Delayed Cellular Immunity in SARS-CoV-2 Antibody-Non-Responders to COVID-19 Vaccination: Rethinking Post-Vaccine Immune Assessment
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Laboratory Procedures
2.2.1. ELISpot Assay for Detection of IFN-γ T-Cell Responses
2.2.2. Measurement of SARS-CoV-2 IgG Antibodies
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Killeen, T.; Kermer, V.; Troxler Saxer, R. mRNA Vaccine Development during the COVID-19 Pandemic: A Retrospective Review from the Perspective of the Swiss Affiliate of a Global Biopharmaceutical Company. J. Pharm. Policy Pract. 2023, 16, 158. [Google Scholar] [CrossRef]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirtaleb, M.S.; Falak, R.; Heshmatnia, J.; Bakhshandeh, B.; Taheri, R.A.; Soleimanjahi, H.; Emameh, R.Z. An insight overview on COVID-19 mRNA vaccines: Advantageous, pharmacology, mechanism of action, and prospective considerations. Int. Immunopharmacol. 2023, 117, 109934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Coppola, C.; Costagli, S.; Pastore, G.; Sicuranza, A.; Tozzi, M.; et al. Longitudinal immunogenicity cohort study of SARS-CoV-2 mRNA vaccines across individuals with different immunocompromising conditions: Heterogeneity in the immune response and crucial role of Omicron-adapted booster doses. EBioMedicine 2025, 113, 105577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhawan, M.; Thakur, N.; Sharma, M.; Rabaan, A.A. The comprehensive insights into the B-cells-mediated immune response against COVID-19 infection amid the ongoing evolution of SARS-CoV-2. Biomed. Pharmacother. 2025, 185, 117936. [Google Scholar] [CrossRef] [PubMed]
- Reekie, J.; Stovring, H.; Nielsen, H.; Johansen, I.S.; Benfield, T.; Wiese, L.; Stærke, N.B.; Iversen, K.; Mustafa, A.B.; Petersen, K.T.; et al. Development of antibody levels and subsequent decline in individuals with vaccine induced and hybrid immunity to SARS-CoV-2. Int. J. Infect. Dis. 2024, 146, 107111. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Vasileiou, I.V.; Konstantinidis, A.D.; Spyrou, N.I.; Tsakris, A. Prolonged SARS-CoV-2 T cell Responses in a Vaccinated COVID-19-Naive Population. Vaccines 2024, 12, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erra, L.; Uriarte, I.; Colado, A.; Paolini, M.V.; Seminario, G.; Fernández, J.B.; Tau, L.; Bernatowiez, J.; Moreira, I.; Vishnopolska, S.; et al. COVID-19 Vaccination Responses with Different Vaccine Platforms in Patients with Inborn Errors of Immunity. J. Clin. Immunol. 2022, 43, 271–285. [Google Scholar] [CrossRef]
- Yang, Z.R.; Jiang, Y.W.; Li, F.X.; Liu, D.; Lin, T.F.; Zhao, Z.Y.; Wei, C.; Jin, Q.Y.; Li, X.M.; Jia, Y.X.; et al. Efficacy of SARS-CoV-2 vaccines and the dose–response relationship with three major antibodies: A systematic review and meta-analysis of randomised controlled trials. Lancet Microbe 2023, 4, e236–e246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.; Tsang, T.K.; Sullivan, S.G.; Cowling, B.J.; Yang, B. Comparative duration of neutralizing responses and protections of COVID-19 vaccination and correlates of protection. Nat. Commun. 2025, 16, 4748. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Theodoridou, K.; Vasileiou, I.V.; Tsakris, A. SARS-CoV-2 T-cell Immunity Responses following Natural Infection and Vaccination. Vaccines 2023, 11, 1186. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478, Erratum in: Lancet 2020, 396, 466. https://doi.org/10.1016/S0140-6736(20)31687-1. Erratum in: Lancet 2020, 396, 1884. https://doi.org/10.1016/S0140-6736(20)32597-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paniskaki, K.; Anft, M.; Meister, T.L.; Marheinecke, C.; Pfaender, S.; Skrzypczyk, S.; Seibert, F.S.; Thieme, C.J.; Konik, M.J.; Dolff, S.; et al. Immune Response in Moderate to Critical Breakthrough COVID-19 Infection After mRNA Vaccination. Front. Immunol. 2022, 13, 816220. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Nikoloudis, D.; Saldari, C.; Konstantinakou, K.E.; Vasileiou, I.V.; Tsakris, A. Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies. Pathogens 2025, 14, 415. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-Specific T Cell Immunity Is Maintained at 6 Months Following Primary Infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599, Erratum in: Nature 2021, 590, E17. https://doi.org/10.1038/s41586-020-03102-w. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.S.; Fukunaga, A.; Yamamoto, S.; Tanaka, A.; Matsuda, K.; Kimura, M.; Kamikawa, A.; Kito, Y.; Maeda, K.; Ueda, G.; et al. SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2: A 9 months longitudinal study. Sci. Rep. 2022, 12, 15447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron–reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [PubMed]
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial Exposure to SARS-CoV-2 Associated with Cellular Immune Response without Seroconversion, France. Emerg. Infect. Dis. 2021, 27, 113–121. [Google Scholar] [CrossRef]
- Bacher, P.; Rosati, E.; Esser, D.; Martini, G.R.; Saggau, C.; Schiminsky, E.; Dargvainiene, J.; Schröder, I.; Wieters, I.; Khodamoradi, Y.; et al. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020, 53, 1258–1271.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marasco, V.; Carniti, C.; Guidetti, A.; Farina, L.; Magni, M.; Miceli, R.; Calabretta, L.; Verderio, P.; Ljevar, S.; Serpenti, F.; et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br. J. Haematol. 2022, 196, 548–558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jay, C.; Ratcliff, J.; Turtle, L.; Goulder, P.; Klenerman, P. Exposed seronegative: Cellular immune responses to SARS-CoV-2 in the absence of seroconversion. Front. Immunol. 2023, 14, 1092910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoang Nguyen, K.H.; Le, N.V.; Nguyen, P.H.; Nguyen, H.H.T.; Hoang, D.M.; Huynh, C.D. Human immune system: Exploring diversity across individuals and populations. Heliyon 2025, 11, e41836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwarz, T.; Tober-Lau, P.; Hillus, D.; Helbig, E.T.; Lippert, L.J.; Thibeault, C.; Koch, W.; Landgraf, I.; Michel, J.; Bergfeld, L.; et al. Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany. Emerg. Infect. Dis. 2021, 27, 2174–2178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- COVID-19 Host Genetics Initiative. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur. J. Hum. Genet. 2020, 28, 715–718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Y.; Lu, Y.; You, J. Antigen transfer and its effect on vaccine-induced immune amplification and tolerance. Theranostics 2022, 12, 5888–5913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathak, G.A.; Singh, K.; Miller-Fleming, T.W.; Wendt, F.R.; Ehsan, N.; Hou, K.; Johnson, R.; Lu, Z.; Gopalan, S.; Yengo, L.; et al. Integrative genomic analyses identify susceptibility genes underlying COVID-19 hospitalization. Nat. Commun. 2021, 12, 4569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliveira, M.H.; Catahay, J.A.; Khan, S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Rostoker, G.; Rouanet, S.; Merzoug, M.; Chakaroun, H.; Griuncelli, M.; Loridon, C.; Boulahia, G.; Gagnon, L. Serological Correlate of Protection Established by Neutralizing Antibodies Differs Among Dialysis Patients with SARS-CoV-2 Variants of Concern. Vaccines 2025, 13, 518. [Google Scholar] [CrossRef]

| Variables | IgG Negatives (N = 230) | IgG Positives (N = 59) | p-Value |
|---|---|---|---|
| Demographic Characteristics | |||
| Sex (F/M) | 131/99 | 32/27 | NS |
| Age (years ± SD) | 58.4 ± 15.5 | 61.8 ± 14.1 | NS |
| Comorbidities | |||
| Respiratory disorders | 33 (14.3) | 4 (6.7) | NS |
| Cardiovascular diseases | 30 (13.0) | 5 (8.4) | NS |
| Central nervous system disorders | 1 (0.4) | 0 | NS |
| Diabetes mellitus | 13 (5.6) | 4 (6.7) | NS |
| Hypertension | 30 (13.0) | 10 (16.9) | NS |
| Lipidemia | 52 (22.6) | 9 (15.2) | NS |
| Obesity | 28 (12.1) | 10 (16.9) | NS |
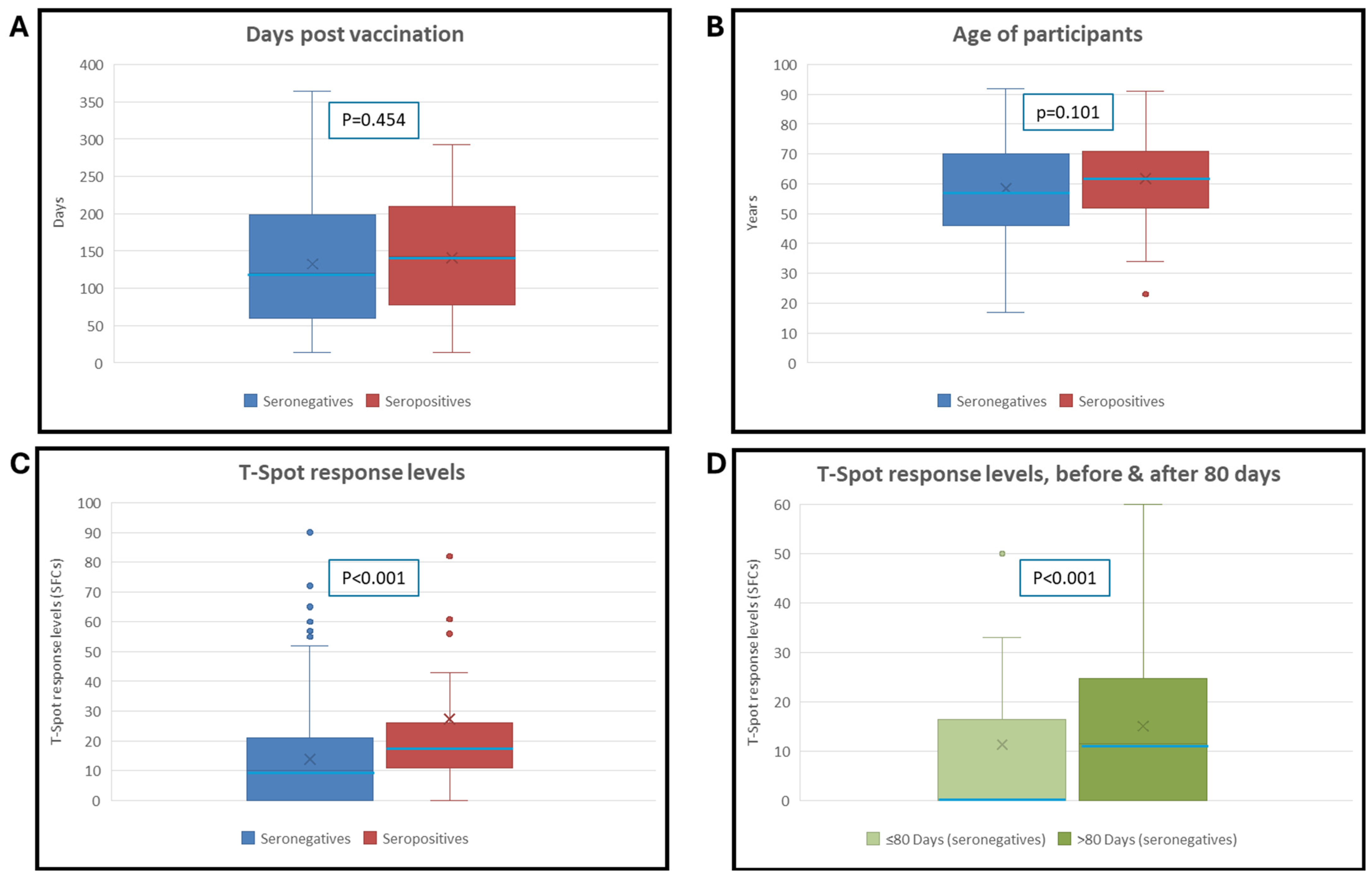
| IgG Negatives (N = 230) | IgG Positives (N = 59) | Statistical Test | p-Value | |
|---|---|---|---|---|
| T-SPOT% positivity rate for S antigen | 57% (132/230) | 88% (52/59) | Χ2 = 19.187 | <0.001 |
| T-SPOT MEDIAN (No of SFC) | 10 | 17 | Mann-Whitney U = 9005 | <0.001 |
| T-SPOT MEAN (No of SFC) for S antigen | 14 | 27 | ||
| Days after vaccination MEDIAN | 120 | 141 | Mann-Whitney U = 7214 | 0.454 |
| Days after vaccination MEAN | 132 | 142 | ||
| Sex (% Female) | 57% (131/230) | 54% (32/59) | Χ2 = 0.141 | 0.713 |
| Age MEDIAN (years) | 57 | 62 | Mann-Whitney U = 7724 | 0.101 |
| Age MEAN (years) | 58 | 62 |
| ≤80 Days | >80 Days | Test Performed | p-Value | |
|---|---|---|---|---|
| No. of samples (Total: 230) | 74 | 156 | ||
| T-SPOT% positivity rate | 38% (28/74) | 67% (104/156) | Χ2 = 17.06 | p < 0.001 |
| T-SPOT MEDIAN (No of SFC) | 0 | 12 | Mann-Whitney U = 4205 | p < 0.001 |
| T-SPOT MEAN (No of SFC) | 11 | 15 | ||
| Sex (% Female) | 61% (45/74) | 55% (86/156) | Χ2 = 0.66 | 0.423 |
| Age MEDIAN (years) | 54 | 59 | Mann-Whitney U = 5274 | 0.291 |
| Age MEAN (years) | 57 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoloudis, D.; Konstantinakou, K.E.; Konstantinidis, A.D.; Spyrou, N.I.; Vasileiou, I.V.; Tsakris, A.; Pitiriga, V.C. Delayed Cellular Immunity in SARS-CoV-2 Antibody-Non-Responders to COVID-19 Vaccination: Rethinking Post-Vaccine Immune Assessment. Vaccines 2025, 13, 1123. https://doi.org/10.3390/vaccines13111123
Nikoloudis D, Konstantinakou KE, Konstantinidis AD, Spyrou NI, Vasileiou IV, Tsakris A, Pitiriga VC. Delayed Cellular Immunity in SARS-CoV-2 Antibody-Non-Responders to COVID-19 Vaccination: Rethinking Post-Vaccine Immune Assessment. Vaccines. 2025; 13(11):1123. https://doi.org/10.3390/vaccines13111123
Chicago/Turabian StyleNikoloudis, Dimitris, Kanella E. Konstantinakou, Alexandros D. Konstantinidis, Natalia I. Spyrou, Irene V. Vasileiou, Athanasios Tsakris, and Vassiliki C. Pitiriga. 2025. "Delayed Cellular Immunity in SARS-CoV-2 Antibody-Non-Responders to COVID-19 Vaccination: Rethinking Post-Vaccine Immune Assessment" Vaccines 13, no. 11: 1123. https://doi.org/10.3390/vaccines13111123
APA StyleNikoloudis, D., Konstantinakou, K. E., Konstantinidis, A. D., Spyrou, N. I., Vasileiou, I. V., Tsakris, A., & Pitiriga, V. C. (2025). Delayed Cellular Immunity in SARS-CoV-2 Antibody-Non-Responders to COVID-19 Vaccination: Rethinking Post-Vaccine Immune Assessment. Vaccines, 13(11), 1123. https://doi.org/10.3390/vaccines13111123








