Long-Term Impact of Pneumococcal Conjugate Vaccines on the Burden of Pneumococcal Meningitis in Mozambique, 2013–2023
Abstract
1. Background
2. Methods
2.1. Study Setting and Population
2.2. Case Definition and Enrollment
2.3. Sample and Data Collection
2.4. Laboratory Methods
2.5. Statistical Analysis
2.6. Ethics Statement
3. Results
3.1. General Characteristics of Study Participants
3.2. Prevalence and Incidence Trends of Pneumococcal Meningitis Among Children with Suspected Meningitis in the Period Between 2013 and 2023
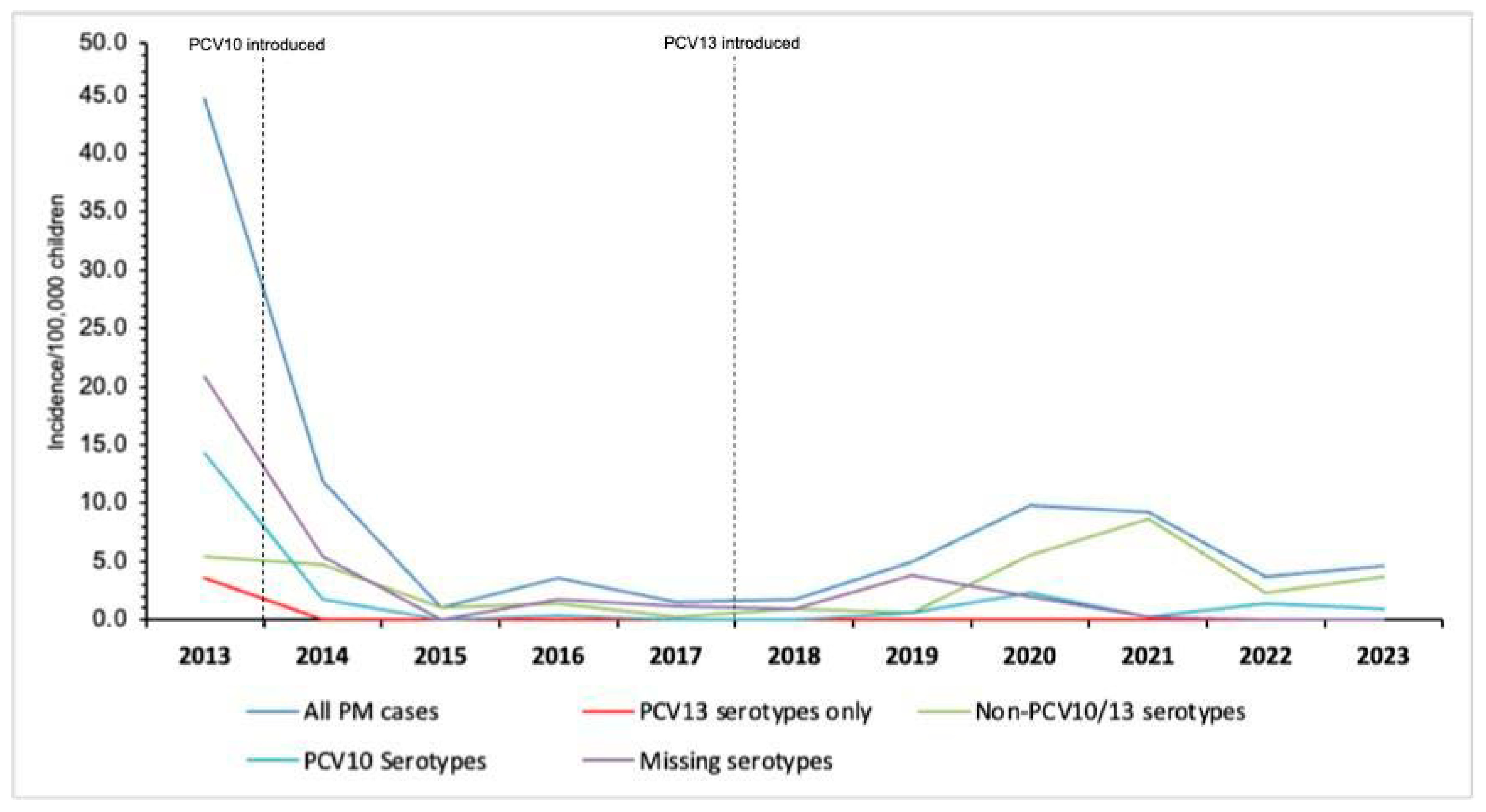
3.3. Incidence of Pneumococcal Meningitis During the PCV10/3+0 Period (2013–2017) and PCV13/2+1 Period (2020–2023)
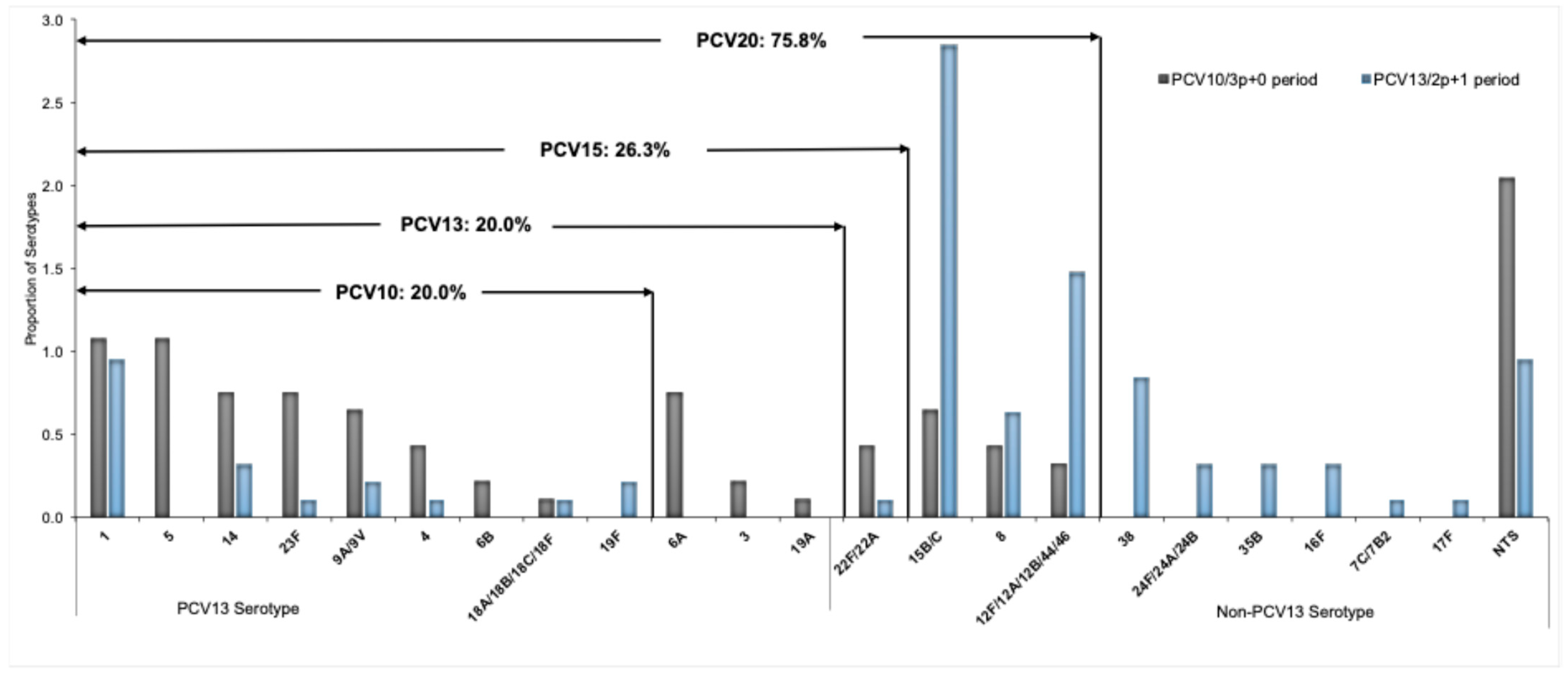
3.4. Trends on Serotype Distribution and Vaccine Formulation Coverage
3.5. Mortality and Case Fatality Ratio
3.6. Predicting Variables Associated with Pneumococcal Meningitis Infection Among Children Under 5 Years Old
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Articles Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef] [PubMed]
- Zunt, J.R.; Kassebaum, N.J.; Blake, N.; Glennie, L.; Wright, C.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Adamu, A.A.; et al. Global, regional, and national burden of meningitis, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 1061–1082. [Google Scholar]
- Mwenda, J.M.; Soda, E.; Weldegebriel, G.; Katsande, R.; Biey, J.N.-M.; Traore, T.; de Gouveia, L.; du Plessis, M.; von Gottberg, A.; Antonio, M.; et al. Pediatric bacterial meningitis surveillance in the World Health Organization african region using the invasive bacterial vaccine-preventable disease surveillance network, 2011–2016. Clin. Infect. Dis. 2019, 69 (Suppl. S2), S49–S57. [Google Scholar] [CrossRef]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper—February 2019. Wkly. Epidemiol. Rec. 2019, 8, 85–104. [Google Scholar]
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Shiri, T.; Datta, S.; Madan, J.; Tsertsvadze, A.; Royle, P.; Keeling, M.J.; McCarthy, N.D.; Petrou, S. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e51–e59. [Google Scholar]
- Perdrizet, J.; Horn, E.K.; Hayford, K.; Grant, L.; Barry, R.; Huang, L.; McDade, C.; Wilson, M. Historical Population-Level Impact of Infant 13-Valent Pneumococcal Conjugate Vaccine (PCV13) National Immunization Programs on Invasive Pneumococcal Disease in Australia, Canada, England and Wales, Israel, and the United States. Infect. Dis. Ther. 2023, 12, 1351–1364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cherian, T.; Cohen, M.; de Oliveira, L.; Farrar, J.L.; Goldblatt, D.; Knoll, M.; Moisi, J.C.; O’Brien, K.L.; Pilishvili, T.; Ramakrishnan, M.; et al. Pneumococcal Conjugate Vaccine (PCV) Review of Impact Evidence (PRIME): Summary of Findings from Systematic Review; Report to Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization; WHO: Geneva, Switzerland, 2017; Volume 1, pp. 1–215. [Google Scholar]
- Nhantumbo, A.A.; Weldegebriel, G.; Katsande, R.; de Gouveia, L.; Comé, C.E.; Cuco, A.Z.; Cantarelli, V.V.; Dias, C.; Caierão, J.; Mathiu, J.M.; et al. Surveillance of impact of PCV-10vaccine on pneumococcal meningitis in Mozambique, 2013–2015. PLoS ONE 2017, 12, e0177746. [Google Scholar] [CrossRef]
- Knoll, M.D.; Bennett, J.C.; Quesada, M.G.; Kagucia, E.W.; Peterson, M.E.; Feikin, D.R.; Cohen, A.L.; Hetrich, M.K.; Yang, Y.; Sinkevitch, J.N.; et al. Global Landscape Review of Serotype-Specific Invasive Pneumococcal Disease Surveillance among Countries Using PCV10/13: The Pneumococcal Serotype Replacement and Distribution Estimation (PSERENADE) Project. Microorganisms 2021, 9, 742. [Google Scholar] [CrossRef]
- Guzman-Holst, A.; de Barros, E.; Rubio, P.; DeAntonio, R.; Cintra, O.; Abreu, A. Impact after 10-year use of pneumococcal conjugate vaccine in the Brazilian national immunization program: An updated systematic literature review from 2015 to 2020. Hum. Vaccin. Immunother. 2022, 18, 1879578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nhantumbo, A.A.; Cantarelli, V.V.; Caireão, J.; Munguambe, A.M.; Comé, C.E.; Pinto, G.d.C.; Zimba, T.F.; Mandomando, I.; Semá, C.B.; Dias, C.; et al. Frequency of Pathogenic Paediatric Bacterial Meningitis in Mozambique: The Critical Role of Multiplex Real-Time Polymerase Chain Reaction to Estimate the Burden of Disease. PLoS ONE 2015, 10, e0138249. [Google Scholar] [CrossRef][Green Version]
- INE. Localização dos Hospital Centrais e Provinciais de Mocambique: Instituto Nacional de Estatística. 2017. Available online: https://www.ine.gov.mz/web/guest/censo-2017 (accessed on 15 November 2023).[Green Version]
- World Health Organization Africa. Standard Operating Procedures for Surveillance of Meningitis Preparedness and Response to Epidemics in Africa. 2018. Available online: https://iris.who.int/bitstream/handle/10665/312141/9789290234241-eng.pdf (accessed on 20 April 2025).[Green Version]
- Ouattara, M.; Whaley, M.J.; Jenkins, L.T.; Schwartz, S.B.; Traoré, R.O.; Diarra, S.; Collard, J.-M.; Sacchi, C.T.; Wang, X. Triplex real-time PCR assay for the detection of Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae directly from clinical specimens without extraction of DNA. Diagn. Microbiol. Infect. Dis. 2019, 93, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.C.; Roundtree, A.; Soysal, A.; Bakir, M.; du Plessis, M.; Wolter, N.; von Gottberg, A.; McGee, L.; Carvalho, M.d.G.; Beall, B. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J. Clin. Microbiol. 2013, 51, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.; Tran, T.; Mongkolrattanothai, T.; Walker, H.; McGee, L.; Beall, B. Expanded sequential quadriplex real-time polymerase chain reaction (PCR) for identifying pneumococcal serotypes, penicillin susceptibility, and resistance markers. Diagn. Microbiol. Infect. Dis. 2020, 97, 115037. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccine-Preventable Diseases Surveillance Standards. 2018. Available online: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-pneumococcus (accessed on 21 June 2019).
- Bar-Zeev, N.; Swarthout, T.D.; Everett, D.B.; Alaerts, M.; Msefula, J.; Brown, C.; Bilima, S.; Mallewa, J.; King, C.; von Gottberg, A.; et al. Impact and effectiveness of 13-valent pneumococcal conjugate vaccine on population incidence of vaccine and non-vaccine serotype invasive pneumococcal disease in Blantyre, Malawi, 2006–2018: Prospective observational time-series and case-control studies. Lancet Glob. Health 2021, 9, e989–e998, Erratum in Lancet Glob. Health 2021, 9, e1657. [Google Scholar] [CrossRef]
- Bar-Zeev, N.; Swarthout, T.D.; Everett, D.B.; Alaerts, M.; Msefula, J.; Brown, C.; Bilima, S.; Mallewa, J.; King, C.; von Gottberg, A.; et al. Impact of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease and pneumonia in The Gambia: 10 years of population-based surveillance. Lancet Infect. Dis. 2021, 21, 1293–1302. [Google Scholar]
- Hammitt, L.L.; Etyang, A.O.; Morpeth, S.C.; Ojal, J.; Mutuku, A.; Mturi, N.; Moisi, J.C.; Adetifa, I.M.; Karani, A.; O Akech, D.; et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: A longitudinal surveillance study. Lancet 2019, 393, 2146–2154. [Google Scholar] [CrossRef]
- Yamba, K.; Mpabalwani, E.; Nakazwe, R.; Mulendele, E.; Weldegebriel, G.; Mwenda, J.M.; Katsande, R.; de Gouveia, L.; Chizema-Kawesha, E.; Chanda, R.; et al. The Burden of Invasive Bacterial Disease and the Impact of 10-Valent Pneumococcal Conjugate Vaccine in Children <5 years Hospitalized for Meningitis in Lusaka, Zambia, 2010–2019. J. Infect. Dis. 2021, 224 (Suppl. S2), S275–S284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waight, P.A.; Andrews, N.J.; Ladhani, N.J.; Sheppard, C.L.; Slack, M.P.; Miller, E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: An observational cohort study. Lancet Infect. Dis. 2015, 15, 535–543. [Google Scholar] [CrossRef]
- Bertran, M.; D’Aeth, J.C.; Abdullahi, F.; Eletu, S.; Andrews, N.J.; E Ramsay, M.; Litt, D.J.; Ladhani, S.N. Invasive pneumococcal disease 3 years after introduction of a reduced 1 + 1 infant 13-valent pneumococcal conjugate vaccine (PCV13) immunisation schedule in England: A prospective national observational surveillance study. Lancet Infect. Dis. 2024, 24, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Choe, Y.J.; Lee, C.Y.; Jung, S.O.; Lee, D.H.; Yoo, J.I. Impact of national pneumococcal vaccination program on invasive pneumococcal diseases in South Korea. Sci. Rep. 2022, 12, 15833. [Google Scholar] [CrossRef] [PubMed]
- Reyburn, R.; Tuivaga, E.; Ratu, F.; Dunne, E.; Nand, D.; Kado, J.; Jenkins, K.; Tikoduadua, L.; Jenney, A.; Howden, B.; et al. The impact of 10-valent pneumococcal vaccine introduction on invasive disease in Fiji. Lancet Reg. Health West Pac. 2022, 20, 100352. [Google Scholar] [CrossRef]
- Moreira, M.; Cintra, O.; Harriague, J.; Hausdorff, W.P.; Hoet, B. Impact of the introduction of the pneumococcal conjugate vaccine in the Brazilian routine childhood national immunization program. Vaccine 2016, 34, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.I.; Castañeda-Orjuela, C.; Brandileone, M.C.d.C.; Echániz-Aviles, G.; Almeida, S.C.G.; Carnalla-Barajas, M.N.; Regueira, M.; Fossati, S.; Alarcón, P.; Araya, P.; et al. The direct effect of pneumococcal conjugate vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006-17): A multicentre, retrospective observational study. Lancet Infect. Dis. 2021, 21, 405–417. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Pneumococcal Disease: Surveillance and Reporting. Available online: https://www.cdc.gov/pneumococcal/php/surveillance/index.html (accessed on 8 August 2023).
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef]
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2017—Conclusions and Recommendations. Available online: https://iris.who.int/bitstream/handle/10665/259533/WER9248.pdf;jsessionid=0650CFB4034DE9A4FD3FDAB46FF35346?sequence=1 (accessed on 20 April 2025).
- Levy, C.; Ouldali, N.; Caeymaex, L.; Angoulvant, F.; Varon, E.; Cohen, R. Diversity of serotype replacement after pneumococcal conjugate vaccine implementation in Europe. J. Pediatr. 2019, 213, 252–253.e3. [Google Scholar] [CrossRef]
- Lo, S.W.; Gladstone, R.A.; van Tonder, A.J.; Lees, J.A.; du Plessis, M.; Benisty, R.; Givon-Lavi, N.; A Hawkins, P.; E Cornick, J.; Kwambana-Adams, B.; et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect. Dis. 2019, 19, 759–769. [Google Scholar] [CrossRef]
- Weinberger, R.; von Kries, R.; van der Linden, M.; Rieck, T.; Siedler, A.; Falkenhorst, G. Invasive pneumococcal disease in children under 16 years of age: Incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine 2018, 36, 572–577. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis 2015, 15, 301–309. [Google Scholar] [CrossRef]
- Brandileone, M.C.; Almeida, S.C.G.; Minamisava, R.; Andrade, A.L. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine 2018, 36, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.M.A.S.; Verani, J.R.; Renoiner, E.I.M.; de Cunto Brandileone, M.C.; Flannery, B.; de Oliveira, L.H.; Santos, J.B.; de Moraes, J.C.; Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: A matched case-control study. Lancet Respir. Med. 2014, 2, 464–471. [Google Scholar] [CrossRef]
- GBD 2019 Meningitis Antimicrobial Resistance Collaborators. Global, regional, and national burden of meningitis and its aetiologies, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 685–711. [Google Scholar] [CrossRef]
- Brueggemann, A.B.; Jansen van Rensburg, M.J.; Shaw, D.; McCarthy, N.D.; Jolley, K.A.; Maiden, M.C.J.; van der Linden, M.P.G.; Amin-Chowdhury, Z.; Bennett, D.E.; Borrow, R.; et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit. Health 2021, 3, e360–e370. [Google Scholar] [PubMed]
- WHO. Defeating Meningitis by 2030: A Global Road Map. 2021. Available online: https://www.who.int/publications-detail-redirect/9789240026407 (accessed on 20 February 2023).
- Rybak, A.; Levy, C.; Angoulvant, F.; Auvrignon, A.; Gembara, P.; Danis, K.; Vaux, S.; Levy-Bruhl, D.; van der Werf, S.; Béchet, S.; et al. Association of Nonpharmaceutical Interventions During the COVID-19 Pandemic With Invasive Pneumococcal Disease, Pneumococcal Carriage, and Respiratory Viral Infections Among Children in France. JAMA Netw. Open 2022, 5, e2218959. [Google Scholar] [CrossRef] [PubMed]
- Casanova, C.; Kuffer, M.; Leib, S.L.; Hilty, M. Re-emergence of invasive pneumococcal disease (IPD) and increase of serotype 23B after easing of COVID-19 measures, Switzerland, 2021. Emerg. Microbes Infect. 2021, 10, 2202–2204. [Google Scholar] [CrossRef]
- Vardanjani, H.M.; Borna, H.; Ahmadi, A. Effectiveness of pneumococcal conjugate vaccination against invasive pneumococcal disease among children with and those without HIV infection: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 685. [Google Scholar] [CrossRef]
- van Aalst, M.; Lötsch, F.; Spijker, R.; Jtm, V.D.M.; Langendam, M.W.; Goorhuis, A.; Grobusch, M.P.; de Bree, G.J. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2018, 24, 89–100. [Google Scholar] [CrossRef]
- Frankel, R.E.; Virata, M.; Hardalo, C.; Altice, F.L.; Friedland, G. Invasive pneumococcal disease: Clinical features, serotypes, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin. Infect. Dis. 1996, 23, 577–584. [Google Scholar] [CrossRef]
- Moore, D.; Nelson, M.; Henderson, D. Pneumococcal vaccination and HIV infection. Int. J. STD AIDS 1998, 9, 1–7. [Google Scholar] [CrossRef]
- Farley, J.J.; King, J.C.; Nair, P.; Hines, S.E.; Tressler, R.L.; Vink, P.E. Invasive pneumococcal disease among infected and uninfected children of mothers with human immunodeficiency virus infection. J. Pediatr. 1994, 124, 853–858. [Google Scholar] [CrossRef] [PubMed]

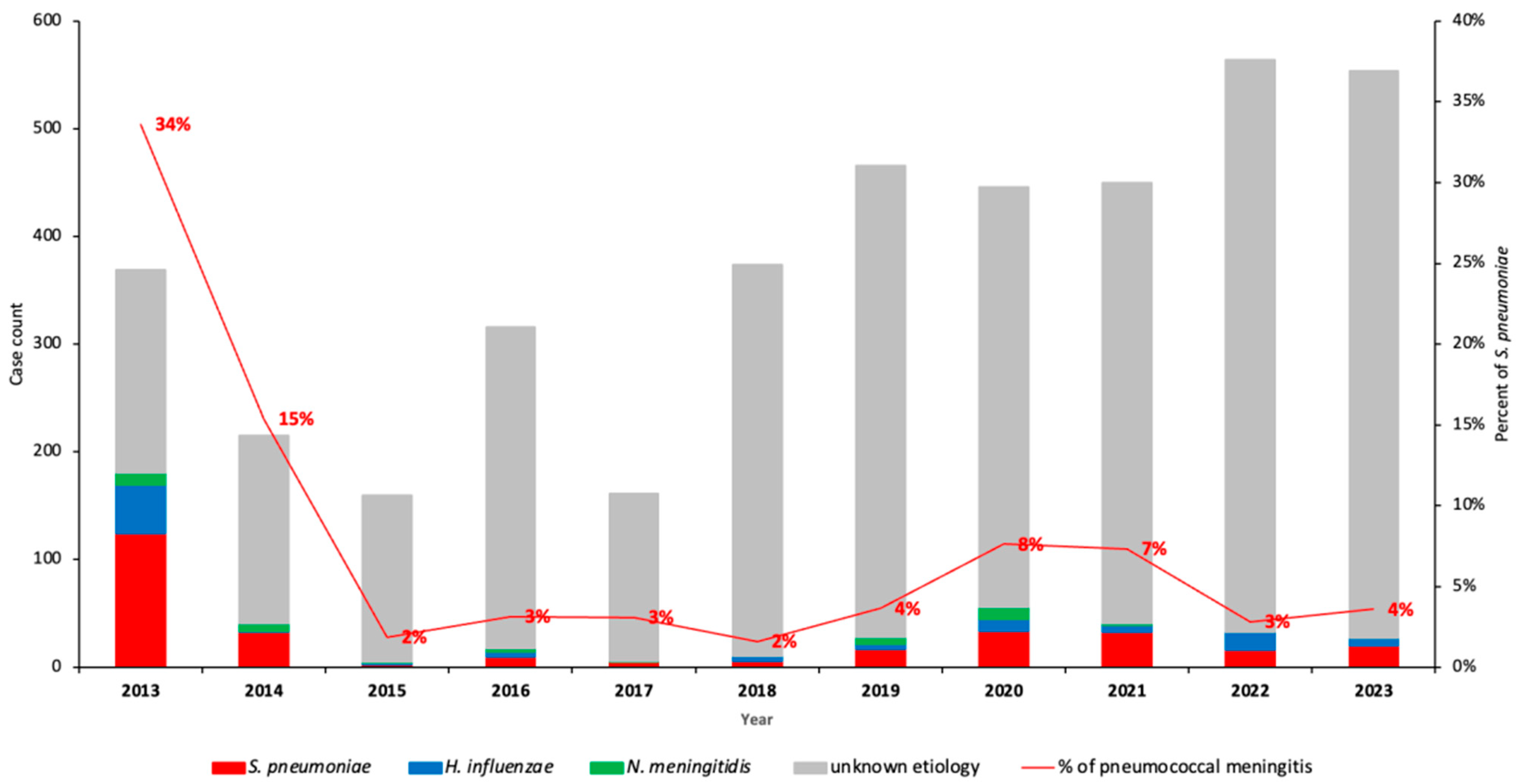
| Characteristics | Total | PCV10/3p+0 Period (2013–2017) | Transition Period (2013–2017) | PCV13/2p+1 Period (2020–2023) | |||
|---|---|---|---|---|---|---|---|
| ABM | S. pneumoniae Positive | ABM | S. pneumoniae Positive | ABM | S. pneumoniae Positive | ||
| Total | 4075 | 1221 | 175 (14.3%) | 840 | 23 (2.7%) | 2014 | 103 (5.1%) |
| Age in months, median (IQR) | 11 (4–25) | 14 (6–36) | 9 (5–23) | 12 (5-26) | 11 (6–36) | 9 (4–24) | 11 (5–24) |
| Age group | |||||||
| 0–23 months | 2712 (66.6%) | 734 (60.1%) | 134 (76.6%) | 302 (36.0%) | 14 (60.9%) | 1440 (71.5%) | 73 (70.9%) |
| 24–59 months | 1363 (33.4%) | 487 (39.9%) | 41 (23.4%) | 538 (64.0%) | 9 (39.1%) | 574 (28.5%) | 30 (29.1%) |
| Gender | |||||||
| Male | 2273 (55.8%) | 683 (55.9%) | 98 (56.0%) | 469 (55.8%) | 14 (60.9%) | 1121 (55.7%) | 57 (55.3%) |
| Female | 1802 (44.2%) | 538 (44.1%) | 77 (44.0%) | 371 (44.2%) | 9 (39.1%) | 893 (44.3%) | 46 (44.7%) |
| Study sites | |||||||
| NCH | 3152 (77.3%) | 694 (56.8%) | 121 (69.1%) | 736 (87.6%) | 18 (78.3%) | 1722 (85.5%) | 89 (86.4%) |
| BCH | 398 (9.8%) | 191 (15.6%) | 5 (2.9%) | 41 (4.9%) | 5 (21.7%) | 166 (8.2%) | 12 (11.7%) |
| MCH | 525 (12.9%) | 336 (27.5%) | 49 (28.0%) | 63 (7.5%) | 0 (0.0%) | 126 (6.3%) | 2 (1.9%) |
| HIV status | |||||||
| Positive | 47 (1.2%) | 18 (1.5%) | 16 (9.1%) | 9 (1.1%) | 1 (4.3%) | 20 (1.0%) | 11 (10.7%) |
| Negative | 4028 (98.8%) | 1203 (98.5%) | 159 (90.9%) | 831 (98.9%) | 22 (95.7%) | 1994 (99.0%) | 92 (89.3%) |
| PCV doses | |||||||
| 0 | 739 (18.1%) | 452 (37.0%) | 155 (88.6%) | 64 (7.6%) | 15 (65.2%) | 223 (11.1%) | 69 (67.0%) |
| 1 or 2 | 857 (21.0%) | 84 (6.9%) | 2 (1.1%) | 118 (14.0%) | 3 (13.0%) | 655 (32.5%) | 12 (11.7%) |
| 3 | 2479 (60.8%) | 685 (56.1%) | 18 (10.3%) | 658 (78.3%) | 5 (21.7%) | 1136 (56.4%) | 22 (21.3%) |
| Case Fatality Rate (%) | 114 (2.8%) | 56 (4.6%) | 32 (18.3%) | 17 (2.0%) | 0 (0.0%) | 41 (2.0%) | 9 (8.8%) |
| Characteristic | PCV10/3p+0 Period (2013–2017) | PCV13/2p+1 Period (2020–2023) | PCV13 Period vs. PCV10 Period | |||||
|---|---|---|---|---|---|---|---|---|
| Cases (n) | Children at Risk | Incidence per 100,000 (95% CI) | Cases (n) | Children at Risk | Incidence per 100,000 (95% CI) | Incidence Rate Ratio (95% CI) | p-Value | |
| Overal S. pneumoniae by age | ||||||||
| All ages | 175 | 1,452,139 | 12.1 (10.3–13.8) | 103 | 1,735,269 | 5.9 (4.8–7.1) | 0.49 (0.4–0.6) | <0.001 |
| 0–23 months | 134 | 849,239 | 15.8 (13.0–18.5) | 73 | 937,445 | 7.8 (4.3–8.9) | 0.49 (0.3–0.5) | <0.001 |
| 24–59 months | 41 | 602,900 | 6.8 (4.7–8.9) | 30 | 797,823 | 3.8 (2.1–4.3) | 0.56 (0.3–0.8) | 0.012 |
| PCV10 ST by ages | ||||||||
| All ages | 47 | 1,452,139 | 3.2 (2.3–4.2) | 19 | 1,735,269 | 1.1 (0.6–1.6) | 0.34 (0.2–0.6) | <0.001 |
| 0–23 months | 32 | 849,239 | 3.8 (2.5–5.1) | 10 | 937,445 | 1.1 (0.3–1.2) | 0.29 (0.1–0.4) | <0.001 |
| 24–59 months | 15 | 602,900 | 2.5 (1.2–3.7) | 9 | 797,823 | 1.1 (0.3–1.6) | 0.44 (0.2–0.9) | 0.05 |
| PCV13 ST by age | ||||||||
| All ages | 10 | 1,452,139 | 0.7 (0.3–1.1) | 0 | 1,735,269 | 0.0 | --- | --- |
| 0–23 months | 8 | 849,239 | 0.9 (0.3–1.6) | 0 | 937,445 | 0.0 | --- | --- |
| 24–59 months | 2 | 602,900 | 0.3 (0.1–0.8) | 0 | 797,823 | 0.0 | --- | --- |
| Non-PCV10/13 | ||||||||
| All ages | 36 | 1,452,139 | 2.5 (1.7–3.3) | 76 | 1,735,269 | 4.4 (3.4–5.5) | 1.76 (1.2–2.6) | 0.004 |
| 0–23 months | 27 | 849,239 | 3.2 (2.0–4.4) | 56 | 937,445 | 6.0 (3.2–5.4) | 1.88 (0.9–2.1) | <0.001 |
| 24–59 months | 9 | 602,900 | 1.5 (0.5–2.5) | 20 | 797,823 | 2.5 (1.2–3.1) | 1.67 (0.6–3.1) | 0.192 |
| PCR Results | PCV10 (3p+0) Period (2013–2017) | Transition Period (2018–2019) | PCV13 (2p+1) Period (2020 –2023) | Total |
|---|---|---|---|---|
| PCV10 serotypes | ||||
| 1 | 10 (10.8%) | 1 (14.3%) | 9 (9.5%) | 20 (10.3%) |
| 5 | 10 (10.8%) | 0 (0.0%) | 0 (0.0%) | 10 (5.1%) |
| 14 | 7 (7.5%) | 0 (0.0%) | 3 (3.2%) | 10 (5.1%) |
| 23F | 7 (7.5%) | 0 (0.0%) | 1 (1.1%) | 8 (4.1%) |
| 9V/9A | 6 (6.5%) | 0 (0.0%) | 2 (2.1%) | 8 (4.1%) |
| 4 | 4 (4.3%) | 0 (0.0%) | 1 (1.1%) | 5 (2.6%) |
| 6B | 2 (2.2%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) |
| 18A/18B/18C/18F | 1 (1.1%) | 1 (14.3%) | 1 (1.1%) | 3 (1.5%) |
| 19F | 0 (0.0%) | 0 (0.0%) | 2 (2.1%) | 2 (1.0%) |
| 7F | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| PCV13 serotypes | ||||
| 6A | 7 (7.5%) | 0 (0.0%) | 0 (0.0%) | 7 (3.6%) |
| 3 | 2 (2.2%) | 0 (0.0%) | 0 (0.0%) | 2 (1.0%) |
| 19A | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) |
| Non PCV10/13 serotypes | ||||
| 15B/C | 6 (6.5%) | 0 (0.0%) | 27 (28.4%) | 33 (16.9%) |
| 8 | 4 (4.3%) | 2 (28.6%) | 6 (6.3%) | 12 (6.2%) |
| 22F/22A | 4 (4.3%) | 1 (14.3%) | 1 (1.1%) | 6 (3.1%) |
| 12 | 3 (3.2%) | 0 (0.0%) | 14 (14.7%) | 17 (8.7%) |
| 38 | 0 (0.0%) | 0 (0.0%) | 8 (8.4%) | 8 (4.1%) |
| 16F | 0 (0.0%) | 0 (0.0%) | 3 (3.2%) | 3 (1.5%) |
| 24F/24A/24B | 0 (0.0%) | 0 (0.0%) | 3 (3.2%) | 3 (1.5%) |
| 35B | 0 (0.0%) | 0 (0.0%) | 3 (3.2%) | 3 (1.5%) |
| 7C/7B2 | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 1 (0.5%) |
| 17F | 0 (0.0%) | 0 (0.0%) | 1 (1.1%) | 1 (0.5%) |
| NTP | 19 (20.4%) | 2 (28.6%) | 9 (9.5%) | 30 (15.4%) |
| Total serotypes | 93 (53.1) | 7 (30.4%) | 95 (92.2%) | 195 (64.8%) |
| Missing serotypes | 82 (46.9%) | 16 (69.6%) | 8 (7.8%) | 106 (35.2%) |
| Total | 175 | 23 | 103 | 301 |
| Variable | PM (n = 301) | CSF Negative (n = 3774) | Unadjusted OR [95% CI] | p-Value | Adjusted OR [95% CI] | p-Value |
|---|---|---|---|---|---|---|
| Age (months) | ||||||
| 0–23 | 221 (73.4%) | 2491 (66.0%) | 1.42 (1.09–1.85) | 0.009 | 1.62 (1.19–2.21) | 0.002 |
| 24–59 * | 80 (26.6%) | 1283 (34.0%) | 1.00 | 1.00 | ||
| Gender | ||||||
| Female * | 132 (43.9%) | 1670 (44.3%) | 1.00 | 1.00 | ||
| Male | 169(56.1%) | 2104 (55.7%) | 1.02 (0.80–1.29) | 0.894 | 1.20 (0.92–1.88) | 0.183 |
| Sites | ||||||
| BCH * | 22 (7.3%) | 376 (10.0%) | 1000 | 1000 | ||
| MCH | 51 (16.9%) | 474 (12.6%) | 1.84 (1.10–3.09) | 0.021 | 1.34 (0.74–2.41) | 0.333 |
| NCH | 228 (75.7%) | 2924 (77.5%) | 1.33 (0.85–2.09) | 0.212 | 1.30 (0.78–2.18) | 0.310 |
| HIV Status | ||||||
| Negative * | 273 (90.7%) | 3755 (99.5%) | 1.00 | 1000 | ||
| Positive | 28 (9.3%) | 19 (0.5%) | 20.27 (11.18–36.76) | <0.001 | 23.00 (10.51–50.35) | <0.001 |
| PCV-13 doses | ||||||
| 1 or 2 * | 17 (5.6)% | 840 (22.3%) | 1000 | 1000 | ||
| 0 | 239 (79.4%) | 500 (13.2%) | 23.62 (14.26–39.10) | <0.001 | 29.41 (17.29–50.02) | <0.001 |
| 3 | 45 (15.0%) | 2434 (64.4%) | 0.91 (0.52–1.60) | 0.753 | 1.20 (0.67–2.15) | 0.551 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nhantumbo, A.A.; Weldegebriel, G.; de Gouveia, L.; Katsande, R.; Comé, C.E.; Munguambe, A.M.; Cantarelli, V.; Dias, C.; Muleia, R.; Sitoe, E.F.; et al. Long-Term Impact of Pneumococcal Conjugate Vaccines on the Burden of Pneumococcal Meningitis in Mozambique, 2013–2023. Vaccines 2025, 13, 1246. https://doi.org/10.3390/vaccines13121246
Nhantumbo AA, Weldegebriel G, de Gouveia L, Katsande R, Comé CE, Munguambe AM, Cantarelli V, Dias C, Muleia R, Sitoe EF, et al. Long-Term Impact of Pneumococcal Conjugate Vaccines on the Burden of Pneumococcal Meningitis in Mozambique, 2013–2023. Vaccines. 2025; 13(12):1246. https://doi.org/10.3390/vaccines13121246
Chicago/Turabian StyleNhantumbo, Aquino Albino, Goitom Weldegebriel, Linda de Gouveia, Reggis Katsande, Charlotte Elizabeth Comé, Alcides Moniz Munguambe, Vlademir Cantarelli, Cícero Dias, Rachid Muleia, Ezequias Fenias Sitoe, and et al. 2025. "Long-Term Impact of Pneumococcal Conjugate Vaccines on the Burden of Pneumococcal Meningitis in Mozambique, 2013–2023" Vaccines 13, no. 12: 1246. https://doi.org/10.3390/vaccines13121246
APA StyleNhantumbo, A. A., Weldegebriel, G., de Gouveia, L., Katsande, R., Comé, C. E., Munguambe, A. M., Cantarelli, V., Dias, C., Muleia, R., Sitoe, E. F., Zeca, E. V., Seni, A., Tambo, A. N., Mussagi, A. C. d. F. N., Maholela, P. I., de Filippis, I., & Gudo, E. S. (2025). Long-Term Impact of Pneumococcal Conjugate Vaccines on the Burden of Pneumococcal Meningitis in Mozambique, 2013–2023. Vaccines, 13(12), 1246. https://doi.org/10.3390/vaccines13121246







