both the exposed and referent groups. In both groups, the majority were male (53.3%, exposed; 54.5%, referent), located in the west (51.4%, exposed; 51.6%, referent), and had commercial insurance (54.9%, exposed; 55.2%, referent). A plurality of patients completed their primary vaccination series in April 2021 (34.8%, both groups) and received their booster vaccine in December 2021 (39.4%, exposed; 39.2%, referent). The most common comorbidities were hypertension, neurologic conditions, and obesity. Less than 10% of the patients had a history of COVID-19 infection.

Table 2.
Baseline characteristics, post-PS matching, for mRNA + Ad26.COV2.S vs. primary series.
3.1.2. Contrast: mRNA + Ad26.COV2.S vs. Ad26.COV2.S + No Boost
After meeting the eligibility criteria, exact matching, and PS matching, the final analytic cohort consisted of a total of 2973 exposed patients (mRNA + Ad26.COV2.S) and 11,568 referent patients (Ad26.COV2.S + no boost) (Table 2). Prior to PS matching, all characteristics were balanced (Supplemental Table S2a). In the PS-matched cohort, the mean age was 47 years, and the majority of patients were male (Table 2). More than half of each group was located in the west, had commercial insurance, and received their primary vaccine in April or May 2021. The most common comorbidities were hypertension, neurologic conditions, and obesity.
3.1.3. Contrast: Ad26.COV2.S + Ad26.COV2.S vs. mRNA + No Boost
After meeting the eligibility criteria, exact matching, and PS matching, the final analytic cohort consisted of 74,628 exposed patients (Ad26.COV2.S + Ad26.COV2.S) and 289,215 referent patients (mRNA + no boost) (Table 3). Of the 50 characteristics used for PS matching, one characteristic (month of primary vaccination: February 2021) was unbalanced pre-PS matching, and all characteristics were balanced post-PS matching (Supplemental Table S2b). Modest differences in demographic characteristics were observed in the contrasts evaluating a homologous Ad26.COV2.S booster vaccine against the heterologous Ad26.COV2.S booster vaccine. After PS matching, the mean age was 51 years in both the exposed and referent groups. Half of the cohort was male (49.5% male in both groups). The majority of the patients were located in the western U.S. (43.4% exposed; 44.1% referent) and had commercial insurance (51.8% exposed; 52.3%). Similarly to the previous comparisons, the majority of the patients completed their primary vaccination series in April 2021 (39.7% exposed; 40.2% referent) and their booster vaccine in November 2021 (34.8% exposed and referent). The most prevalent comorbidities were hypertension, neurologic conditions, and obesity.

Table 3.
Baseline characteristics, post-PS matching, for Ad26.COV2.S + Ad26.COV2.S vs. primary series.
3.1.4. Contrast: Ad26.COV2.S + Ad26.COV2.S vs. Ad26.COV2.S + No Boost
After meeting the eligibility criteria, exact matching, and PS matching, a total of 43,072 exposed patients (Ad26.COV2.S + Ad26.COV2.S) and 166,907 referent patients (Ad26.COV2.S + no boost) were included in the analytic cohort. Two characteristics (recent medical claim and recent pharmacy claim) were unbalanced pre-PS matching (Supplemental Table S2b); all characteristics were balanced after PS matching (Table 3). After PS matching, the mean age was 49 years in both the exposed and referent groups. Half of the cohort was male; additionally, the cohort was predominantly located in the western U.S., and the majority had commercial insurance. The majority of the patients completed their primary vaccination series in April 2021 and their booster vaccine in November 2021. The most prevalent comorbidities were hypertension, neurologic conditions, and obesity.
3.2. Main Results
The incidence rate of COVID-19-related hospitalization was 12.3 hospitalizations per 1000 PY among those with an mRNA + Ad26.COV2.S exposure, compared to 18.9 hospitalizations per 1000 PY among those with an mRNA vaccine with no booster (Table 4). The adjusted HR for COVID-19-related hospitalization was 0.67 (95% CI: 0.43, 1.06). Similarly, the medically attended COVID-19 incidence rates for the exposed and referent groups were 138.2 cases and 170.7 cases per 1000 PY, respectively, resulting in an HR of 0.84 (95% CI: 0.73, 0.97).

Table 4.
Adjusted hazard ratios for COVID-19-related hospitalization and medically attended COVID-19.
The incidence rate of COVID-19-related hospitalization was 12.3 cases per 1000 PY among those with an mRNA + Ad26.COV2.S exposure, compared to 15.7 cases per 1000 PY among those with an Ad26.COV2.S vaccine with no booster (HR 0.81, 95% CI: 0.51, 1.27, Table 4). The HR of medically attended COVID-19 was 0.88 (95% CI: 0.76, 1.01).
Among those in the Ad26.COV2.S + Ad26.COV2.S exposure group, the incidence rate of COVID-19-related hospitalization was 10.6 per 1000 PY; in the mRNA + no boost referent group, the incidence rate was 13.9 per 1000 PY (Table 4), resulting in an HR of 0.82 (95% CI: 0.75, 0.90). A similarly higher rate of events was observed for medically attended COVID-19. The HR of medically attended COVID-19 was 0.93 (95% CI: 0.90, 0.96).
The risk of COVID-19-related hospitalization was lower among those with an Ad26.COV2.S + Ad26.COV2.S exposure than among those with an Ad26.COV2.S vaccine with no booster (HR 0.63, 95% CI: 0.56, 0.72). The HR of medically attended COVID-19 was 0.94 (95% CI: 0.91, 0.97).
We observed similar incidence rates and hazard ratios across all four contrasts of interest when limiting the study cohort to those in the California state registry (Supplemental Table S5).
4. Discussion
To the best of our knowledge, this is the largest study to date evaluating the real-world comparative effectiveness of heterologous and homologous booster vaccinations with Ad26.COV2.S for the prevention of COVID-19-related outcomes in the 12 months following the FDA emergency use authorization of a booster dose among a sample of U.S. individuals with a completed COVID-19 primary vaccination. Overall, the findings of this analysis demonstrate that booster vaccinations are effective in preventing COVID-19-related outcomes. The results of this study demonstrate the public health importance of boosting to prevent serious COVID-19 outcomes, including COVID-19-related hospitalization and medically attended COVID-19.
Although the Ad26.COV2.S EUA has recently expired and the vaccine is no longer available in the U.S., the findings of this study contribute to an understanding of the importance of booster vaccinations as a whole as a preventative public health measure in reducing the burden of COVID-19. Heterologous and homologous booster vaccines were effective in reducing the risk of medically attended COVID-19 by between 6% and 14%, depending on the primary vaccination type. When considering all COVID-19 booster vaccine platforms together, observational studies demonstrate higher VE against COVID-19-related hospitalization in individuals who received a booster compared to unboosted individuals when both groups (boosted and unboosted) were compared to unvaccinated individuals.
A test-negative case–control study (n = 1572 cases, n = 1609 controls) reported a VE of 77% (95% CI: 71–82%) for a primary series and booster vaccine compared to unvaccinated persons and a VE of 44% (95% CI: 31–54%; p < 0.001) for a primary series alone compared to unvaccinated persons [16]. Other studies of the relative effectiveness estimates of the Ad26.COV2.S booster vaccine have limited precision mainly due to their small sample sizes. A test-negative study of vaccine effectiveness (VE) among individuals hospitalized in the U.S. from 25 December 2021 to 4 April 2022, the period during which the Omicron variant was the most prevalent, reported a relative effectiveness estimate of 49% (95% CI: −9–76%) for the Ad26.COV2.S booster vaccine compared to an Ad26.COV2.S primary series vaccine alone [17]. The sample size for the number of individuals with an Ad26.COV2.S primary series was 96, and the sample size for the number of individuals with an Ad26.COV2.S primary series plus a booster was 65. A different U.S.-based test-negative VE study, which compared both individuals boosted with an Ad26.COV2.S vaccine and individuals with a primary Ad26.COV2.S vaccine alone to individuals with no COVID-19 vaccine, did not report an increased VE (Ad26.COV2.S boosted: VE 35% (95% CI: −54–73%); Ad26.COV2.S primary series only: VE 32% (95% CI: 1–54%; p = 0.79)) [16]. The sample size of individuals who received an Ad26.COV2.S booster dose was 25, making it difficult to derive meaningful inferences. A strength of the current study is the relatively larger sample size for both heterologous and homologous COVID-19 booster groups.
This study has several limitations. COVID-19 vaccination status may be under-ascertained. The data source leveraged for this study captures real-world vaccine status in the study sample using CPT or NDC codes in procedure and/or pharmacy claims, respectively. Not all providers in the U.S. submit for reimbursement for vaccine administration due to no-cost government vaccination programs.
Similarly, COVID-19 infection status may be under-ascertained due to the impact of at-home testing. We caution that the results of this study focus on more severe COVID-19 outcomes of interest rather than on non-serious outcomes; as a result, the incidence rates of the outcomes observed in this study may be lower than those observed in general COVID-19-related infections that may be captured in a clinical trial setting. However, the use of different data sources to ascertain outcomes (e.g., evidence of diagnoses, procedures, and lab test results) allows for improved capture of COVID-19 events.
There may be unmeasured or residual confounding in this observational study. Unlike in a clinical trial, exposure assignment was not randomized, resulting in the possibility of unmeasured confounding of an unknown magnitude of the exposure–outcome association. Disruptions to the healthcare system as a result of the COVID-19 pandemic, along with the accompanying influence of COVID-19 on disease outcomes, precluded the use of methods such as negative controls to assess for such potential confounding. However, at baseline, the exposure groups were overall similar, with two or fewer measured characteristics imbalanced prior to PS matching. To mitigate bias and facilitate confounding control, this study measured a large set of demographic, clinical, and utility-related characteristics. PS matching and doubly robust model specification were then applied to this set of characteristics, mimicking a clinical trial’s randomization and minimizing the chance of spurious effect estimates due to potential confounders.
This study used data from a large and generalizable U.S. population-based dataset. Real-world data are generated during routine clinical care and are representative of the underlying populations. The source data include 16 individual-linked data sources of medical and pharmacy claims data, hospital transactional records for inpatient and outpatient hospital encounters (also known as chargemaster data), and laboratory data, and they include populations covered by commercial insurance, Medicare Advantage, and Medicaid. The results of this study may be generalizable to the broader U.S. adult population.
5. Conclusions
This population-based observational cohort study from clinical practice in the U.S. demonstrates the effectiveness of an Ad26.COV2.S booster vaccination for at least 12 months. In the U.S., individuals who were boosted with Ad26.COV2.S were at a lower risk of COVID-19 hospitalization and medically attended COVID-19 than unboosted individuals. This study adds to the volume of evidence that demonstrates the added benefit of COVID-19 vaccine booster doses that is critical to guiding the development of sound public health policy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13020166/s1, Supplemental Table S1. Variable definitions. Supplemental Table S2a: Baseline characteristics, pre-PS matching, for mRNA + Ad26.COV2.S vs. primary series. Supplemental Table S2b: Baseline characteristics, pre-PS matching, for Ad26.COV2.S + Ad26.COV2.S vs. primary series. Supplemental Table S3a. Baseline characteristics, pre-PS matching, for mRNA + Ad26.COV2.S vs. primary series among patients in the California state registry. Supplemental Table S3b. Baseline characteristics, pre-PS matching, for Ad26.COV2.S + Ad26.COV2.S vs. primary series among patients in the California state registry. Supplemental Table S4a. Baseline characteristics, post-PS matching, for mRNA + Ad26.COV2.S vs. primary series among patients in the California state registry. Supplemental Table S4b. Baseline characteristics, post-PS matching, for Ad26.COV2.S + Ad26.COV2.S vs. primary series among patients in the California state registry. Supplemental Table S5. Adjusted hazard ratios for COVID-19-related hospitalization and medically attended COVID-19 among patients in the California state registry. Figure S1. Study population flow diagram, mRNA + Ad26.COV2.S vs. mRNA + no boost. Figure S2. Study population flow diagram, mRNA + Ad26.COV2.S vs. Ad26.COV2.S + no boost. Figure S3. Study population flow diagram, Ad26.COV2.S + Ad26.COV2.S vs. mRNA + no boost. Figure S4. Study population flow diagram, Ad26.COV2.S + Ad26.COV2.S vs. Ad26.COV2.S + no boost.
Author Contributions
Conceptualization, A.M., L.R., L.S.Y., P.S., R.A.H., M.N., and D.R.; methodology, A.M., L.R., L.S.Y., P.S., R.A.H., M.N., and D.R.; formal analysis, L.S.Y., A.M., P.S., M.I., and T.Z.; investigation, L.S.Y., L.R., A.M., M.I., and T.Z.; writing—original draft, L.S.Y., A.M., and M.I.; writing—review and editing, A.M., M.N., R.A.H., and D.R.; supervision, A.M. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Johnson & Johnson Innovative Medicine.
Institutional Review Board Statement
This study was granted exemption from IRB review by the Sterling IRB pursuant to the terms of the U.S. Health and Human Services’ Policy for the Protection of Human Research Subjects at 45 C.F.R. §46.104(d). The IRB determined that a Category 4 Exemption (DHHS) was applicable to the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from HealthVerity and are available from the authors with the permission of HealthVerity.
Conflicts of Interest
A.M., L.S.Y., L.R., P.S., M.I., and T.Z. were employees and/or held stock options or equity in Aetion, a software-enabled healthcare analytics company, at the time this paper was written. M.N., R.A.H., and D.R. are employees of Johnson & Johnson Innovative Medicine, the manufacturer of the studied vaccine Ad26.COV2.S, and hold stock options or equity in the company.
References
- Steele, M.K.; Couture, A.; Reed, C.; Iuliano, D.; Whitaker, M.; Fast, H.; Hall, A.J.; MacNeil, A.; Cadwell, B.; Marks, K.J.; et al. Estimated Number of COVID-19 Infections, Hospitalizations, and Deaths Prevented Among Vaccinated Persons in the US, December 2020 to September 2021. JAMA Netw. Open 2022, 5, e2220385. [Google Scholar] [CrossRef]
- Yamana, T.K.; Galanti, M.; Pei, S.; Di Fusco, M.; Angulo, F.J.; Moran, M.M.; Khan, F.; Swerdlow, D.L.; Shaman, J. The Impact of COVID-19 Vaccination in the US: Averted Burden of SARS-CoV-2-Related Cases, Hospitalizations and Deaths. PLoS ONE 2023, 18, e0275699. [Google Scholar] [CrossRef]
- Authorizations of Emergency Use of Certain Biological Products During the COVID-19 Pandemic Availability. Available online: https://www.federalregister.gov/documents/2021/05/27/2021-11234/authorizations-of-emergency-use-of-certain-biological-products-during-the-covid-19-pandemic (accessed on 15 February 2024).
- Vaccine Monitor: Eagerness to Get Vaccinated Begins to Level Off as Most People Who Want a Vaccine Have Gotten One, but Republicans Show Biggest Shift Toward Vaccination. KFF. 2021. Available online: https://www.kff.org/coronavirus-covid-19/press-release/vaccine-monitor-eagerness-to-get-vaccinated-begins-to-level-off-as-most-people-who-want-a-vaccine-have-gotten-one-but-republicans-show-biggest-shift-toward-vaccination/ (accessed on 3 January 2025).
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 8 November 2021).
- Coronavirus (COVID-19) Update: FDA Expands Eligibility for COVID-19 Vaccine Boosters. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters (accessed on 27 January 2025).
- Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (accessed on 15 February 2024).
- Coronavirus (COVID-19) Update: 29 October 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-october-29-2021 (accessed on 15 February 2024).
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.G.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Hardt, K.; Vandebosch, A.; Sadoff, J.; Le Gars, M.; Truyers, C.; Lowson, D.; Van Dromme, I.; Vingerhoets, J.; Kamphuis, T.; Scheper, G.; et al. Efficacy, Safety, and Immunogenicity of a Booster Regimen of Ad26.COV2.S Vaccine against COVID-19 (ENSEMBLE2): Results of a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Infect. Dis. 2022, 22, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Vaccines and Related Biological Products Advisory Committee 14–15 October 2021 Meeting Presentation. Presented at the Vaccines and Related Biological Products Advisory Committee Meeting. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-october-14-15-2021-meeting-announcement (accessed on 3 January 2025).
- Hung Nguyen, V.; Boileau, C.; Bogdanov, A.; Sredl, M.; Bonafede, M.; Ducruet, T.; Chavers, S.; Rosen, A.; Martin, D.; Buck, P.; et al. Relative Effectiveness of BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines and Homologous Boosting in Preventing COVID-19 in Adults in the US. Open Forum Infect. Dis. 2023, 10, ofad288. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-Term Effectiveness of COVID-19 Vaccines against Infections, Hospitalisations, and Mortality in Adults: Findings from a Rapid Living Systematic Evidence Synthesis and Meta-Analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Adams, K.; Rhoads, J.P.; Surie, D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Talbot, H.K.; Casey, J.D.; Zepeski, A.; Shapiro, N.I.; et al. Vaccine Effectiveness of Primary Series and Booster Doses against COVID-19 Associated Hospital Admissions in the United States: Living Test Negative Design Study. BMJ 2022, 379, e072065. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.M.; Murray, N.; Adams, K.; Surie, D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; et al. Absolute and Relative Vaccine Effectiveness of Primary and Booster Series of COVID-19 Vaccines (mRNA and Adenovirus Vector) Against COVID-19 Hospitalizations in the United States, December 2021–April 2022. Open Forum Infect. Dis. 2023, 10, ofac698. [Google Scholar] [CrossRef]
- Table HIC-4_ACS. Health Insurance Coverage Status and Type of Coverage by State—All Persons: 2008 to 2019, September 2020. Available online: https://www.census.gov/library/publications/2023/demo/p60-281.html (accessed on 2 January 2025).
- Centers for Disease Control and Prevention. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): Pfizer-BioNTech, Moderna, and Janssen COVID-19 Booster Doses. 2024. Available online: https://www.cdc.gov/acip/grade/covid-19-booster-doses.html#:~:text=COV2.S%20Vaccine%20(5%C3%971010%20viral%20particles%2C%20IM)%20should,%E2%89%A518%20years%20who%20completed%20a%20COVID%2D19%20vaccine (accessed on 6 December 2024).
- Gagne, J.J.; Glynn, R.J.; Avorn, J.; Levin, R.; Schneeweiss, S. A Combined Comorbidity Score Predicted Mortality in Elderly Patients Better than Existing Scores. J. Clin. Epidemiol. 2011, 64, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lash, T.L.; VanderWeele, T.J.; Haneuse, S. Modern Epidemiology, 4th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021; ISBN 978-1-4511-9328-2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).