Nineteen-Year Evidence on Measles–Mumps–Rubella Immunization in Mexico: Programmatic Lessons and Policy Implications
Abstract
1. Introduction
2. Material and Methods
- Theoretical Target Population: the estimated number of individuals eligible for vaccination, calculated based on each vaccine’s specific indications and the population assigned to the corresponding healthcare institution.
- Annual Procurement and Year-on-Year Change: total number of vaccine doses procured each year, along with the percentage change compared to the previous year.
- PUR (Procurement-to-Target Ratio): the proportion of vaccine doses acquired relative to the theoretical target population.
- APP (Application-to-Procurement Ratio): the proportion of administered doses compared to the total number of doses acquired.
- COV (Coverage Rate): the proportion of administered doses in relation to the theoretical target population.
- Total theoretical target population: the combined number of individuals aged 12 months and 6 years for all relevant years, along with those aged 18 months for cohorts born in 2021 and later, following MMR vaccination guidelines.
- IMSS: total theoretical target population × annual proportion of the population affiliated with IMSS.
- ISSSTE: total theoretical target population × annual proportion of the population affiliated with ISSSTE.
- SSA: total theoretical target population × annual proportion of the population covered by SSA.
- %PUR (Procurement-to-Target Ratio): (Doses acquired/Theoretical target population) × 100
- %APP (Application-to-Procurement Ratio): (Doses administered/Doses acquired) × 100
- %COV (Coverage Rate): (Doses administered/Theoretical target population) × 100
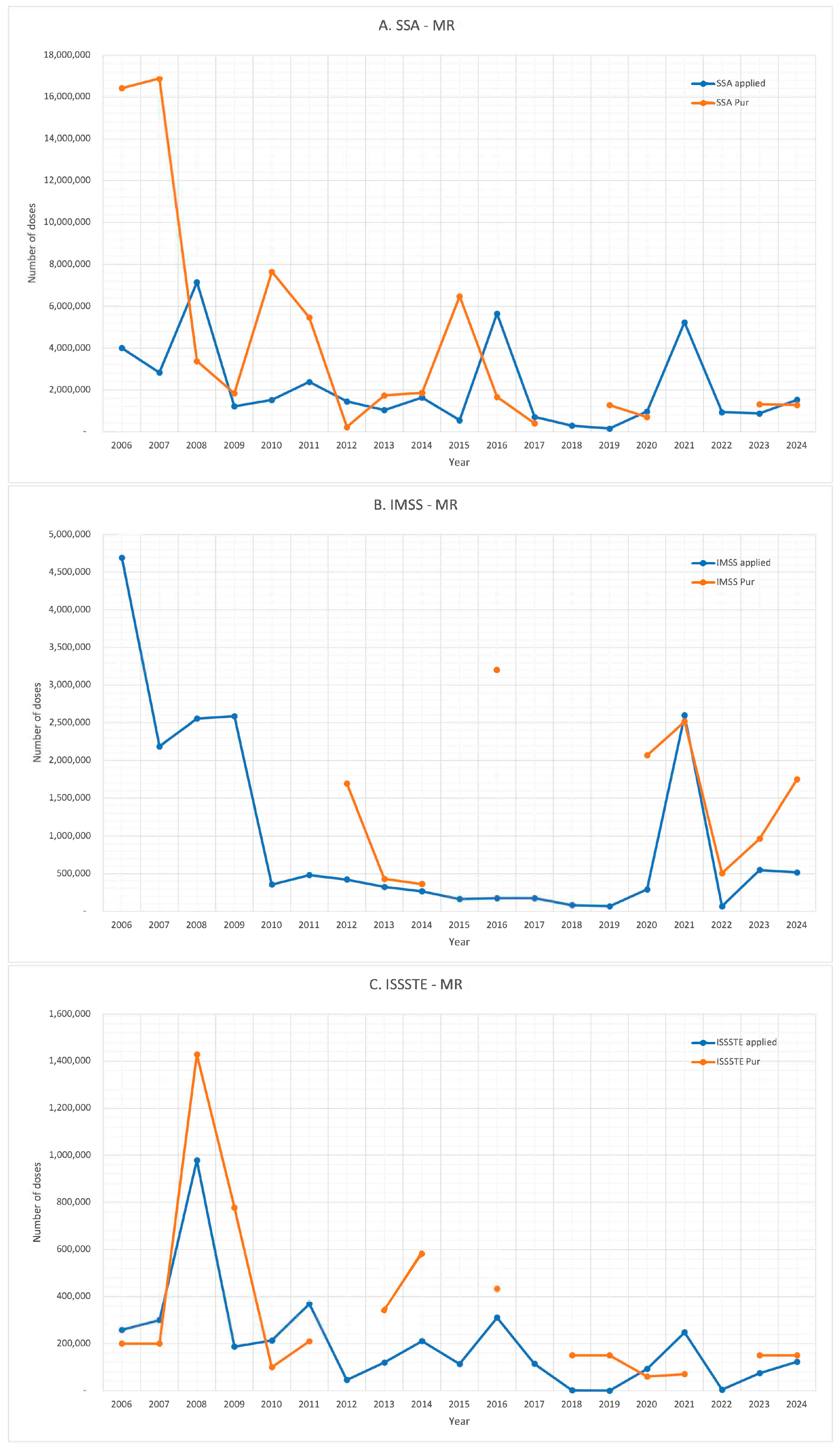
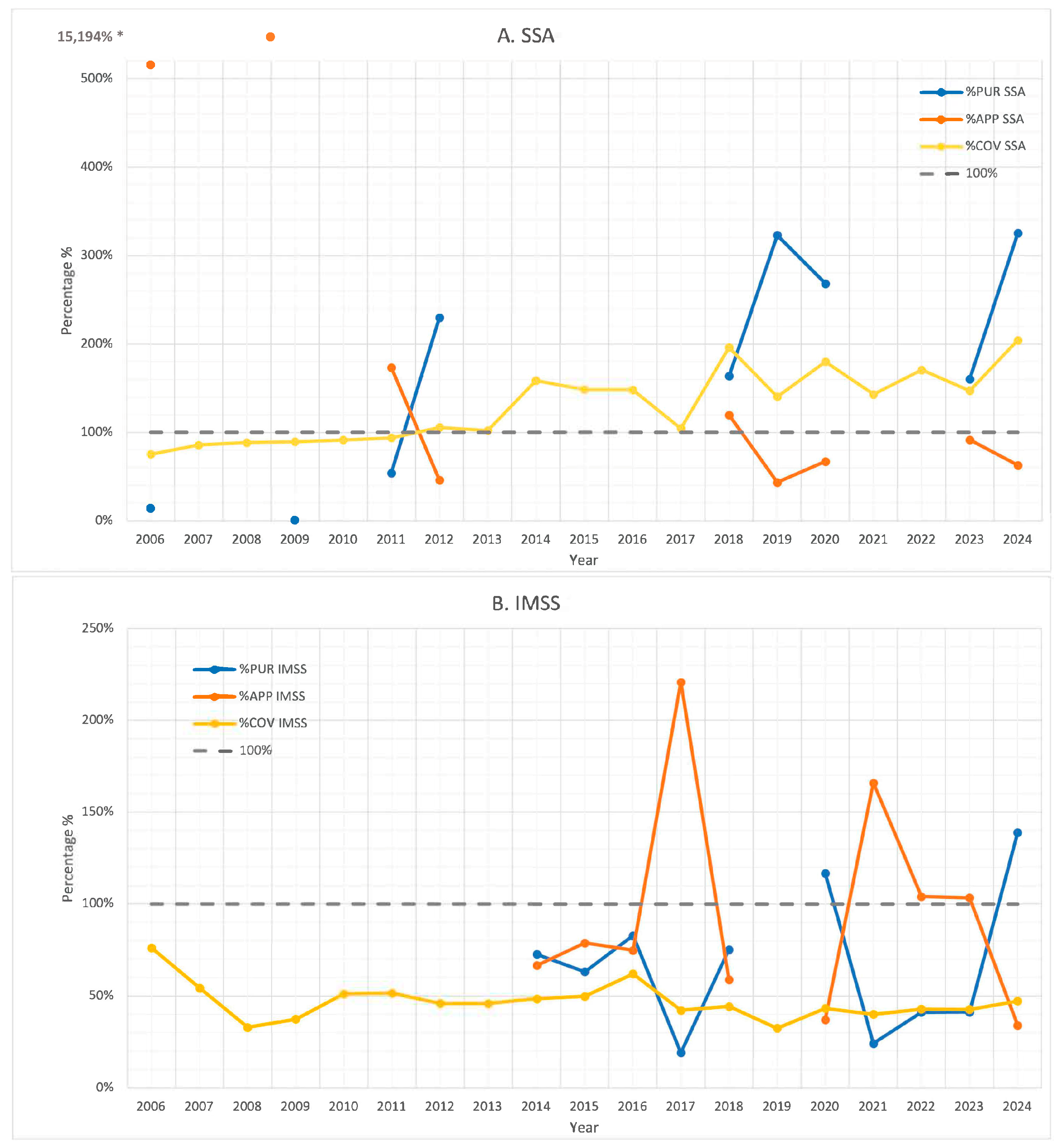
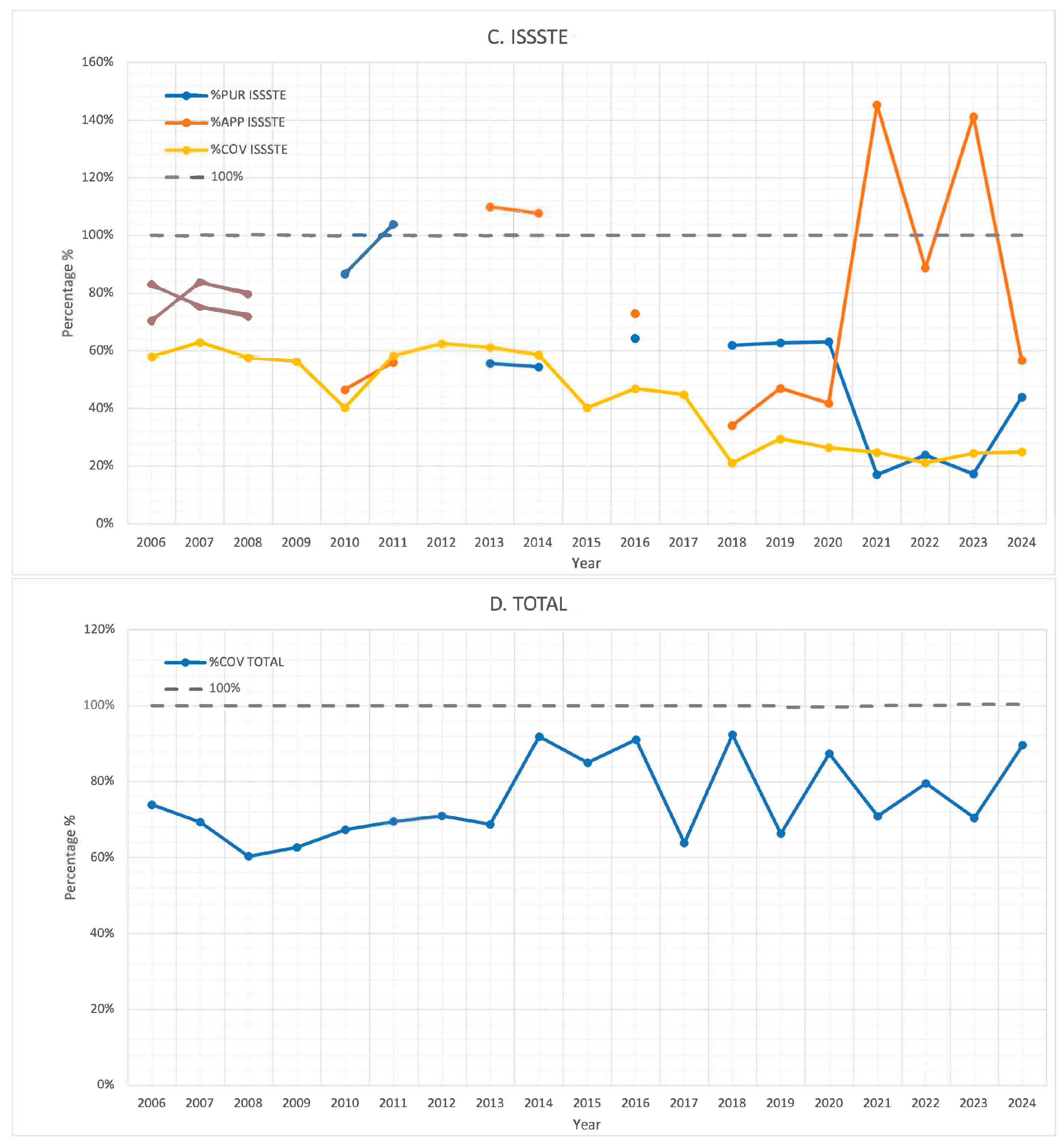
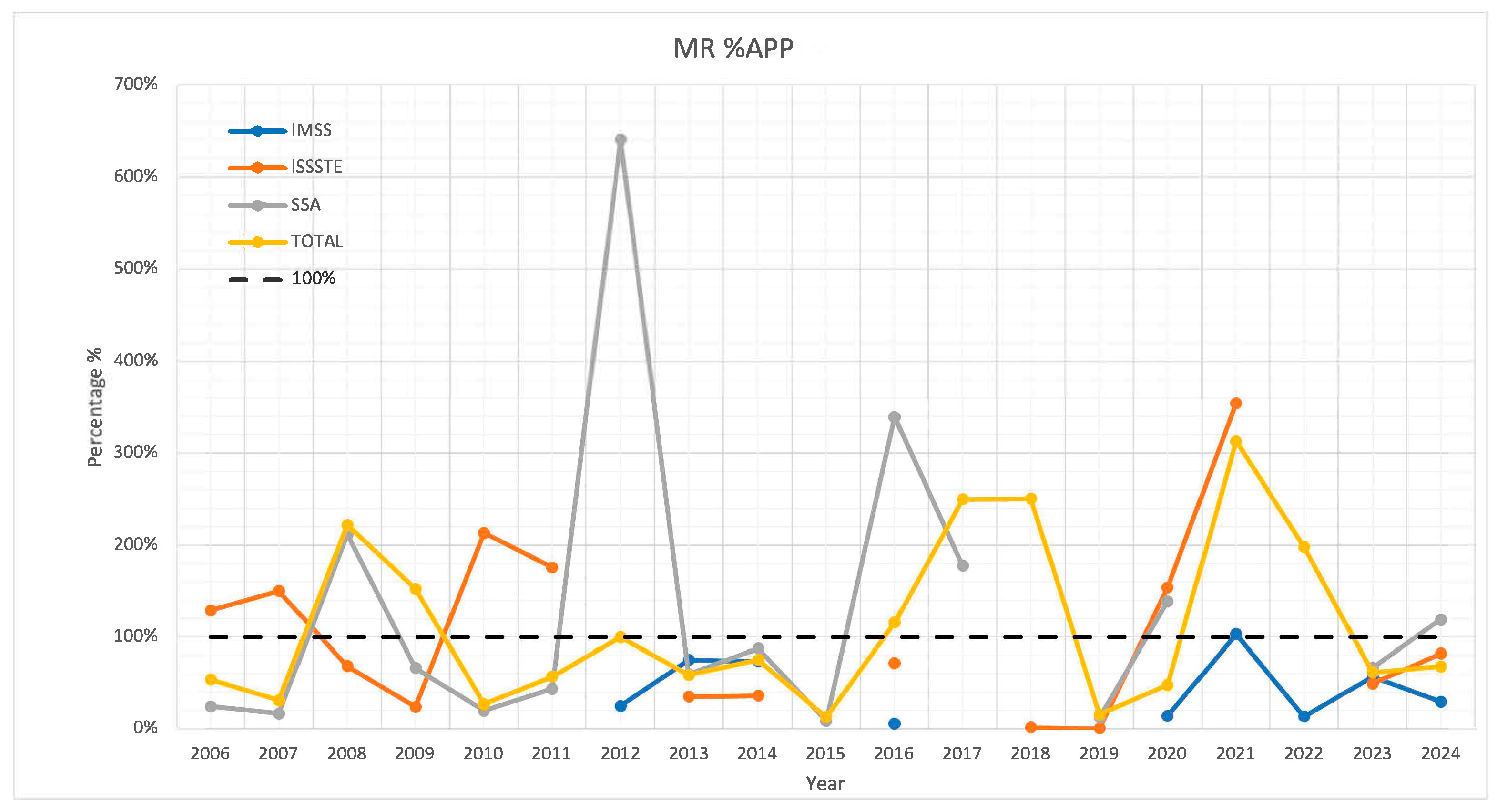
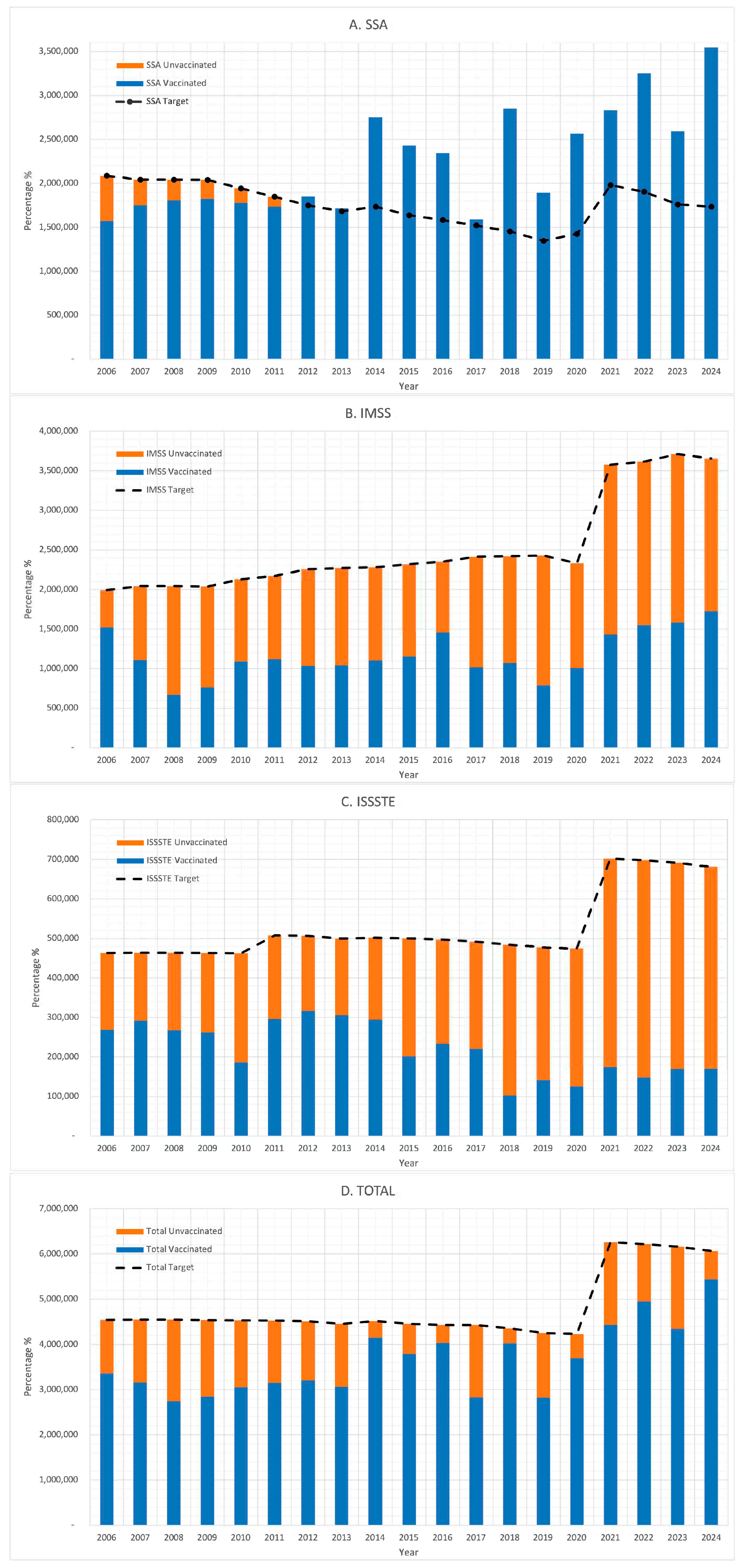
3. Results
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. History of Measles Vaccination; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/history-of-measles-vaccination (accessed on 23 April 2025).
- The College of Physicians of Philadelphia. Mumps. History of Vaccines. 17 April 2022. Available online: https://historyofvaccines.org/diseases/mumps (accessed on 23 April 2025).
- Lanzieri, T.; Haber, P.; Icenogle, J.P.; Patel, M. Chapter 20: Rubella. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-20-rubella.html (accessed on 23 April 2025).
- Secretaría de Salud. Manual de Vacunación 2021; Secretaría de Salud: Mexico City, México, 2021. Available online: https://drive.google.com/file/d/19am3cMC-88a28QxUjb1OO34vO_jLkFh3/view (accessed on 24 April 2025).
- Pan American Health Organization. Elimination of Measles and Rubella in the Americas [Special Issue]. Rev Panam Salud Publica. 2024. Available online: https://journal.paho.org/en/special-issues/elimination-measles-and-rubella-americas (accessed on 29 April 2025).
- Román-Pedroza, J.F.; Cruz-Ramírez, E.; Landín-Martínez, K.E.; Salas-García, M.; López-Ortiz, E.; Ramírez-González, J.E.; López-Martínez, I.; Díaz-Quiñonez, J.A. Diagnostic algorithm for the confirmation of cases of measles and rubella in Mexico. Gac. Med. Mex. 2019, 155, 492–495. [Google Scholar] [CrossRef]
- World Health Organization. Measles Vaccination Coverage; WHO Immunization Data Portal: Geneva, Switzerland, 2024; Available online: https://immunizationdata.who.int/global/wiise-detail-page/measles-vaccination-coverage?CODE=MEX&ANTIGEN=MCV1+MCV2&YEAR= (accessed on 29 April 2025).
- Mongua-Rodríguez, N.; Delgado-Sánchez, G.; Ferreira-Guerrero, E.; Ferreyra-Reyes, L.; Martínez-Hernández, M.; Canizales-Quintero, S.; Téllez-Vázquez, N.A.; García-García, L. Vaccination in children under five years of age. Salud Publica Mex. 2024, 66, 368–380. [Google Scholar] [CrossRef]
- Mongua-Rodríguez, N.; Delgado-Sánchez, G.; Ferreira-Guerrero, E.; Ferreyra-Reyes, L.; Martínez-Hernández, M.; Canizales-Quintero, S.; Téllez-Vázquez, N.A.; García-García, L. Vaccination coverage in children and adolescents in Mexico. Salud Publica Mex. 2023, 65 (Suppl. 1), s23–s33. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Cuevas-Nasu, L.; Romero-Martínez, M.; Gómez-Acosta, L.M.; Gaona-Pineda, E.B.; Méndez-Gómez-Humarán, I.; Mendoza-Alvarado, L.R.; Rivera-Dommarco, J.A. National Health and Nutrition Survey 2018–19: National Results; National Institute of Public Health: Cuernavaca, Mexico, 2020; Available online: https://ensanut.insp.mx/encuestas/ensanut2018/doctos/informes/ensanut_2018_informe_final.pdf (accessed on 29 April 2025).
- Gutiérrez, J.P.; Rivera-Dommarco, J.; Shamah-Levy, T.; Villalpando-Hernández, S.; Franco, A.; Cuevas-Nasu, L.; Romero-Martínez, M.; Hernández-Ávila, M. National Health and Nutrition Survey 2012: National Results; National Institute of Public Health: Cuernavaca, Mexico, 2012; Available online: https://ensanut.insp.mx/encuestas/ensanut2012/doctos/informes/ENSANUT2012ResultadosNacionales.pdf (accessed on 29 April 2025).
- Olaiz-Fernández, G.; Rivera-Dommarco, J.; Shamah-Levy, T.; Rojas, R.; Villalpando-Hernández, S.; Hernández-Ávila, M.; Sepúlveda-Amor, J. National Health and Nutrition Survey 2006; National Institute of Public Health: Cuernavaca, Mexico, 2006; Available online: https://ensanut.insp.mx/encuestas/ensanut2006/doctos/informes/ensanut2006.pdf (accessed on 24 April 2025).
- Secretaría de Salud. Epidemiological Bulletin: National Epidemiological Surveillance System—Single Information System; Dirección General de Epidemiología: Mexico City, Mexico, 2025. Available online: https://www.gob.mx/salud/documentos/boletinepidemiologico-sistema-nacional-de-vigilancia-epidemiologica-sistema-unico-de-informacion-387843 (accessed on 29 April 2025).
- Secretaría de Salud. Daily Report on the Measles Outbreak in Mexico 2025; Government of Mexico: Mexico City, Mexico, 2025. Available online: https://www.gob.mx/salud/documentos/informe-diario-del-brote-de-sarampion-en-mexico-2025 (accessed on 25 October 2025).
- Romero-Feregrino, R.; Romero-Cabello, R.; Rodríguez-León, M.A.; Rocha-Rocha, V.M.; Romero-Feregrino, R.; Muñoz-Cordero, B. Report of the Influenza Vaccination Program in Mexico (2006–2022) and proposals for its improvement. Vaccines 2023, 11, 1686. [Google Scholar] [CrossRef]
- Romero-Feregrino, R.; Romero-Cabello, R.; Rodríguez-León, M.A.; Romero-Feregrino, R.; Muñoz-Cordero, B.; Aguilar-Feregrino, J.I. Report of 16 years of the BCG vaccine under the Expanded Program on Immunizations in Mexico (2006–2021). Vaccines 2023, 11, 337. [Google Scholar] [CrossRef]
- Instituto Mexicano del Seguro Social (IMSS). Chapter II—Entitled Population. In Statistical Yearbook 2023; IMSS: Mexico City, Mexico, 2024. Available online: https://www.imss.gob.mx/conoce-al-imss/memoria-estadistica-2024 (accessed on 30 April 2025).
- Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE). Preventive Health Care—Seasonal Influenza Vaccine Doses Administered by State Representation, Age Group, and Entitlement Status. In Statistical Yearbook 2023; ISSSTE: Mexico City, Mexico, 2024. Available online: https://www.issste.gob.mx/datosabiertos/anuarios/anuarios2023.html (accessed on 30 April 2025).
- Consejo Nacional de Población (CONAPO). Population Projections of Mexico and the Federal Entities, 2020–2070; CONAPO: Mexico City, Mexico, 2023. Available online: https://www.gob.mx/conapo/documentos/bases-de-datos-de-la-conciliacion-demografica-1950-a-2019-y-proyecciones-de-la-poblacion-de-mexico-2020-a-2070 (accessed on 30 April 2025).
- Consejo Nacional de Población (CONAPO). Population projections of Mexico and the federal entities, 2020–2070 [Internet]; Government of Mexico: Mexico City, Mexico, 2023. Available online: https://datos.gob.mx/dataset/proyecciones-de-poblacion/resource/7783e6e6-f1be-4b81-b59b-ebd039837104 (accessed on 30 April 2025).
- Instituto Nacional de Transparencia, Acceso a la Información y Protección de Datos Personales (INAI). Response to Request for Information to ISSSTE. Folio No. 0063700269721; Plataforma Nacional de Transparencia: Mexico city, Mexico, May 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA-CENSIA. Folio No. 1200900005421; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to ISSSTE. Folio No. 0063700269921; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA-CENSIA. Folio No. 1200900005521; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to Secretaría de Salud. Folio No. 0001200257221; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS. Folio No. 0064101313621; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS. Folio No. 0064101314321; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to Secretaría de Salud. Folio No. 0001200257321; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA-CENSIA. Folio No. 1200900009821; Plataforma Nacional de Transparencia: Mexico city, Mexico, September 2021. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA-CENSIA. Folio No. 330006522000148; Plataforma Nacional de Transparencia: Mexico city, Mexico, June 2022. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS. Folio No. 330018022022911; Plataforma Nacional de Transparencia: Mexico city, Mexico, September 2022. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS. Folio No. 330018023009330; Plataforma Nacional de Transparencia: Mexico city, Mexico, March 2023. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA-CENSIA. Folio No. 330006523000062; Plataforma Nacional de Transparencia: Mexico city, Mexico, March 2023. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to ISSSTE. Folio No. 330017123002081; Plataforma Nacional de Transparencia: Mexico city, Mexico, March 2023. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS. Folio No. 330018024031763; Plataforma Nacional de Transparencia: Mexico city, Mexico, October 2024. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to SSA. Folio No. 330026924002659; Plataforma Nacional de Transparencia: Mexico city, Mexico, October 2024. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to ISSSTE. Folio No. 330017124007467; Plataforma Nacional de Transparencia: Mexico city, Mexico, October 2023. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- INAI. Response to Request for Information to IMSS-Bienestar. Folio No. 333021324001553; Plataforma Nacional de Transparencia: Mexico city, Mexico, October 2024. Available online: https://www.plataformadetransparencia.org.mx (accessed on 30 April 2025).
- Instituto Mexicano del Seguro Social (IMSS). Chapter VI—Public Health. In Statistical Yearbook 2023; IMSS: Mexico City, Mexico city, Mexico, 2023. Available online: https://www.imss.gob.mx/conoce-al-imss/memoria-estadistica-2023 (accessed on 30 April 2025).
- Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE). Chapter 17—Preventive Health Care. In Statistical Yearbook 2006–2023; ISSSTE: Mexico City, Mexico, 2023. Available online: https://www.gob.mx/issste/documentos/anuarios-estadisticos (accessed on 30 April 2025).
- Secretaría de Salud. Dirección General de Información en Salud. Dynamic Data Cubes; Secretaría de Salud: Mexico City, Mexico, 2024. Available online: https://sinba.salud.gob.mx/CubosDinamicos (accessed on 30 April 2025).
- Hernández-Ávila, M.; Palacio-Mejía, L.S.; Hernández-Ávila, J.E.; Charvel, S. Vaccination in Mexico: Imprecise coverages and deficiency in the follow-up of children with incomplete immunization. Salud Publica Mex. 2020, 62, 215–224. [Google Scholar] [CrossRef]
- Ríos-Blancas, M.J.; Lamadrid-Figueroa, H.; Betancourt-Cravioto, M.; Lozano, R. Vaccination coverage estimation in Mexico in children under five years old: Trends and associated factors. PLoS ONE 2021, 16, e0250172. [Google Scholar] [CrossRef]
- Carnalla, M.; Gaspar-Castillo, C.; Dimas-González, J.; Aparicio-Antonio, R.; Justo-Berrueta, P.S.; López-Martínez, I.; Shamah-Levy, T.; Lazcano-Ponce, E.; Barrientos-Gutiérrez, T.; Alpuche-Aranda, C.M.; et al. A population-based measles serosurvey in Mexico: Implications for re-emergence. Vaccine 2025, 51, 126886. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Epidemiología. Boletín informativo No. 29. Situación Epidemiológica de Enfermedades Prevenibles por Vacunación en México, SE 42.24 de Octubre de 2025; Secretaría de Salud: Ciudad de México, Mexico, 2025. Available online: https://www.gob.mx/cms/uploads/attachment/file/1032160/Boletin_informativo-29_EPV_SE42_20251024.pdf (accessed on 27 October 2025).
- Griffore, K.A.; Bowra, A.; Guilcher, S.J.T.; Kohler, J. Corruption risks in health procurement during the COVID-19 pandemic and anti-corruption, transparency and accountability (ACTA) mechanisms to reduce these risks: A rapid review. Glob. Health 2023, 19, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farzanegan, M.R.; Hofmann, H.P. Effect of public corruption on the COVID-19 immunization progress. Sci. Rep. 2021, 11, 23423. [Google Scholar] [CrossRef]
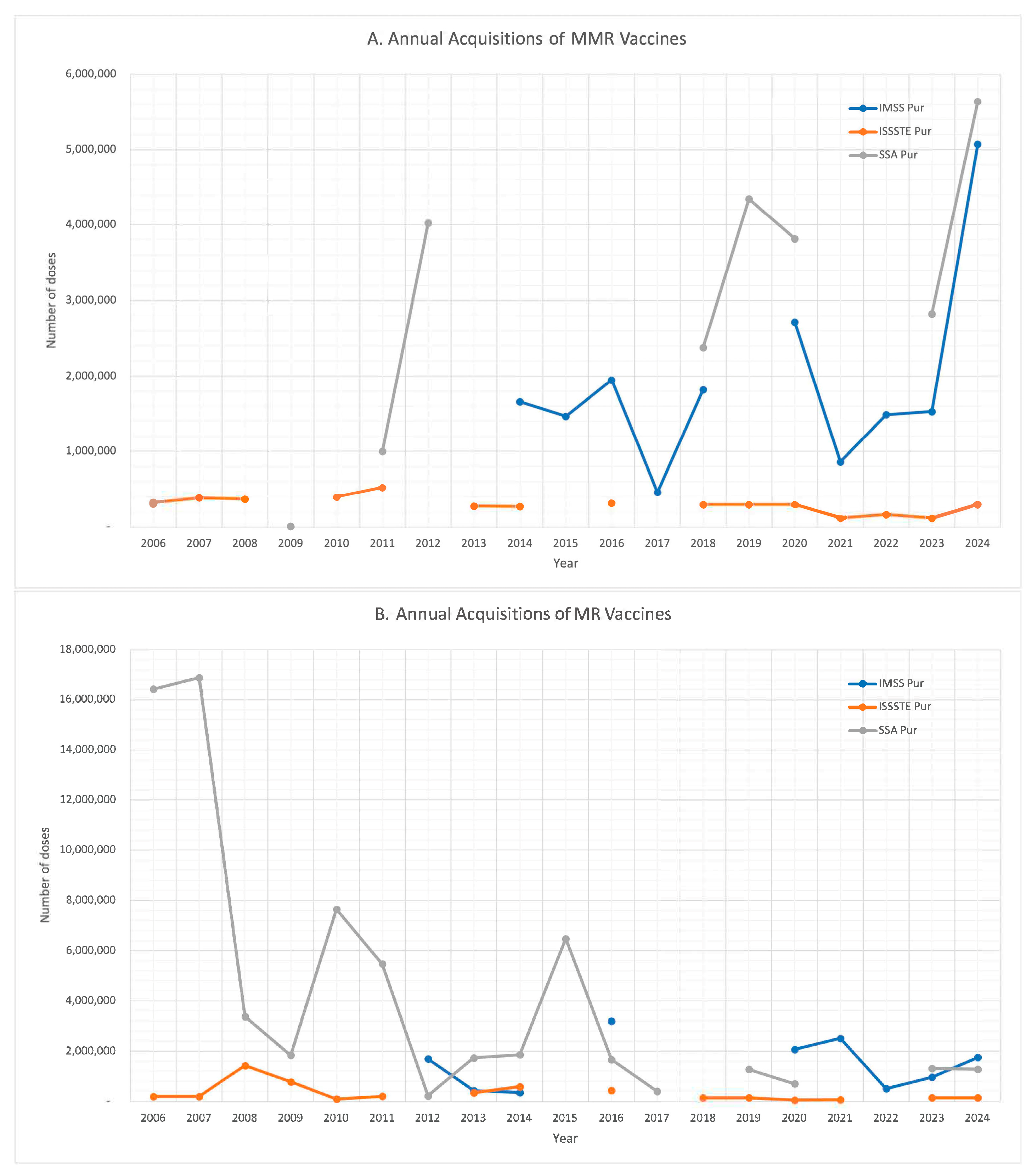
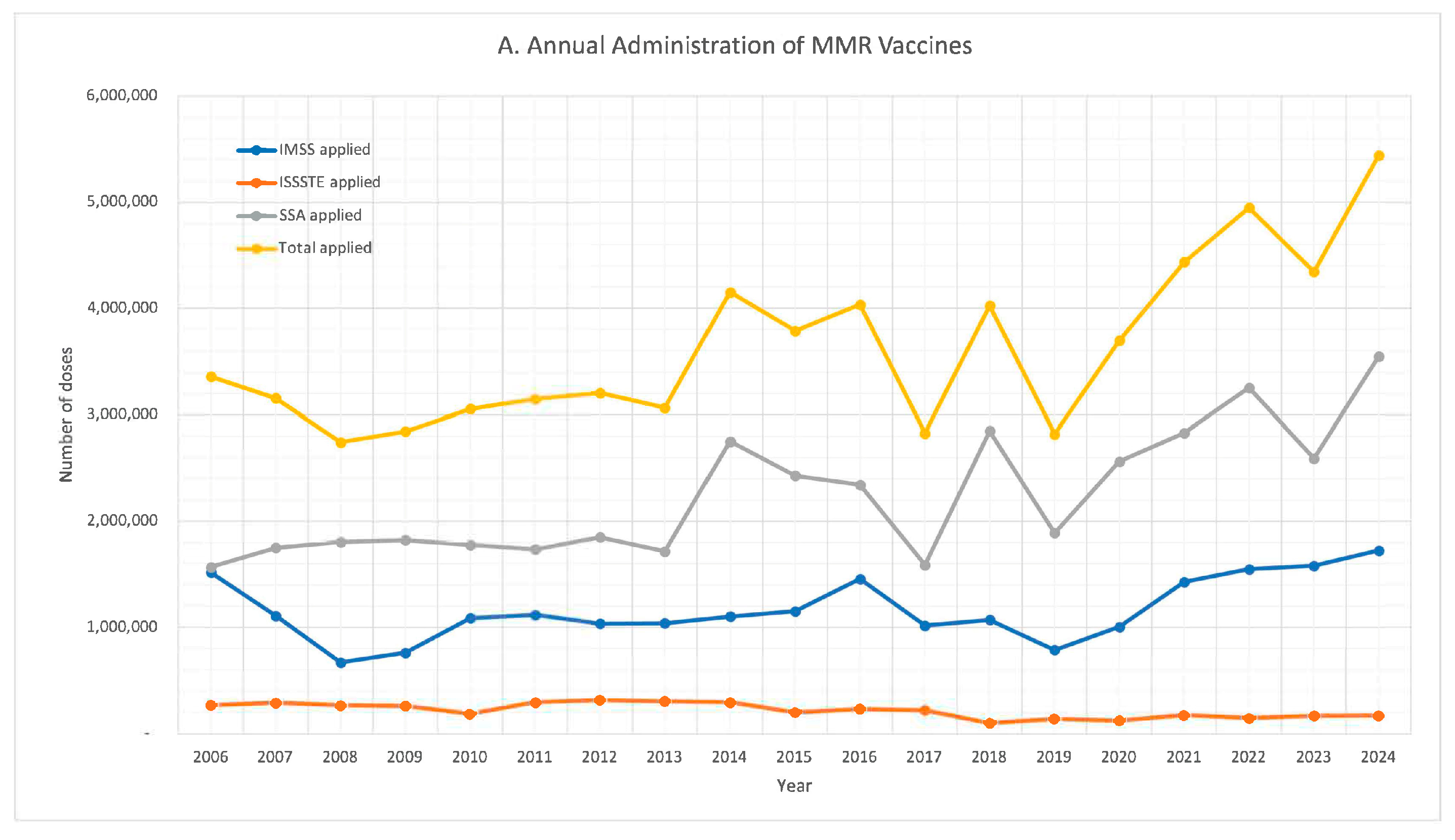
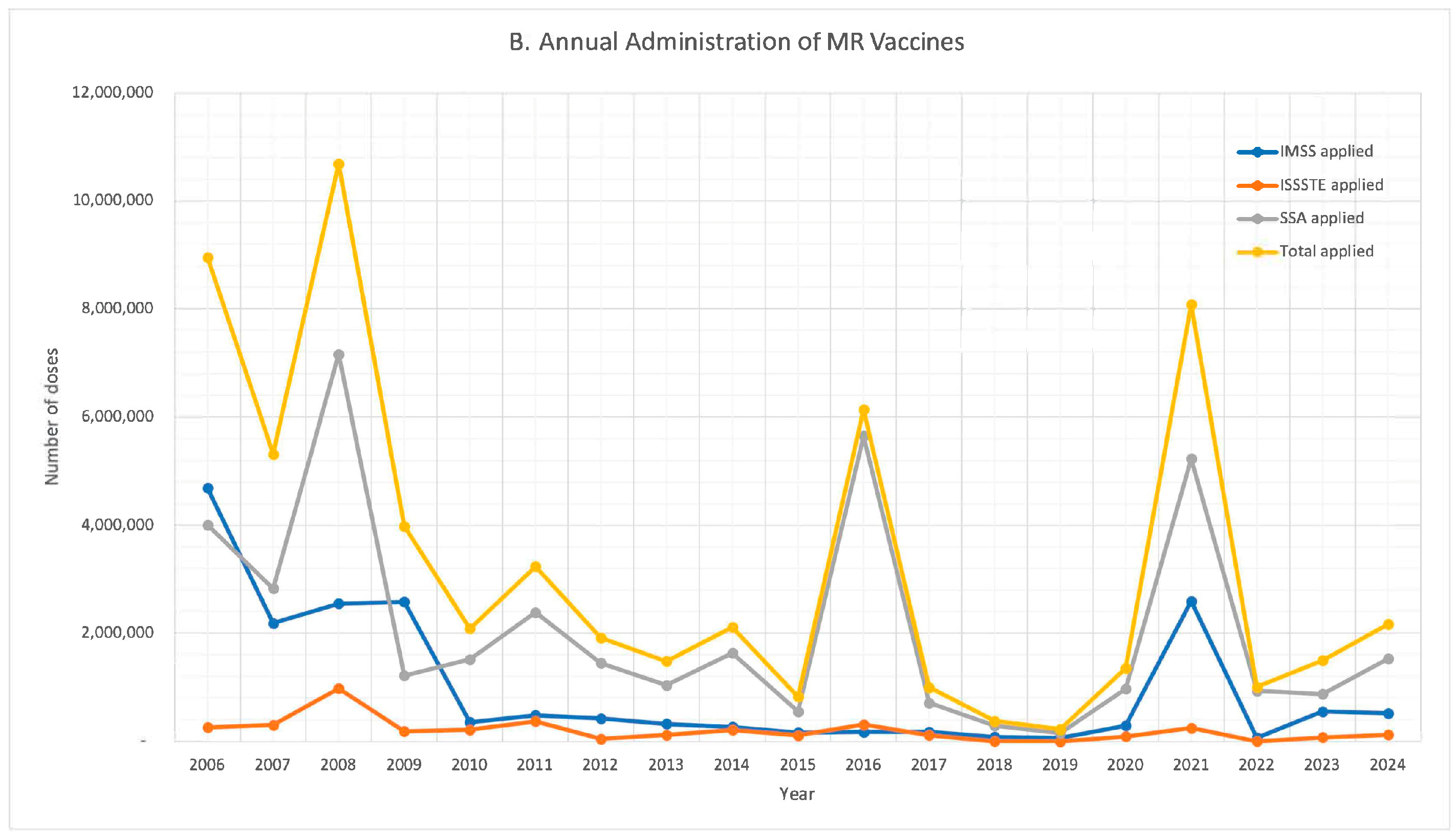
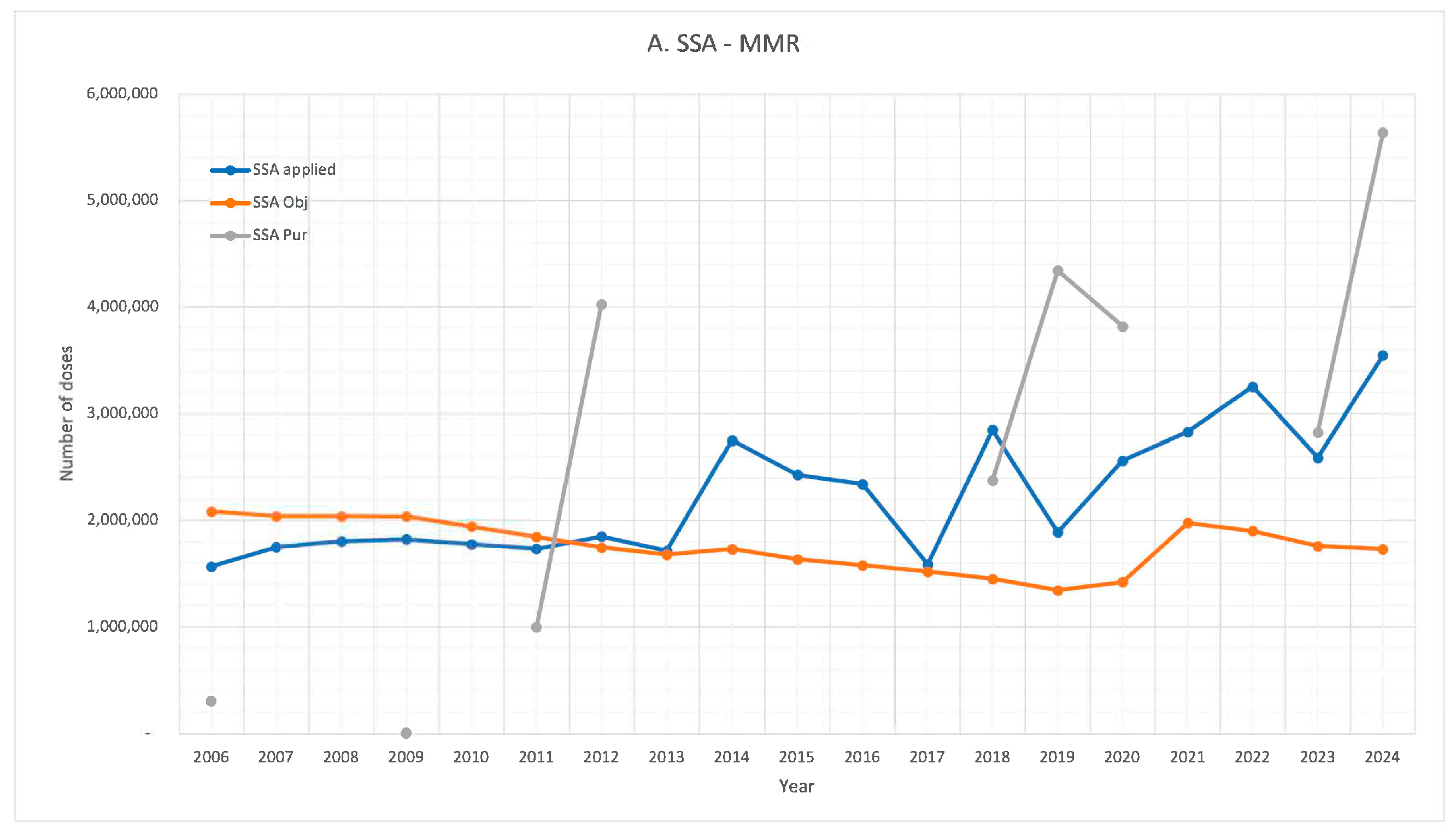



| Year | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| WHO | 76.4 | 73.7 | 82.5 | 85.8 | 103 | 97 | 83.3 | 104 | 73 | 73 | 97 | 99 | 62 | 76 | 98.3 | 96.5 | 100.9 | 96.1 |
| EN * | 71.3 | 61.8 | 72.6 | 64.8 | ||||||||||||||
| Year | 2014 | 2013 | 2012 | 2011 | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||
| Dose | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| WHO | 98 | 96 | 88.62 | 75.7 | 98.8 | 91.8 | 98.4 | 96.6 | 91 | 95.2 | 92.1 | 96 | 94.3 | 95.5 | 85.3 | 96 | 96 | 55.2 |
| EN * | 81.2 | 78.4 | ||||||||||||||||
| Year | 2025 | 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|
| Rubella | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
| Mumps | 1040 | 3748 | 2027 | 2734 | 2301 | 3494 | 8009 | 8818 | 4519 | 3601 |
| Measles | 5053 [14] | 0 | 0 | 0 | 0 | 196 | 1 | 5 | 0 | 0 |
| Year | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|---|---|
| 12 months | 2,299,151 | 2,298,258 | 2,307,115 | 2,319,419 | 2,329,577 | 2,329,754 | 2,315,845 | 2,288,969 | 2,258,535 | 2,235,734 |
| 6 years | 2,334,716 | 2,342,092 | 2,333,358 | 2,313,057 | 2,296,540 | 2,289,510 | 2,290,080 | 2,255,036 | 2,305,548 | 2,311,233 |
| Target | 4,633,867 | 4,640,350 | 4,640,473 | 4,632,476 | 4,626,117 | 4,619,264 | 4,605,925 | 4,544,005 | 4,564,083 | 4,546,967 |
| Year | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 * | 2022 * | 2023 * | 2024 * |
| 12 months | 2,235,734 | 2,214,966 | 2,186,443 | 2,142,836 | 2,112,354 | 2,109,981 | 2,098,926 | 2,091,094 | 2,083,347 | 2,050,080 |
| 6 years | 2,311,233 | 2,306,483 | 2,287,586 | 2,259,450 | 2,227,893 | 2,206,235 | 2,188,443 | 2,163,565 | 2,122,316 | 2,093,173 |
| Target | 4,546,967 | 4,521,449 | 4,474,029 | 4,402,286 | 4,340,247 | 4,316,216 | 6,386,295 | 6,345,753 | 6,289,010 | 6,193,333 |
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMSS | 44 | 44 | 44 | 44 | 46 | 47 | 49 | 50 | 50 | 51 | 52 | 54 | 55 | 56 | 54 | 56 | 57 | 59 | 59 |
| ISSSTE | 10 | 10 | 10 | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 |
| Other | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| SSA | 44 | 44 | 44 | 44 | 42 | 40 | 38 | 37 | 38 | 36 | 35 | 34 | 33 | 31 | 33 | 31 | 30 | 28 | 28 |
| Institution | MMR: Years Available | MMR: % Availability | MR: Years Available | MR: % Availability |
|---|---|---|---|---|
| SSA | 9 | 47% | 16 | 84% |
| IMSS | 10 | 53% | 9 | 47% |
| ISSSTE | 15 | 79% | 15 | 79% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Feregrino, R.; Romero-Feregrino, R.; Romero-Cabello, R.; Muñoz-Cordero, B.; Madrigal-Alonso, B.; Rocha-Rocha, V.M. Nineteen-Year Evidence on Measles–Mumps–Rubella Immunization in Mexico: Programmatic Lessons and Policy Implications. Vaccines 2025, 13, 1126. https://doi.org/10.3390/vaccines13111126
Romero-Feregrino R, Romero-Feregrino R, Romero-Cabello R, Muñoz-Cordero B, Madrigal-Alonso B, Rocha-Rocha VM. Nineteen-Year Evidence on Measles–Mumps–Rubella Immunization in Mexico: Programmatic Lessons and Policy Implications. Vaccines. 2025; 13(11):1126. https://doi.org/10.3390/vaccines13111126
Chicago/Turabian StyleRomero-Feregrino, Rodrigo, Raul Romero-Feregrino, Raul Romero-Cabello, Berenice Muñoz-Cordero, Benjamin Madrigal-Alonso, and Valeria Magali Rocha-Rocha. 2025. "Nineteen-Year Evidence on Measles–Mumps–Rubella Immunization in Mexico: Programmatic Lessons and Policy Implications" Vaccines 13, no. 11: 1126. https://doi.org/10.3390/vaccines13111126
APA StyleRomero-Feregrino, R., Romero-Feregrino, R., Romero-Cabello, R., Muñoz-Cordero, B., Madrigal-Alonso, B., & Rocha-Rocha, V. M. (2025). Nineteen-Year Evidence on Measles–Mumps–Rubella Immunization in Mexico: Programmatic Lessons and Policy Implications. Vaccines, 13(11), 1126. https://doi.org/10.3390/vaccines13111126






