1. Introduction
As a member of the B7 family, V-set and immunoglobulin domain-containing 4 (VSIG4) plays a critical role in the regulation of immune responses [
1]. VSIG4 is selectively expressed in resting tissue-resident macrophages and serves as a key marker that facilitates the clearance of bacterial pathogens by binding to complement components C3b or inactivated C3b (iC3b) [
2]. Moreover, VSIG4 plays a role in suppressing macrophage-driven inflammation by transcriptionally inhibiting Nlrp3 and IL-1β expression in the context of inflammatory diseases such as multiple sclerosis (MS) and autoimmune arthritis [
3]. Studies using animal models of autoimmune type 1 diabetes have demonstrated that VSIG4 inhibits T cell proliferation and facilitates the differentiation of Foxp3
+ regulatory T cells (Tregs) through interaction with an unidentified receptor on T cells [
4]. Currently, most research on macrophages focuses on tissue damage and disease onset induced by tissue-specific chronic excessive inflammation [
5,
6,
7]. As an important marker of tissue-resident populations, the function and mechanism of VISG4 in these disease models have been described [
8,
9]. However, the potential role of VSIG4 in controlling acute viral infection, as well as its underlying mechanisms, has yet to be elucidated.
Under infected or autoimmune disease conditions, macrophages sense environmental stimuli and subsequently induce primary T cell response to defend against pathogens or induce tissue damage [
10]. Thus, maintaining immune balance has become the predominant topic in infection, cancer, and autoimmune disease. Macrophages may modulate T cell responses through multiple mechanisms. The most important mechanism is that T cells are regulated by some stimulated molecules secreted by macrophages in tissues. Macrophages, especially tissue-resident macrophages, can express some coinhibitory molecules of the CD28 and TNFR families. These molecules have been proven functional in regulating T cells or T-reg cells in a few studies, such as PD-1 and TIM-4 [
11,
12]. However, the influence of the tissue microenvironment and immune checkpoints on T cell responses remains inadequately understood.
The influenza virus represents a leading cause of severe viral respiratory illness, and the secondary bacterial infections that frequently follow can result in substantial morbidity and mortality, impacting 3 to 5 million individuals each year [
13]. Understanding the mechanisms by which VSIG4 mediates this acute viral infection and how VSIG4 facilitates respiratory immunity is of growing interest. Here, we used an influenza virus-infected VSIG4-deficient animal model to define the detailed mechanisms involved in acute virus infection. The study demonstrated that VSIG4 deficiency heightens the vulnerability of pulmonary immunity to the influenza virus. This heightened susceptibility is associated with elevated numbers of CD8
+ T cells and excessive production of pro-inflammatory cytokines, ultimately leading to tissue damage. DC-SIGNR1 (CD209), a member of the type C lectin receptor family, interacts with VSIG4 to suppress excessive Th1 immune responses. The defects observed in the mouse models employed in this study provide strong evidence that VSIG4 is essential for maintaining innate immune homeostasis in defense against pulmonary infection.
2. Materials and Methods
2.1. Mice
All animal studies were conducted in accordance with Beijing Institute of Microbiology and Epidemiology Animal Care and Use Committee guidelines. VSIG4 knockout mice on a C57BL/6J background were generated by our Laboratory Animal Center using the CRISPR/Cas9 gene editing technique to delete a 624 bp fragment in exon 2. The primers used for verification were as follows: Forward, 5′-cacacaa-tagctagcacaaccagtg-3′; Reverse, 5′-aaattgggagatgtacttggtggga-3′. Ten mice heterozygous for the VSIG4 gene (VSIG4+/−, Heter) and ten homozygous knockout mice (VSIG4−/−, KO) were randomly selected. The mice were acclimated for one week under grouped housing conditions. Fresh fecal samples (3–6 pellets per mouse) were collected into sterile microcentrifuge tubes, labeled, immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis. C57BL/6 wild-type mice (5-week-old, weighing 14–16 g) were obtained from Weitonglihua Company, Beijing, China. All experimental mice were bred in a specific pathogen-free facility at our institute. The experimental mice were matched for age and sex and cared for according to the guidelines of the institute (IACUC-IME-2024-030).
2.2. Virus Infections
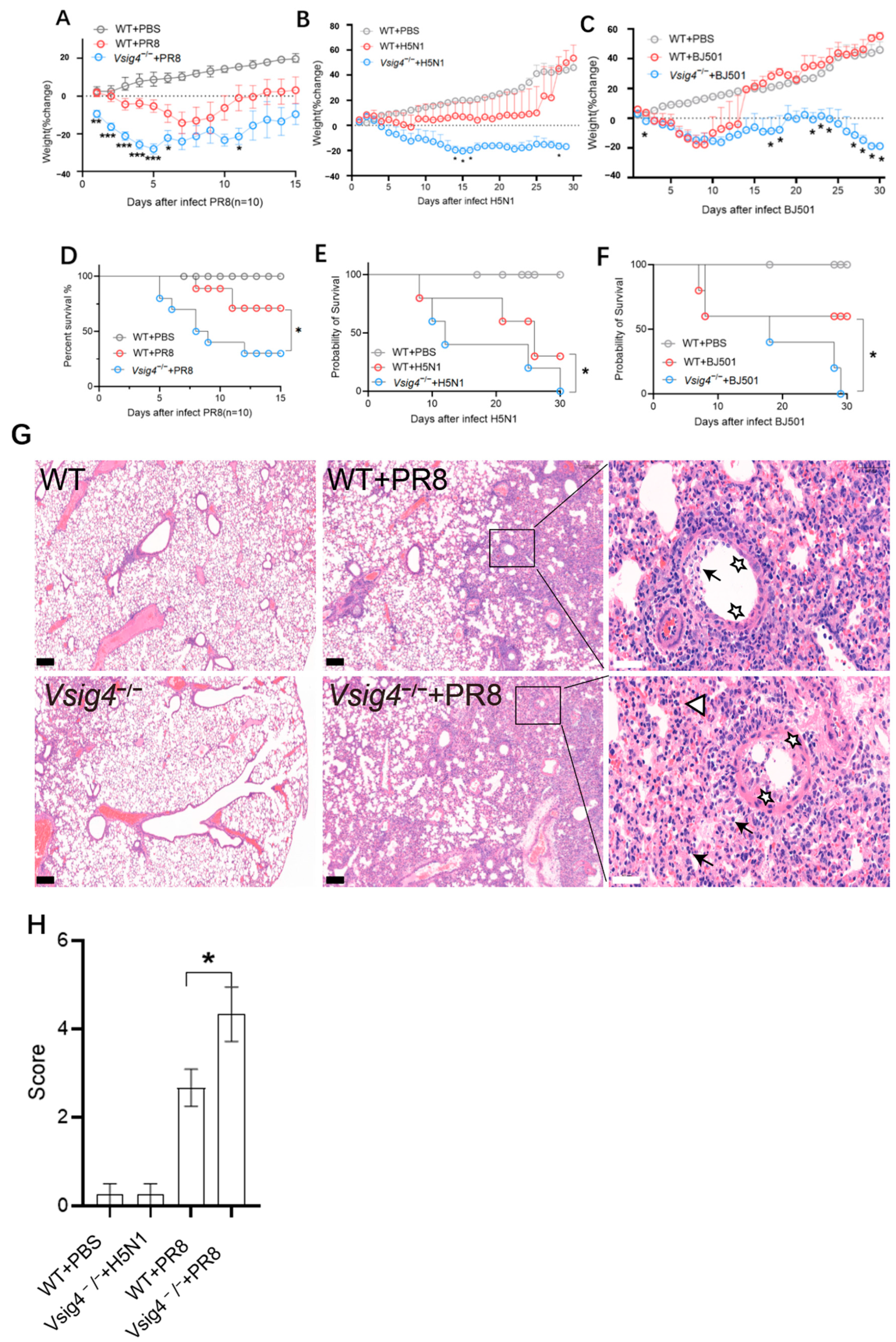
Influenza A virus (IAV) strains A/Puerto Rico/8/1934 (PR8), A/Vietnam/1194/2004 (H5N1) and A/Beijing/501/2009 (BJ501) were propagated in 10-day-old specific pathogen-free chicken embryos. The mice were infected intranasally with 103.5 TCID50 influenza PR8, 103.5 TCID50 H5N1, and 105.5 TCID50 BJ501. They were monitored and weighed at least once daily after infection. The mice that became recumbent or lost more than 30% of their body weight were considered moribund and were euthanized. When indicated, the wild-type mice were challenged intranasally with 103.5 TCID50 influenza PR8 and then supplied with 10 μg VSIG4 protein in vivo by i.v. injection (SinoBiological, Beijing, China) every day; the control mice received injections of PBS. All mice were observed daily and harvested on day 8 post-infection. The viral titers in the lungs were evaluated by qRT-PCR to test the influenza gene M1 expression (M1-Forward primer: AAGACCAATCCTGTCACCTCTG; M1-Reverse primer: CAAAACGTCTACGCTGCAGTCC), and the levels of inflammatory cytokines and chemokines in whole lung tissue were determined by Luminex assay.
2.3. Flow Cytometry
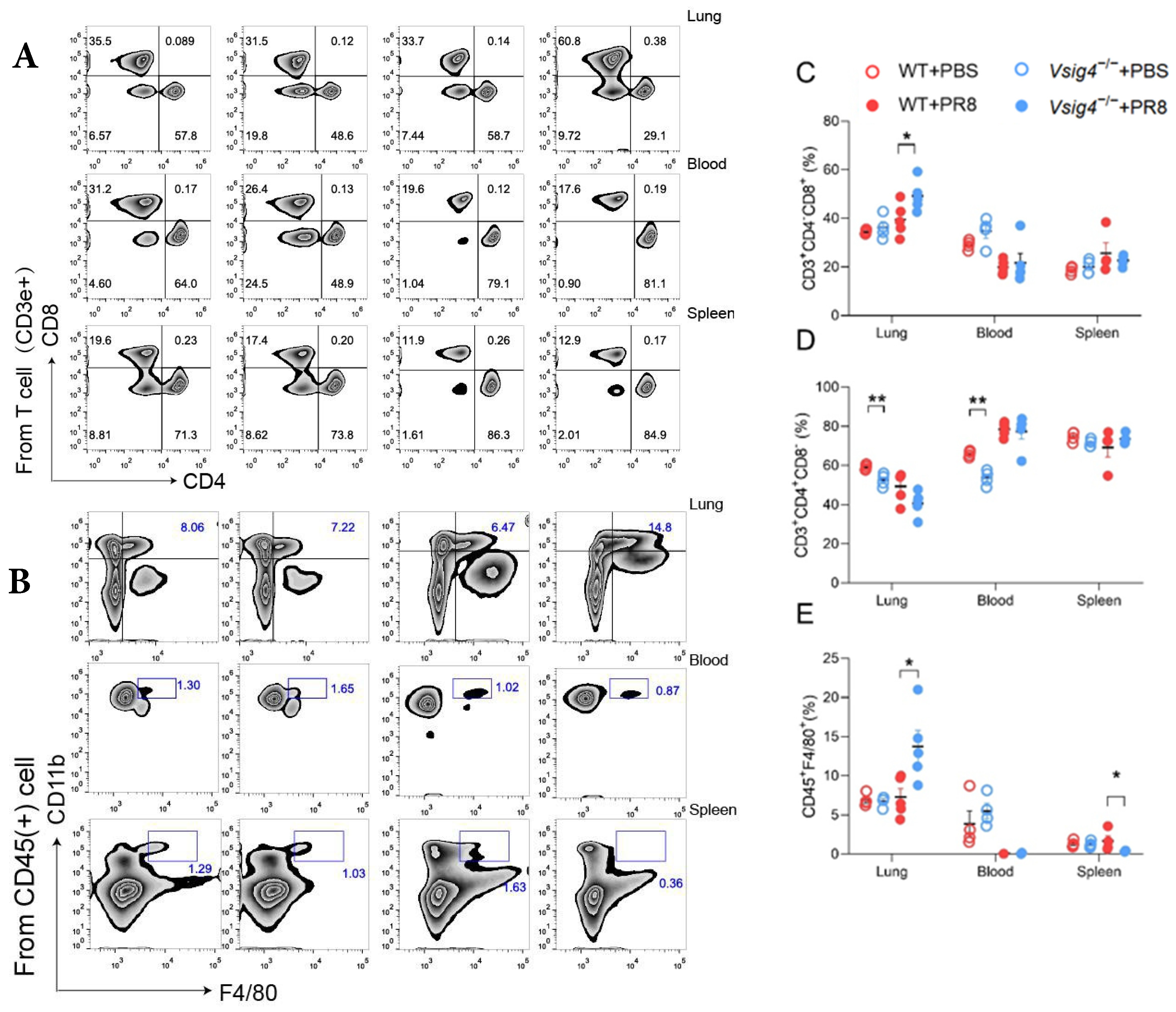
Preparation of lung single cells is described below. In brief, cells isolated from lung tissues were ground with glass rods and incubated with Fc Block (clone 2.4G2) for 15 min at 4 °C, then washed 3 times. For enumeration of CD45 cells, macrophages, and neutrophils, pulmonary lymphocytes were stained on ice with anti-CD45 (clone RM-5), anti-CD11b (clone M1/70), and anti-Ly6G (clone 1A8), anti-Gr-1 (clone RB6-8C5), anti-CD3 (clone 17A2), anti-CD4 (clone RM4-5), anti-CD8a (clone 53-6.7), and anti-VSIG4 (eBioscience, San Diego, CA, USA). The gating strategies for T cells range from lymphocytes to single cells, to live cells, to CD45+ cells, to CD3e+/CD4+/CD8+. The gating strategies for macrophages are from lymphocytes to single cells, to live cells, to CD45+ cells, to F4/80+/CD11b+. Data were gated for forward scatter/side scatter, collected on an FACSCanto II (BD Biosciences, San Jose, CA, USA), and analyzed using Flow Jo software 10.0 (Tree Star, Ashland, OR, USA). Flow cytometry data were visualized using cloud plots, and the frequencies of T cells and macrophages were quantified with Flow Jo software 10.0. Statistical differences among the groups were analyzed by one-way ANOVA.
2.4. Luminex Multiplex Cytokine Test
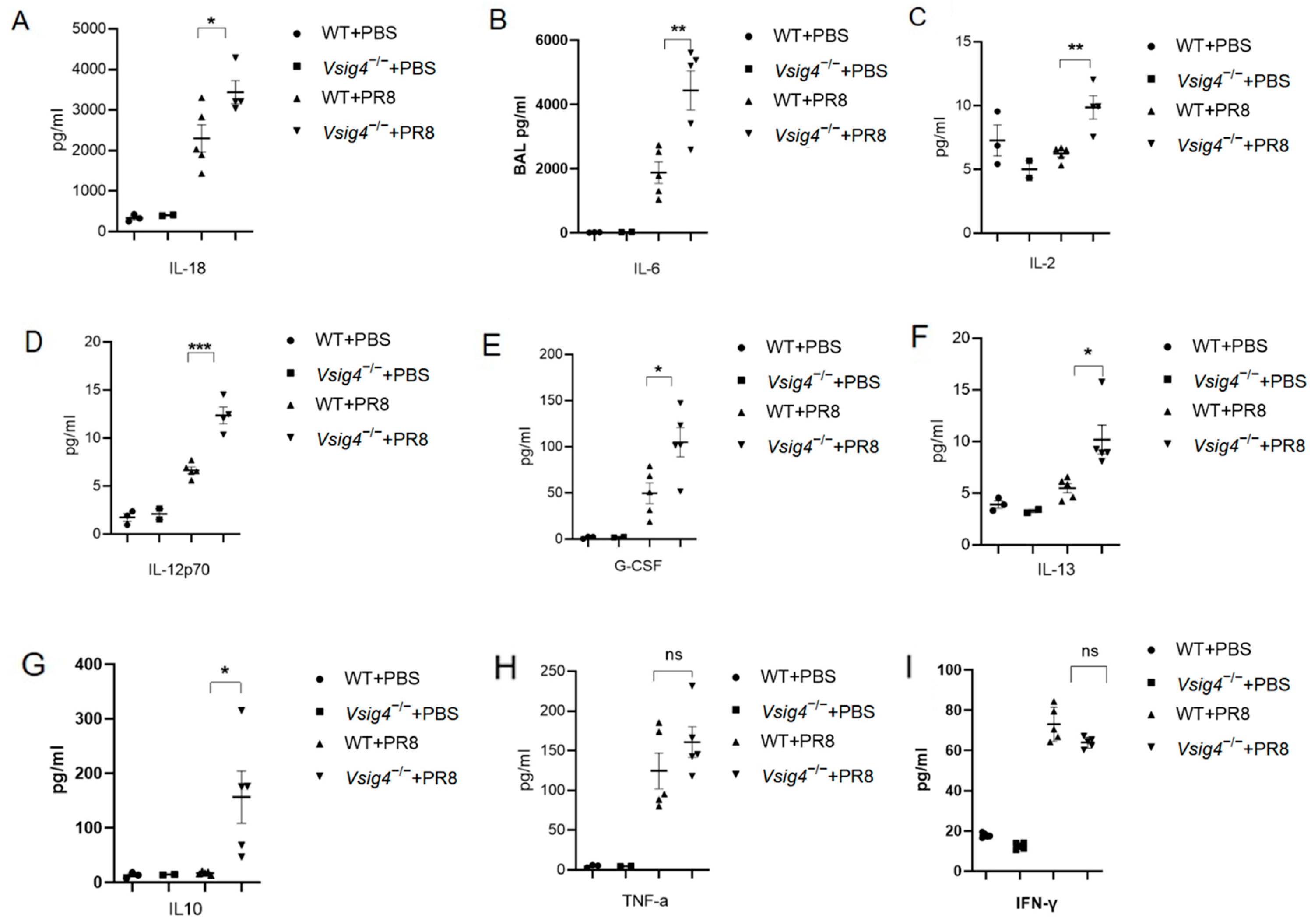
The levels of 34 cytokine molecules were measured using a Luminex-based bead array, the ProcartaPlex Hu Cytokine/Chemokine Panel 34 plex (Invitrogen, Thermo Fisher Scientific, Wilmington, DE, USA), for the following biomarkers: IL-18, IL-6, IL-2, G-CSF, IL-12p70, IL-13, IL-10, IFN-γ, TNF-α, IL-1β, and so on. In brief, 25 µL Broncho alveolar lavage fluid (BALF) and serum samples were analyzed for cytokine levels using a multiplex immunoassay with fluorescent-labeled beads conjugated to specific monoclonal antibodies targeting the molecules of interest, following the manufacturer’s instructions [
14]. Appropriate standards, including 34 beads and quality control (provided by the manufacturers with the test sample plate), were used. The results were interpreted from the standards using the Luminex 200 system. The software determined and interpreted the data and concentrations of analytes in the samples based on the standards run in the test.
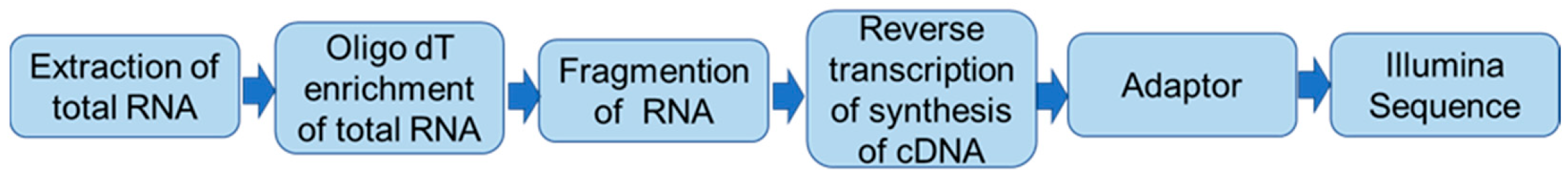
2.5. Transcriptome Sequencing
Total RNA from the lungs of VSIG4
−/− and wild-type mice was isolated using RNAiso plus reagent (Takara, Dalian, China) in accordance with the manufacturer’s instructions. DNase I digestion was used to eliminate genomic DNA contamination. The quality and purity of RNA was checked using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). A total of 1 μg RNA per sample was sent to Majorbio (Shanghai, China) for RNA sample preparation, sequence mapping, assembly, and gene functional annotation. The detailed workflow of transcriptome sequencing is shown in
Scheme 1.
2.6. Construction of Recombinant Adenovirus Encoding mVSIG4
The recombinant adenovirus vector encoding VSIG4 was generated using the Adeno-X™ Expression System (Clontech, Palo Alto, CA, USA) following the manufacturer’s instructions. Briefly, the mVSIG4 cDNA was cloned into the shuttle vector FV012 (pCDNA3.1) containing EGFP. The desired replication-deficient adenovirus containing the full-length cDNA of mVSIG4 was generated by homologous recombination through co-transfection of plasmids pCDNA3.1-mVSIG4 and pBHG1oXE1 and 3Cre in HEK 293 cells using the DOTAP liposome reagent (Roche, Mannheim, Germany). After several rounds of plaque purification, the adenovirus containing the GFP-mVSIG4-3*flag (Ad-GFP-mVSIG4-3*flag) gene was amplified and purified from the cell lysates by banding twice in CsCl density gradients. Viral products were desalted and stored at −80 °C in PBS containing 10% glycerol (v/v). A second recombinant El- and E3-deleted adenovirus carrying the LacZ protein under the control of the CMV promoter (rAd-LacZ) was used as a control vector for DC transduction.
2.7. Cellular Infection
The day before infection, approximately 1 × 106 of RAW264.7 cells (mouse colon macrophage) were seeded in DMEM (10% fetal bovine serum, Gibco, Grand Island, NE, USA) on six-well plates or coverslips without antibiotics. VSIG4 recombinant protein (SinoBiological, Beijing, China) was added to fresh DMEM medium at a 5 μg/mL concentration, and the recombinant Fc protein (SinoBiological, Beijing, China) served as a control. Meanwhile, the cells were infected with PR8 at a multiplicity of infection (MOI) = 0.01. Phosphate-buffered saline (PBS)-treated cells were used as controls. The supernatants and cells were collected for viral burden, levels of inflammatory cytokines, and chemokines. Ad-GFP-mVSIG4-3*flag virus was added to the wells at a series of MOIs—10, 20, 30, and 40—and the RAW264.7 cells were harvested after 24 h of incubation.
2.8. Immunoprecipitation and Immunoblotting Assay
Peritoneal macrophages (PETs) were isolated from the mouse peritoneal cavity 4 days after thioglycolate (Sigma, St. Louis, MO, USA) injection. The macrophages were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) and penicillin–streptomycin (100 U/mL). For the immunoprecipitation assays, PETs were collected after 48 h and then lysed in lysis buffer (25 mM Tris∙HCl pH 7.4, 150 mM NaCl, 1%Nonidet P-40, 1 mM EDTA, 5% glycerol) supplemented with protease inhibitor mixture (Thermo Fisher Scientific) on ice for 30 min with periodic mixing. The whole cell lysates were centrifuged and then incubated with an anti-Flag (Sigma) or anti-mVSIG4Ab overnight at 4 °C, followed by further incubation with protein A beads (Sigma) for 2 h at 4 °C. The cell lysates were removed from the refrigerator and thawed on ice. The sample detection mixture was prepared. Then, following the manufacturer’s instructions, the primary antibody dilution for the target protein, secondary antibody, and chemiluminescent substrate were sequentially prepared. The prepared reagents were sequentially added to the detection plate. The plate was covered and centrifuged at 25,000 rpm at room temperature, ensuring that it was properly balanced. The detection plate was placed into the new instrument (Protein sample, WesTM WS-3493), the capillaries were retrieved and loaded into the automated Western blot analyzer (ProteinSimple, San Jose, CA, USA), the computer was connected to it, the parameters were set, and the program was started. Upon completion of the program, protein expression levels were analyzed using Compass for SW software 5.0. Primary antibodies used included anti-CD209 (Biolegend,147802), anti-Ac, anti-phospho-Ser/Thr, anti-phospho-Tyr, anti-Ub, anti-VSIG4 (Abcam, Cambridge, UK, ab252933), anti-BCL3, anti-IKKE, anti-LSP1, and anti-CYLD (1:1000; ABclonal, Wuhan, China). After three washes with TBS containing 0.1% Triton X-100, goat anti-rabbit or anti-mouse HRP-conjugated secondary Ab (1:5000; Cell Signaling Technology, Danvers, MA, USA) was incubated for 1 h at room temperature. The final detection of protein was performed using Compass for SW software 5.0.
2.9. Confocal Immunofluorescence Microscopy
PEMs were fixed with 4% paraformaldehyde at room temperature for 15 min, permeabilized with PBS containing 10% FBS, 3% BSA, and 0.5% Triton X-100 for 15 min, and then incubated with diluted primary Abs overnight at 4 °C. After two washes with PBS, the cells were incubated with secondary Abs conjugated to Alexa Flour® 488 and Alexa Flour® 555 (Abcam) at room temperature for 1 h. Cell nuclei were stained with DAPI (Roche) for 10 min, followed by three PBS washes. Images were captured using fluorescence microscopy (Zeiss LSM880, Carl Zeiss AG, Oberkochen, Germany).
2.10. Histology
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Pathological foci in each section—defined as areas with extensive inflammatory cell infiltration accompanied by submucosal edema—were evaluated. Representative photomicrographs were captured at ×100 magnification.
2.11. Statistical Analysis
Statistical analyses were performed using the program Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Values are expressed as mean ± SD. Data were analyzed by unpaired Student’s t-test (normal distribution) or one-way ANOVA, followed by Dunnett’s multiple comparison tests. Survival data were analyzed by log rank tests, and p < 0.05 was considered to be statistically significant.
4. Discussion
While inflammatory responses are essential for host defense against pathogens, uncontrolled expression of pro-inflammatory cytokines may cause tissue damage [
18]. The identification and characterization of molecules and pathways that modulate inflammation and eliminate excessive inflammatory cells are therefore important in preventing acute inflammation-induced tissue damage [
19].
In our study, the VSIG4
−/− mice became more susceptible to influenza virus infection. The control mice readily survived sublethal dose intranasal challenge with influenza virus. Meanwhile, we observed elevated expression of pro-inflammatory cytokines and prominent inflammatory foci with varying degrees of lung damage in the VSIG4-deficient mice following influenza infection. The increased proliferation of CD8
+ T cells and tissue-resident memory T cells was associated with heightened inflammation. Our findings demonstrate that VSIG4, a B7 family-associated protein specifically expressed in resting macrophages, plays a regulatory role in this process [
1]. However, the potential clinical treatment with modulating factors involved in the inflammatory signal pathway has become a much-debated topic in recent years. These results indicate that modulation of VSIG4 signaling may offer a promising therapeutic strategy for managing inflammation during acute viral infections.
Based on our observation, one useful way to manipulate the pro-inflammatory cytokine expression is by supplying VSIG4 recombinant protein. We purchased this recombinant protein from a company and treated wild-type mice after infection. Our observations demonstrated that although the results of the in vitro assay (
Supplementary Materials Figure S3A–C) and body weight changes (
Supplementary Materials Figure S3D) were negative, supplying recombinant VSIG4 protein conferred slight protection from influenza virus infections (
Supplementary Materials Figure S3E). This suggests that recombinant VSIG4, or agents capable of enhancing its expression, may serve as promising therapeutic strategies for controlling inflammation, particularly during acute viral infections. Although this anti-inflammatory effect is evident, the precise mechanisms underlying VSIG4-mediated regulation remain to be fully elucidated. Notably, the clinical use of anti-inflammatory treatments based on recombinant proteins is not without precedent. Our study uncovers a previously underappreciated mechanism of protection in the context of influenza infection—namely, the role of immune homeostasis in conferring resistance to viral challenge. Together with existing clinical and experimental evidence, our results underscore the potential of adoptive cell therapies and immune-modulating strategies to enhance public health responses during outbreaks of emerging or severe viral infections.