1. Introduction
Pancreatic cancer (PC), with its high mortality rate and dismal prognosis, is the third leading cause of cancer-related death in the U.S. Key risk factors include smoking, obesity, and diabetes. While traditional treatments like surgery and chemotherapy are available, their effectiveness is limited due to late-stage diagnosis. Although immunotherapy has revolutionized the treatment of many cancers, its effectiveness in alleviating the burden of PC has been met with many challenges. Several factors complicate the immune system’s ability to recognize and attack cancer cells, including the dense tumor microenvironment, which inhibits the infiltration of immune cells and acts as a physical barrier limiting the presence of immunosuppressive cells as well as the tumor’s low antigenicity [
1,
2]. Arguably the most potent antigen-presenting cell (APC) type that bridges innate and adaptive immunity, the Dendritic cell (DC) is capable of priming naïve T cells and initiating effective adaptive immune responses against infection and cancer [
3]. Over the last two decades, DC-based therapies have been explored extensively as an immunotherapeutic treatment option for a wide variety of cancers, most notably melanoma, glioblastoma, prostate cancer, and PC [
4,
5]. Clinical studies have shown that ex vivo-generated DCs loaded with tumor antigens can induce tumor-specific cytotoxic T lymphocyte (CTL) responses and, in some cases, durable clinical remissions [
6,
7]. For instance, the approval of Sipuleucel-T (Provenge
®) for metastatic prostate cancer established the feasibility of autologous DC vaccines as a therapeutic approach [
8]. However, autologous DC therapies face or are limited by multiple challenges, particularly when it comes to the treatment of solid tumors. These include the time and labor-intensive manufacturing process, variability of patients, limited availability of circulating monocytes as a starting material, and insufficient immunogenicity in the highly suppressive tumor microenvironment (TME) [
9]. Solid tumors, such as PC, present additional challenges due to a dense desmoplastic stroma, low effector T-cell infiltration, and the presence of immunosuppressive cells that inhibit anti-tumor immune responses [
10,
11]. Recent studies were conducted with efforts to improve DC therapies that were focused on enhancing antigen loading, co-stimulation, cytokine secretion, and migratory capacity, often through genetic engineering approaches.
In our recently published study [
12], we have described a novel CD34
+ hematopoietic stem cell (HSC)-derived allogeneic DC therapy that was lentivirally engineered to express three key immunomodulatory molecules: CD93, CD40-ligand (CD40L), and Chemokine (C-X-C motif) ligand-13 (CXCL13) via lentiviral transduction. CD93, a C-type lectin-like protein, promotes immune cell infiltration and phagocytosis, critical for overcoming the immunosuppressive tumor microenvironment [
13]. CD40L, expressed on activated T cells and DCs, amplifies T cell priming through bidirectional CD40-CD40L interactions [
14]. CXCL13, a B-cell chemoattractant, enhances lymphoid tissue recruitment, potentially boosting humoral and T-helper responses [
15]. Engineered DCs expressing these three factors exhibited enhanced capacity for antigen presentation, T-cell activation, and recruitment of immune effector cells. In humanized mouse models of orthotopic PC, treatment with these Engineered DCs led to significant tumor reduction, increased tumor-infiltrating lymphocytes (CD4
+ and CD8
+ T cells), and improved survival. Lentiviral vector (LVV) was used to deliver the three target genes. LVV could efficiently deliver and permanently integrate genetic material into the genome of the cells, lowering stable long-term expression of the therapeutic genes [
16]. It has been used clinically in the chimeric receptor antigen T-cell (CAR-T) therapies. The major risk of LVV is insertional mutagenesis from random integration into the host genome. However, the current clinical experience of the LVV showed that insertional oncogenesis was rare. Allogeneic DCs edited by the LVV impose even lower risk, as the DCs would be quickly removed by the host immune responses.
In this follow-up study, we optimized the LVV design to enhance the three target gene expressions and to incorporate a clinical compliant design for future potential clinical development. We rearranged transgenes in the order CD40L-CD93-CXCL13 and utilized a strong EF1α promoter to drive robust expression of these three transgenes. We hypothesized that these optimizations would improve DC maturation, cytokine production, and T-cell priming and translate into superior anti-tumor responses. Additionally, the LVV was modified for potential future clinical applications with the resistance gene changed from ampicillin to kanamycin and the expression regulatory element changed from wPRE to oPRE. While comparable in terms of transgene expression, oPRE is often preferred due to its smaller size and safety profile, which are essential for regulatory compliance [
17,
18]. Furthermore, in the optimized LVV, the antibiotic resistance was changed to Kanamycin to minimize the risk of β-lactam–induced hypersensitivity reactions in patients and to reduce the potential spread of β-lactamase resistance genes to environmental microbes, which is considered more stable and broadly accepted for use in vectors intended for biological production [
19,
20]. The allogeneic DCs transduced with the optimized 2nd generation (Gen) LVV were tested both in vitro and in vivo to ensure they have comparable or better performance than the 1st Gen product.
Here, we report preliminary observational data that this 2nd Gen Engineered DC therapy not only induces robust immune activation but also achieves complete regression of both primary pancreatic tumors and metastatic lesions in vivo in a head-to-head comparison with our 1st Gen Engineered DC therapy. Furthermore, we optimized our pancreatic tumor model by using Luciferase-expressing MIA PaCa-2 (MP2-Luc) cells, enabling visualization of tumors in live mice and analysis of primary and metastatic lesions. This disease model with MP2-Luc cells mimicked a late-stage pancreatic tumor model with metastatic lesions and poor survival. Treatment of these MP2-Luc tumor-bearing humanized mice revealed that optimized Engineered DCs not only reduce tumor burden but also achieve complete regression of both primary pancreatic tumors and metastatic lesions. Although these results are observational preliminary findings, we believe this tumor-killing response warrants further investigation into the specific immune activation mechanics driving this platform and showcases potential for the treatment of PC and other solid tumor types.
2. Materials and Methods
2.1. Cells and Reagents
Standard conditions were used to culture all cells at 37 °C and 5% CO2 in a humidified incubator. Prior to use in experiments, all cells were evaluated for mycoplasma contamination. Dulbecco’s Modified Essential Medium (DMEM) (Gibco, Billings, MA, USA) enriched with fetal bovine serum (FBS) (ThermoFisher, Waltham, MA, USA, Cat# A5669701) to a final concentration of 10% was used to culture pancreas carcinoma MP2 cancer cells (American Type Culture Collection (ATCC), Manassas, VA, USA, Cat# CRL-1420). MP2-Luc cells (GenTarget INC, San Diego, CA, USA, Cat# SC079-LG) were cultured as per the provider’s recommendations using DMEM supplemented with 10% heat-inactivated FBS + 1% Penicillin–Streptomycin (Gibco, Cat #1514012). RPMI 1610 Medium (Gibco, Cat# 11875093) supplemented with FBS to a final concentration of 10% was used to culture Jurkat T-cells [Clone E6-1, TIB-152] (ATCC), Peripheral Blood Mononucleated Cells (PBMCs) (isolated from mice), splenocytes (isolated from mice), and T cells (ATCC [PCS-800-016]).
2.2. Lentiviral Vectors and Engineered DC Generation
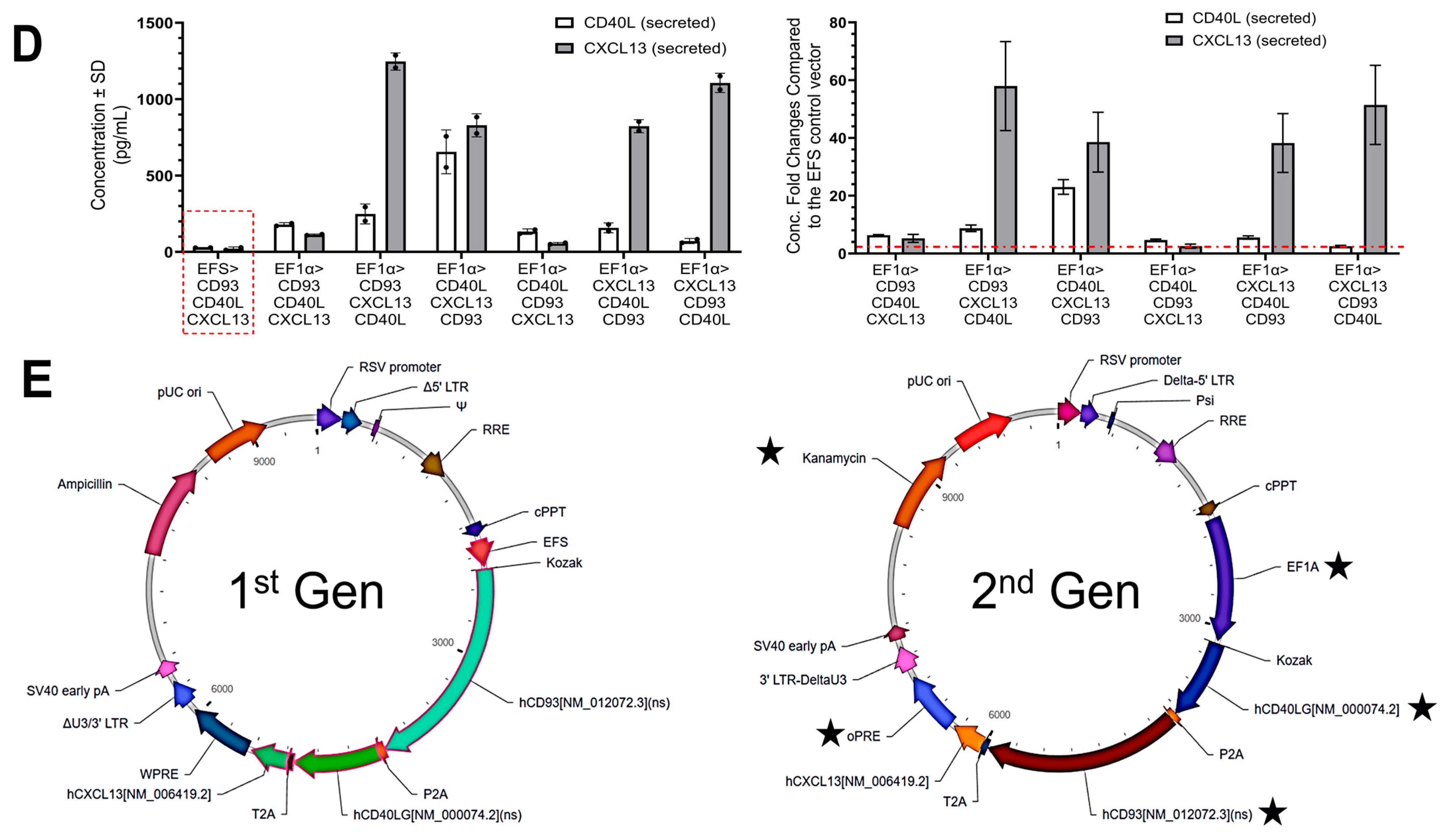
The 1st Gen LVV was constructed at VectorBuilder (Chicago, IL, USA) and included transgenes separated by P2A and T2A linkers in the order of CD93, CD40L, and CXCL13, as described in our previous publication [
12]. The transgenes were expressed under EFS promoter, and the vector contained an ampicillin resistance gene. Three major modifications were made in the development of the 2nd Gen LVV. First, promoter EF1α was selected to enhance transgene expression. Second, while the same three genes were used, their order was changed to CD40L–CD93–CXCL13. Third, the Ampicillin resistance gene was replaced by the Kanamycin gene for the antibiotic selection, enabling regulatory, safety, and practical considerations relevant to clinical applications and gene therapy. In addition, woodchuck hepatitis virus posttranscriptional regulatory element (wPRE) was replaced by an optimized and shorter version, oPRE [
21].
Human CD34+ HSCs were obtained from healthy donors under IRB-approved protocols and transduced with the optimized 2nd Gen LVV. All transductions using LVV were performed as described before in our previous publication [
12], and said engineered cells were further expanded for 120 h following the 24 h incubation with LVVs. The cells were further differentiated into monocytes and then DCs as per the previously published protocol [
12]. Experimental parameters, including culture conditions and incubation times, were consistent with those described previously [
12]. Quality control measures, including flow cytometry and quantitative PCR (qPCR), were adapted and used to characterize cell phenotype and transduction efficiency. Controls, such as untransduced (UTD) cells and cells transduced with a lentiviral stuffer-sequence, were included to confirm specificity. Lysate production from MP2 cells or tumor tissue samples, which consists of 10 freeze-dry cycles and sonication of cells, and pulsing of mature DCs (mDCs) with the lysate, was performed as described [
12]. The antigen education or pusling of the cells consists of incubating the DCs with the whole cell tumor lysate for 18 h at 18 µg per 10
6 cells. We classify the resulting DCs as Engineered DCs.
2.3. Antibodies and Flow Cytometry
Flow cytometry was utilized to perform immunophenotyping of cells. REA monoclonal antibodies (mAbs) (Miltenyi Biotec, Bergisch Gladbach, Germany), including allophycocyanin-Cy7 (APC-Cy7)-conjugated anti-CD40L (clone REA238), fluorescein isothiocyanate (FITC)- and VioBlue (VB)-conjugated anti-CD3 (clone REA613), FITC-conjugated anti-CD93 (clone REA1111), and VB-conjugated anti-CD34 (clone REA1164) were utilized to perform cell surface staining as recommended by manufacturers. Following the manufacturer’s recommendations, human anti-CD3/CD28 mAbs for T cells were used and obtained from STEMCELL Technologies. Propidium iodide or 7AAD (Miltenyi Biotec) was used to evaluate cell viability. MACSQuant Analyzer 10 (Miltenyi) or Beckman Coulter Epics XL Cytometer (Brea, CA, USA) was used to perform flow cytometry, and this data was analyzed using FlowJo software (v10.10.0).
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
All ELISAs were conducted as outlined in prior reports [
12]. CXCL13/BLC/BCA-1 ELISA Kit (ThermoFisher, Cat# EHCXCL13) and CD40 Ligand/TNFSF5 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA, Cat# DCDL40) were used following manufacturers’ recommendations to quantify secreted CXCL13 and CD40L collected from engineered cell supernatants. Human interferon-gamma (IFN-γ) ELISA Kit (ThermoFisher, Cat# KHC4021) was used to quantify IFN-γ secretion in response to activation caused by treatment with Engineered DCs in isolated ex vivo experiments. ELISA plates were analyzed using Varioskan Lux plate reader (ThermoFisher).
2.5. Mixed Lymphocyte Reaction (MLR) and T-Cell Proliferation
A one-way MLR assay was employed to measure T-cell proliferation against tumor-antigen-pulsed engineered DCs carrying the 2nd Gen vector. This protocol was adapted from Mangelinck et al. [
22] with some modifications. Cryopreserved T cells were thawed at 37 °C, transferred dropwise into T-cell medium (3% human AB (ThermoFisher Cat# J66674.03), 97% TexMACS medium (Miltenyi Biotec, Cat# 130-097-196). These cells were centrifuged at 500×
g, resuspended in 1× DPBS, and stained with 10 μM CellTrace Violet (ThermoFisher Cat# C34557). The Engineered DCs were used as stimulatory cells and co-cultured with CellTrace Violet-labeled T cells at different ratios, including 1:1, 1:2, 1:4, and 1:8 (DC: T cell) in 96-well plates. The cultures were incubated at 37 °C with 5% CO
2 for 94 h. T-cell activation was assessed by flow cytometry by evaluating the CellTrace Violet dye (dilution) as a measurement of cell proliferation. Controls for this experiment included unstimulated T cells, no antigen-pulsed DC, and CD34
+ HSCs. Analyzed using NovoExpress Software 1.6.3.
2.6. Enzyme-Linked Immunospot (ELISpot)
IFN-γ ELISpot assay was performed using Human IFN-γ ELISpot Plus kit (Mabtech, Nacka Strand, Sweden, Cat#3420-4APW-2) according to the manufacturer’s protocol. The assay was performed on a 96-well polyvinylidene fluoride-backed microtiter plate coated with capture antibody specific to IFN-γ in PBS. After incubating overnight at 4 °C, the plates were washed with PBS to remove unbound antibody and blocked with RPMI-1640 (supplemented with 10% FBS). After decanting the supernatant in the plates, the T cells and Engineered DCs were added to the wells and incubated at 37 °C in a humidified incubator for 48 h. Post-stimulation, the plates were washed five times with PBS, and 1 µg/mL of biotinylated anti-IFN-γ detection antibody was added. Plates were incubated at room temperature for 2 h and washed five times with PBS afterwards. Streptavidin-ALP diluted to 1:1000 in PBS was added to the plate and incubated at room temperature for 1 hr. After five additional washes, substrate solution was added to each well and incubated for 20 min at room temperature in the dark until spots developed. The plates were washed with distilled water to stop color development. Finally, the wells were air-dried at room temperature, spot-forming cells (SFCs) were quantified using Mabtech ASTOR ELISpot reader, and analysis was performed using Mabtech Apex™ RAWspot™ software v1.
2.7. Orthotopic Tumor Implantations and Treatment with DCs in Hu-BLT Mice
The University of California, Los Angeles (UCLA) animal facility provided the Humanized-Bone–Liver–Thymus (Hu-BLT) mice. Orthotopic implantation of pancreatic tumors using MP2 cells was performed according to previously described methods [
12,
23]. 12 Mice (3 per experimental group) were allowed to acclimate to the environment 168 h prior to the start of the experiment. Sample size was chosen to minimize costs and for statistical significance. Mice were individually identified and housed in groups of 2-to-5 in ventilated cages (type II, 16 × 19 × 35 cm, floor area = 500 cm
2) under the following controlled conditions such room temperature (22 ± 2 °C), hygrometry (55 ± 10%), Photoperiod (12:12 h light–dark cycle 7 am:7 pm). Mouse handling, care, and treatment plan was performed as described in this study [
12]. Water and food were available ad libitum. Mice were randomly divided into four groups: those receiving 2 injections of 10
6 tumor-antigen-pulsed 1st Gen Engineered DCs, those receiving 2 injections of 10
6 tumor-antigen-pulsed 2nd Gen Engineered DCs, or those receiving 2 injections of 10
6 tumor-antigen-pulsed UTD DCs via intradermal (i.d.) injection adjacent to the surgical wound near the lymph node, and one group left untreated as a control. Mice were monitored for 5 weeks post-treatment for health and tumor responses. At the study endpoint, blood and tissue samples were collected and analyzed, and metastases and ascites were evaluated as described herein [
24]. All animal housing and experimental procedures were reviewed and approved by the UCLA Animal Research Committee (ARC) and conducted in full compliance with state, federal, and institutional animal welfare guidelines. Ethic Committee Name: UCLA Animal Research Committee. Approval Code: ARC-2012-101. Approval Date: 18 December 2013.
2.8. PBMC and Splenocyte Isolation and Ex Vivo Activation Assay
PBMCs and splenocytes were isolated using the ficoll-hypaque method as described here [
25,
26]. The purity and viability of the cells were assessed using flow cytometry and trypan blue exclusion, respectively. Cells from each group were cultured for 120 h in the presence of anti-CD3/28. Supernatants were collected on day (D) 5 by centrifugation at 575×
g for 5 min followed by aspirating the supernatant from the pelleted cells, and ELISA was conducted to analyze IFN-γ secretion. IFN-γ levels (pg/mL) are presented as mean ± standard deviation (SD).
2.9. Orthotopic Implantation of Luciferase-Expressing MP2 Tumors in CD34+ NCG Humanized Mice, Treatment Regimen with DCs, and In Vivo Live Imaging of Tumor Progression
Experiments were carried out with female NOD-Prkdc
em26Cd52Il2rg
em26Cd22/GptCrl immunodeficient (NCG) mice from Charles River Laboratories (Wilmington, MA, USA). Mice were humanized by using CD34
+ HSCs from two donors that were isolated from human cord blood samples (TransCure, Farmers Branch, TX, USA). Only mice with an above 25% humanization rate (hCD45/totalCD45) were used for the study. The HLA-A2 status of the donors was recorded. Orthotopic injection of luciferase-encoded PC cells was performed as described here [
27]. Six Mice (three per experimental group) were allowed to acclimate to the environment 168 h prior to the start of the experiment. Mice were individually identified and housed by groups of 2-to-5 in ventilated cages (type II, 16 × 19 × 35 cm, floor area = 500 cm
2) under the following controlled conditions such as room temperature (22 ± 2 °C), hygrometry (55 ± 10%), photoperiod (12:12 h light–dark cycle 7 am:7 pm), and water and food available ad libitum.
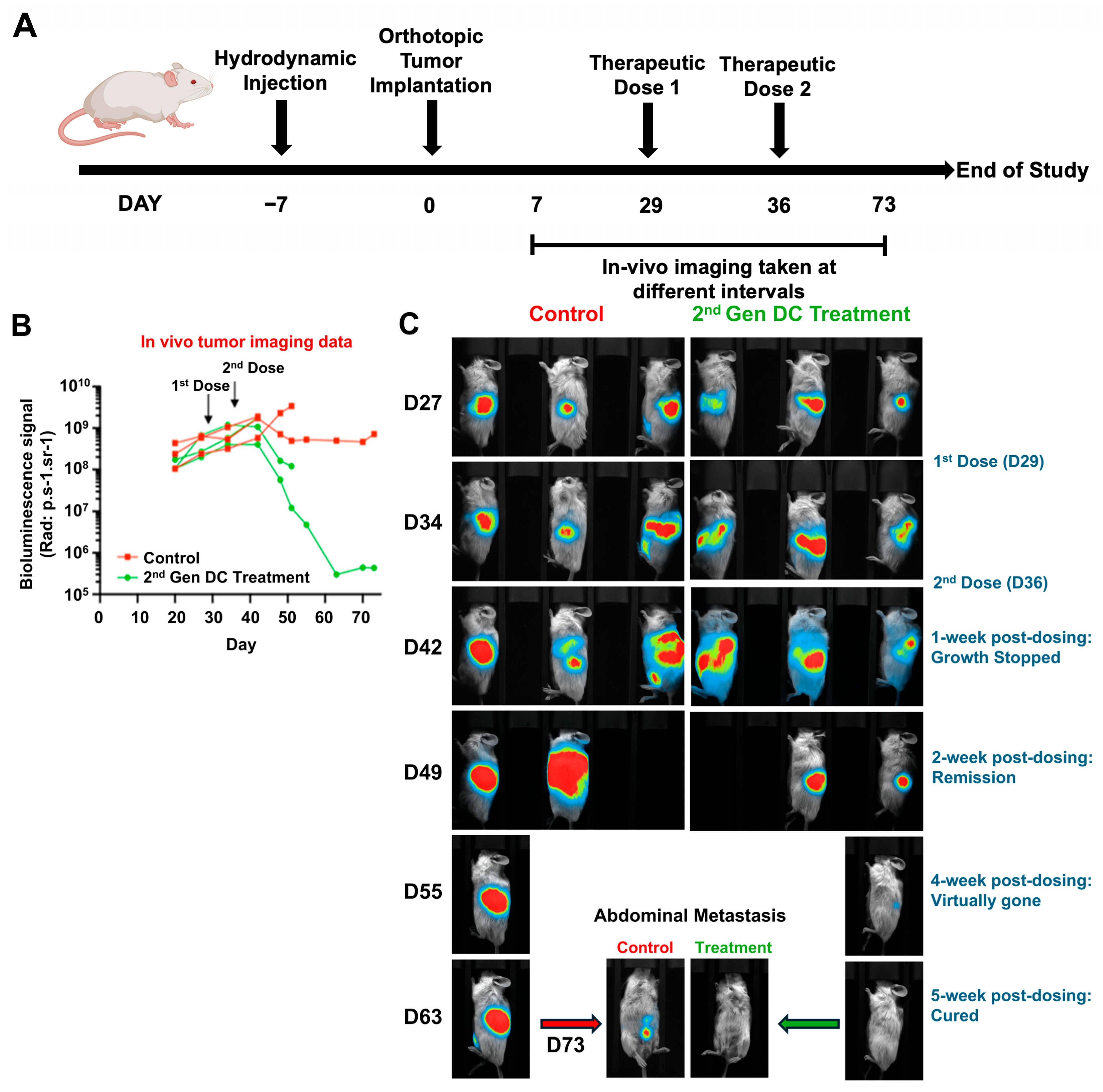
Dosing of 2nd Gen Engineered DC treatment was performed post intra-pancreatic tumor implantations on D29 and repeated on D36; a total of two doses/group. Treatment consisted of 100 μL (2 × 50 μL) intradermal injections at a site close to lymph nodes on the right flank of the mouse. Mice were treated with 106 cells for each treatment. Progression of luciferase-expressing tumors was monitored by in vivo imaging using a Vilber Newton 7.0. Prior to each imaging timepoint, mice were anesthetized with isoflurane and injected intraperitoneally with 150 mg/kg luciferin. All procedures described in this study have been reviewed and approved by the Local Ethics Committee for Animal Experimentation (CELEAG) under compliance with the Institutional Animal Care and Use Committee (IACUC). The Ethic Committee Name: CELEAG-TCS. Approval Code: A7418324. Approval Date: 9 December 2021. No data regarding animals was excluded.
2.10. Statistical Analyses
Statistical analysis of the results was performed by using Student’s t test (two groups), ANOVA (analysis of variance), and/or Tukey’s test (three or more groups) (****—p value < 0.0001, ***—p value < 0.001, **—p value < 0.001–0.01, and *—p value < 0.01–0.05). The Mann–Whitney U test was performed for non-normal data for the comparison of two groups. For comparing multiple groups, bonferroni correction was utilized and adjusted α = 0.05/2 = 0.025. Statistical analysis was performed using GraphPad Prism-10.3.0.507 software.
4. Discussion
In the present study, we optimized the lentiviral construct by altering its properties like promoters, post-transcriptional regulatory element, transgene orientation, and antibiotic resistance to evaluate the therapeutic potential of 1st and 2nd Gen Engineered DCs in orthotopic humanized pancreatic tumor models, including early- and late-stage disease settings. The 2nd Gen vector construct was determined by evaluating the varying expression of the transgenes and potential cytotoxic effects while also considering lentiviral titer and protein secretion (
Figure 1). Ultimately, the vector with CD40L in the 1st ORF, CD93 in the 2nd ORF, and CXCL13 in the 3rd ORF proved to increase transgene expression more than the original construct without sacrificing lentiviral titer and cell viability. Furthermore, protein secretion of CD40L and CXCL13 was greater compared to the 1st Gen vector. After selecting the new vector, its therapeutic efficacy was evaluated in vitro and then compared head-to-head in humanized mouse models.
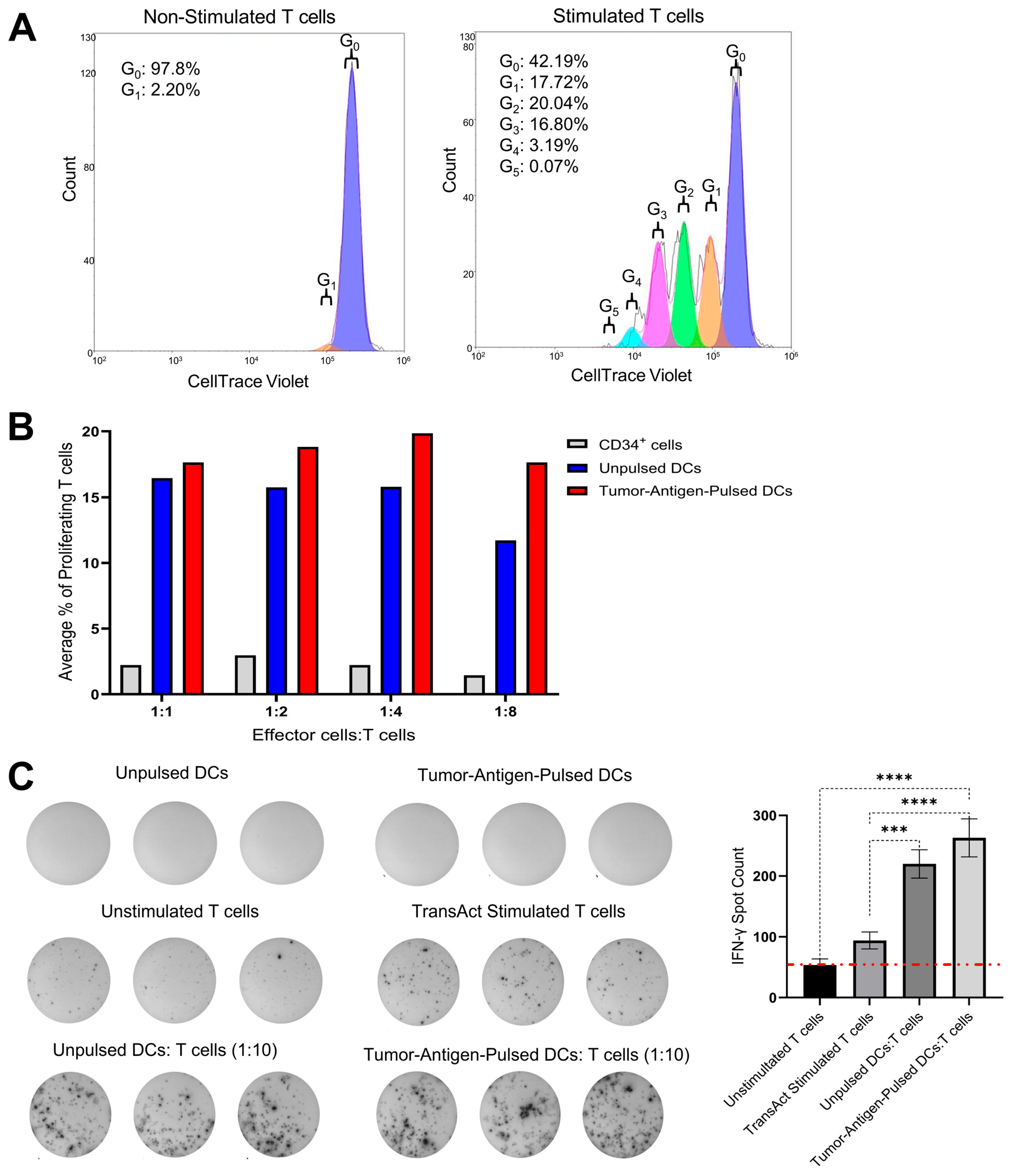
To evaluate the immunostimulatory potential of our Engineered DCs carrying the 2nd Gen vector, we conducted MLR and ELISpot assays (
Figure 2). In the MLR assay, T cells co-cultured with Engineered DCs at decreasing DC:T cell ratios exhibited a dose-dependent increase in cell proliferation, indicating one individual DC was capable of greater T-cell activation. These findings suggest that our Engineered DCs effectively stimulate T-cell responses in a ratio-dependent manner, indicating and confirming previously published data that a single DC can engage with and activate as many as 10 T cells simultaneously. This interaction is crucial for initiating and modulating immune responses for a proper anti-tumor immunity [
28,
29]. At the lowest DC:T cell ratio (1:8), the difference in total proliferation T cells in tumor-antigen-pulsed Engineered DCs relative to unpulsed Engineered DCs began to exhibit larger differences, suggesting the need for exploration in lower ratios. Future studies will explore even lower DC:T cell ratios to further characterize the proliferative response and determine the optimal conditions for T-cell activation. Although this limitation suggests this observational data should be taken lightly, these results further exemplify DC’s capacity to stimulate multiple cells. Similarly, the ELISpot assay revealed that tumor-antigen-pulsed Engineered DCs induced more IFN-γ-secreting T cells compared to unpulsed DCs. T-cell IFN-γ-secretion was observed in the unpulsed Engineered DC co-culture, which may be caused by an allogeneic response. The tumor-antigen-pulsed optimized DCs led to higher IFN-γ-secretion, suggesting that the antigen-specific response combined with the immunomodulating effects of the three transgenes [
30]. Although the difference between pulsed and unpulsed DCs was not statistically significant, this trend underscores the robust T-cell-stimulatory capacity of our tumor-antigen-pulsed DCs transduced with the 2nd Gen vector. These results collectively highlight the potential of our Engineered DCs as potent inducers of T cell responses, warranting further investigation into their application in animal models.
MP2 lysate-pulsed 1st and 2nd Gen DCs significantly reduced tumor burden compared to untreated or UTD DC-treated mice, highlighting the capacity of antigen-loaded DCs engineered to overexpress CD93, CD40L, and CXCL13 to drive robust anti-tumor immunity in the Hu-BLT model. Across three independent experiments, the 1st Gen and 2nd Gen Engineered DCs achieved comparable suppression of primary tumor growth (76.5% and 78.3% reduction in volume, respectively) and tumor mass (65.7% and 65.9% reduction in weight, respectively). The partial anti-tumor effect seen with UTD DCs (~32.6% tumor volume reduction) suggests that allogeneic DCs may exert baseline immune-stimulatory effects independent of transgene expression (
Figure 3B). The expression of three transgenes in the Engineered DCs leads to significant tumor reduction in both generations of Engineered DCs, showcasing the importance and role of the immune-modulating properties of CD40L, CD93, and CXCL13.
In our preliminary assessment, a key distinction emerged in the context of metastatic disease control. While the 1st Gen Engineered DCs inhibited most metastatic events, liver metastases remained detectable in some animals. In contrast, the optimized 2nd Gen Engineered DCs not only suppressed primary pancreatic tumor growth but also completely prevented metastatic spread to the liver, stomach, and intestine (
Figure 3D). This superior anti-metastatic activity is likely attributable to the combinatorial expression of CD40L, CD93, and CXCL13 and the promoter optimization of the LVV, which may enhance antigen presentation, T cell recruitment, and improved immune modulation in the tumor microenvironment. This is supported by the observation that 2nd Gen Engineered DC treatment induced higher IFN-γ secretion from PBMCs upon stimulation, indicating more potent systemic immune activation.
We further extended these findings to a late-stage disease setting using MP2-Luc tumor cells to enable real-time, non-invasive monitoring of tumor growth and metastatic spread (
Figure 4A–B). In this model, treatment with 2nd Gen Engineered DCs initiated on D29 after tumor implantation suppressed tumor progression within one week of the second dose (D36), followed by a trend toward tumor regression (
Figure 4B–C). Remarkably, by D63–D73, the single surviving mouse in the treatment arm exhibited complete elimination of luciferase signal, indicating eradication of both primary and metastatic lesions, whereas control mice showed progressive disease with widespread metastases. These results demonstrate that 2nd Gen Engineered DC therapy retains efficacy even against established advanced-stage tumors and may induce durable tumor clearance. These preliminary observational results are highly encouraging and report the effectiveness of the gene-modified allogeneic DCs in treating pancreatic tumors, one of the devastating cancer types.
Our findings are consistent with the results of an already commercially available allogeneic DC vaccine (DCP-001) for acute myeloid leukemia (AML) treatment [
31]. In the clinical trial, allogeneic DCs directly presented tumor antigens on allogeneic MHC class I and II to recipient T cells, leveraging the alloimmune response as a natural adjuvant to enhance CD8
+ and CD4
+ T cell activation. Additionally, upon rapid clearance by the recipient’s immune system, dying allogeneic DCs release antigens that were taken up by host APCs for indirect presentation on matched MHC molecules, with cross-presentation by host DCs further driving cytotoxic T cell responses against tumor cells. The MHC mismatch itself promoted a pro-inflammatory microenvironment, amplifying T cell priming via cytokines such as IFN-γ and co-stimulatory signals (CD80/CD86), resulting in robust anti-tumor immunity, as evidenced by 70% of patients in NCT03697707 showing WT1-specific T cell responses and 45% achieving MRD-negative status, with minimal toxicities like GVHD.
LVV is commonly used as a gene delivery approach in immunotherapies such as CAR-T products. CAR-T is revolutionary in treating cancers; however, it faces limitations, including variable patient responses, immune-related adverse events like colitis or pneumonitis, and high costs limiting accessibility. LVVs are effective as a gene delivery cargo for ex vivo gene therapy but are hindered by risks of insertional mutagenesis. Our DC vaccine can address the above safety and cost issues. The allogeneic DC is capable of being manufactured at a scalable and significantly lower cost per patient. DC vaccines have been demonstrated to be safe by previous clinical studies, and the allogeneic nature will ensure the LVV gene-edited DCs have a short life span in vivo [
17,
32,
33].
Although encouraging, these findings should be interpreted with caution. The Hu-BLT mouse model provides a partially humanized immune system but does not fully replicate the complexity, heterogeneity, and immune suppression observed in human PC [
34]. Additionally, the small cohort sizes, particularly in the late-stage treatment study, limit statistical power and the ability to capture rare outcomes or treatment-associated variability. Additional studies with larger cohorts of mice are needed to fully evaluate the effectiveness of the 2nd Gen Engineered DCs. In this brief report, we are presenting the preliminary observational data that the absence of metastases in the 2nd Gen group is striking; however, the molecular and cellular mechanisms driving this effect remain to be fully elucidated. Additional studies with larger cohorts of mice and immune cell analyses at multiple different timepoints are needed to completely understand the phenomenon.
These studies include validation of current findings in larger and more genetically diverse tumor models, including patient-derived xenografts, coupled with immune cell profiling to identify effector cell subsets, cytokine signaling, associated with metastatic suppression. Detailed mechanistic studies could provide insights into the individual/relative contribution of each transgene (CD40L, CD93, and CXCL13) and how the optimization of their expression leads to therapeutic efficacy.