Comparative Analysis of Pneumococcal Serotypes for 10 Years (2014–2024) in the Comunidad Valenciana Region, Spain, and How They Are Correlated with PCV13, PCV20, and PCV21
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Sociodemographic and Analytical Data
2.3. Serotyping
2.4. Statistical Analysis
- 0.00–0.09: negligible;
- 0.10–0.29: small;
- 0.30–0.49: medium;
- ≥0.50: large.
3. Results
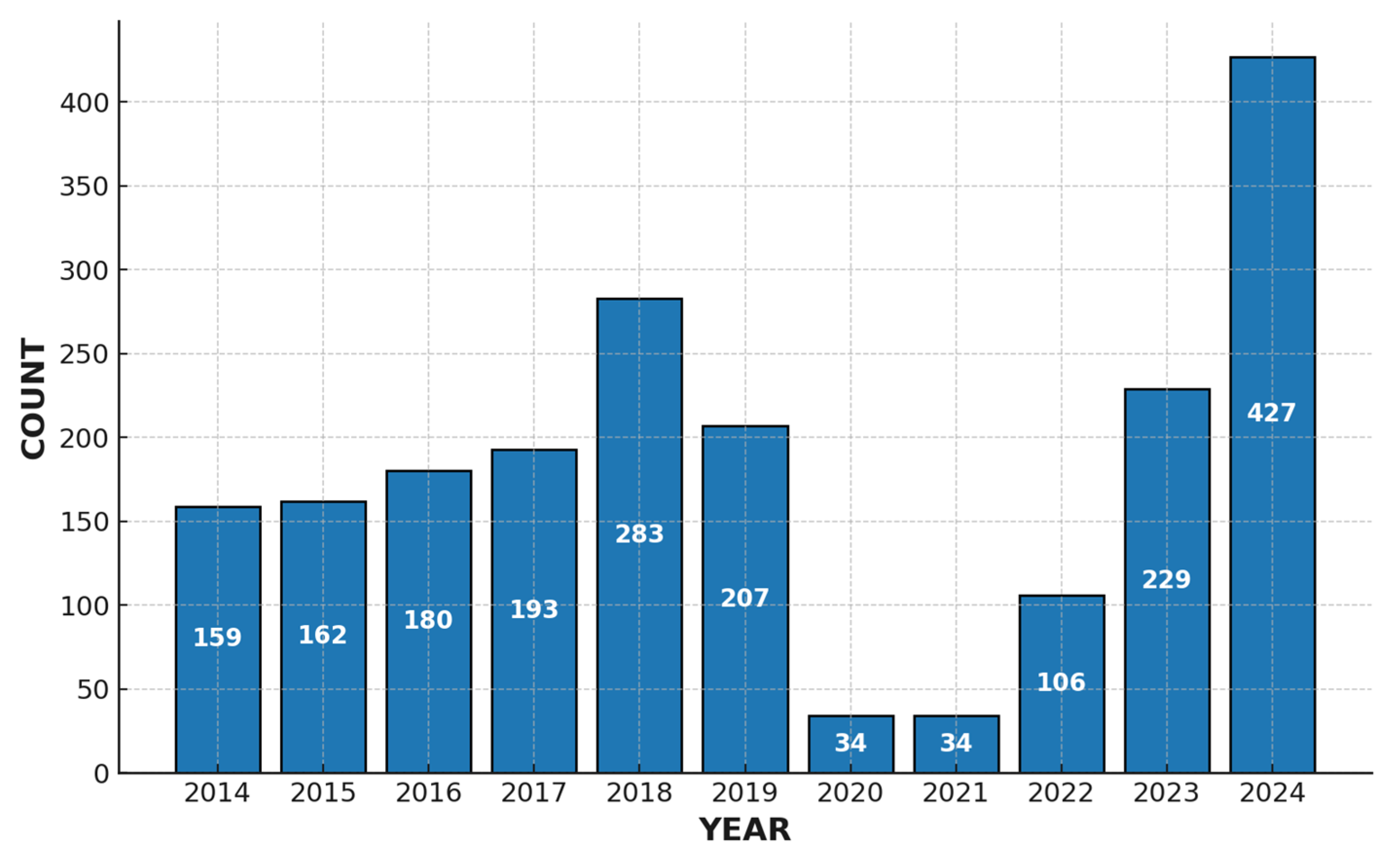
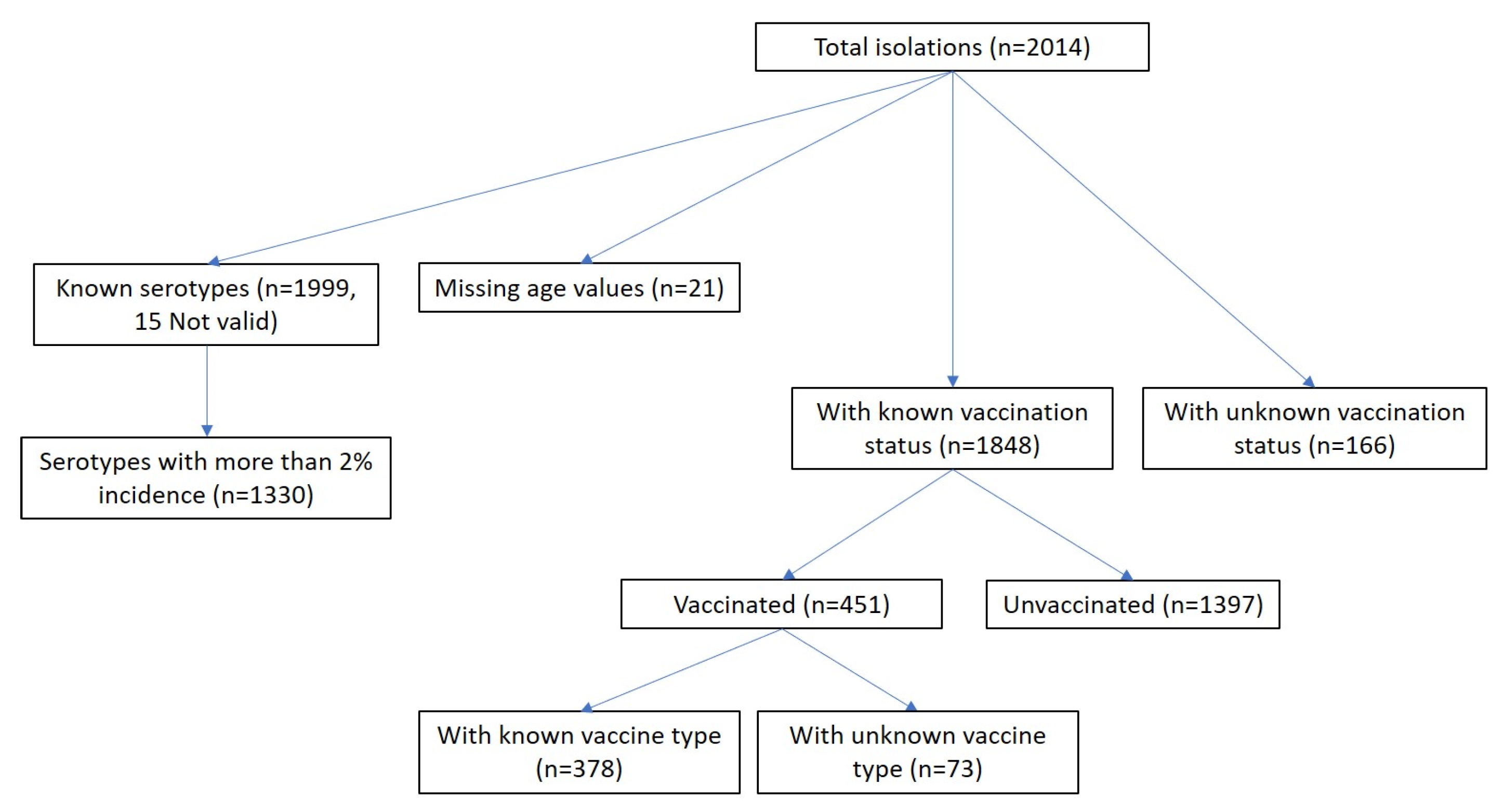
3.1. Vaccination Status Overview
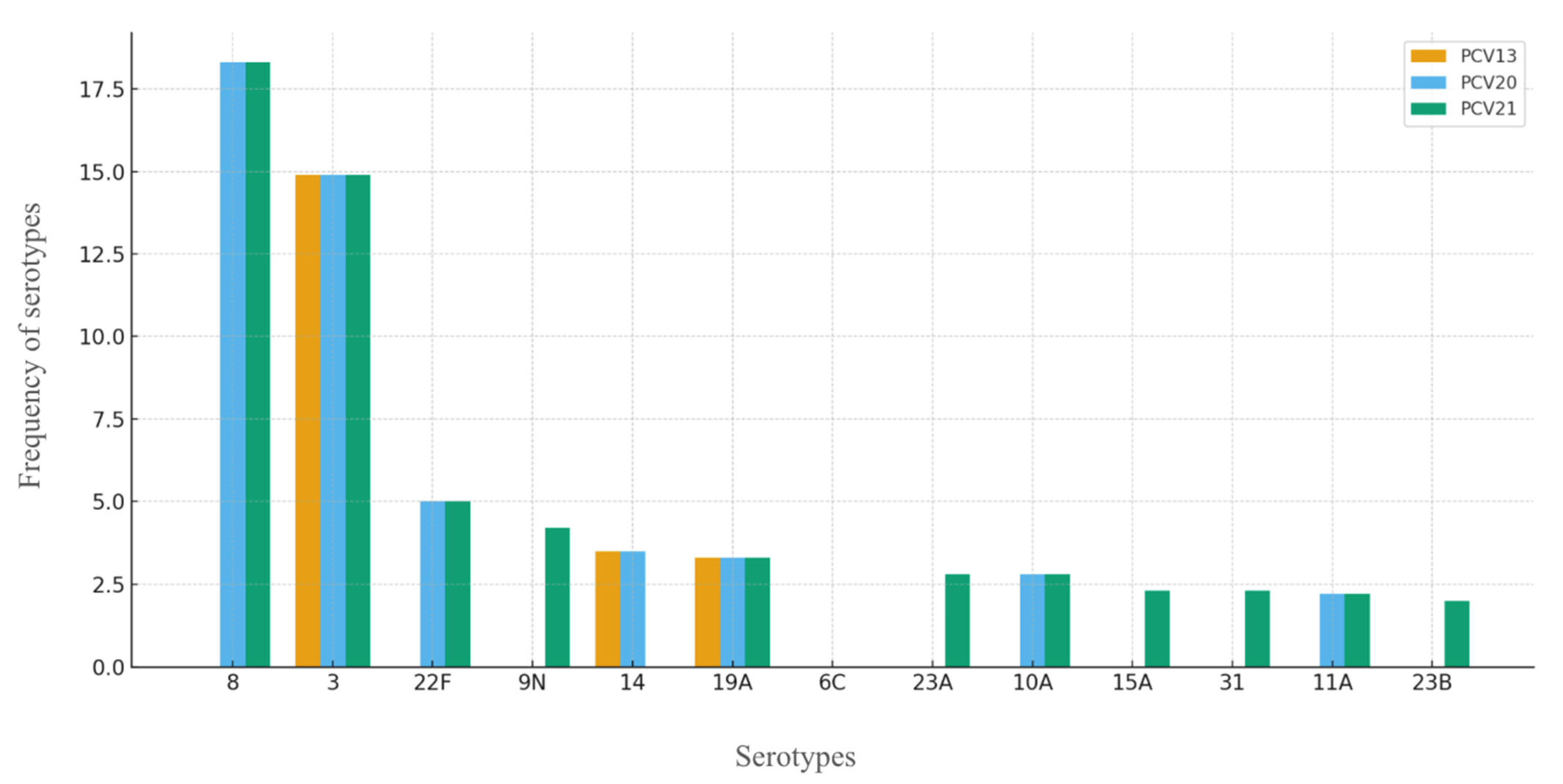
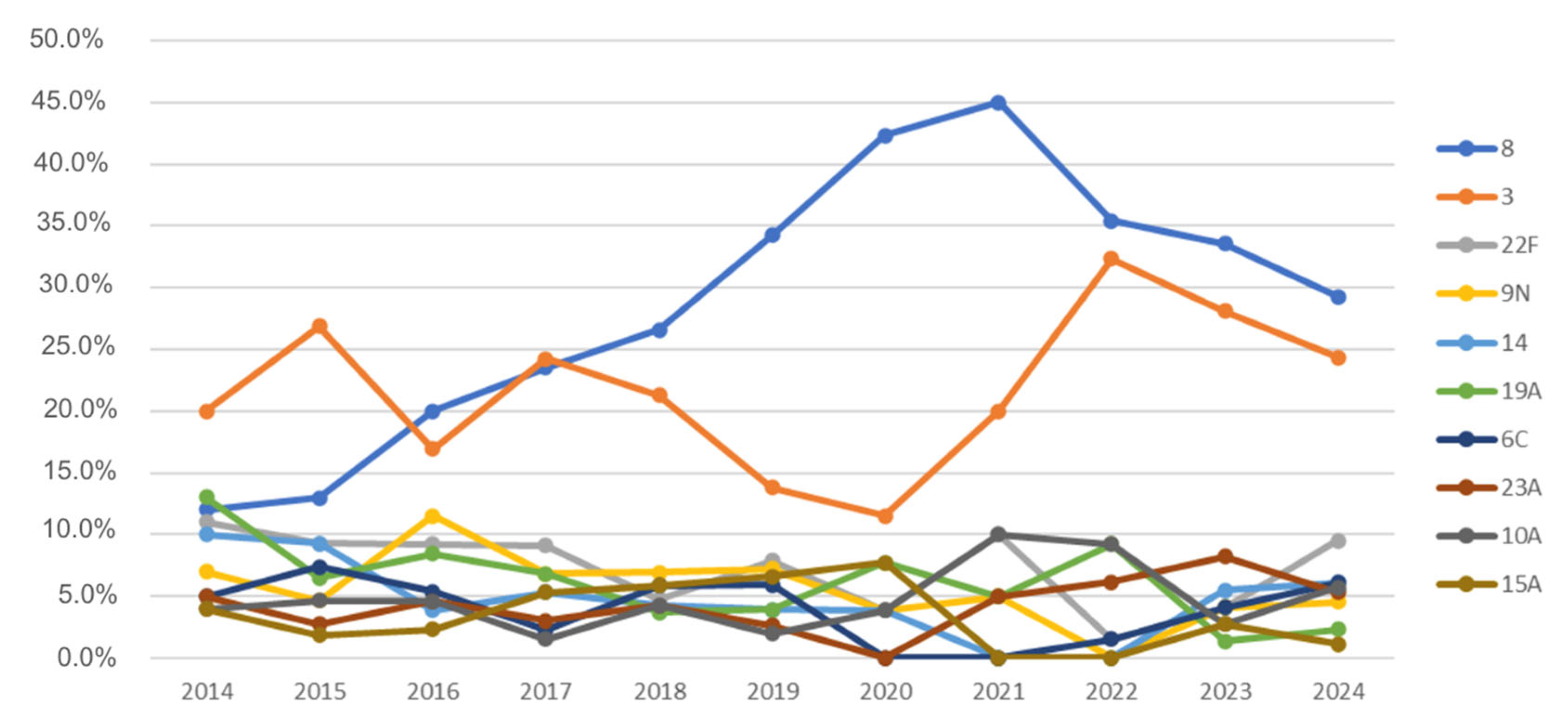
3.2. Pneumococcal Serotypes
- Serotype 8 is more common among individuals older than 10 years (particularly in the 10–64 age group) (<10 vs. 10–64: p < 0.001 (B-H and Bf); <10 vs. >64: p = 0.037 (Bf) and p = 0.012 (B-H));
- Serotype 3 is more frequent in individuals older than 64 years compared to patients aged 10–64 years (>64 vs. 10–64: p = 0.033 (B-H and Bf));
- Serotypes 10A and 23B are more frequent in children under 10 years of age (10A: <10 vs. 10–64: p < 0.001 (B-H and Bf); <10 vs. >64: p < 0.001 (B-H and Bf); 23B: <10 vs. 10–64: p = 0.029 (B-H)/p = 0.015 (Bf); <10 vs. >64: p = 0.002 (B-H and Bf)).
| Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | <10 | 10–64 | >64 | ||||||
| Count | N % | Count | N % | Count | N % | Count | N % | ||
| Serotypes | Total | 1993 | 100.0% | 112 | 100.0% | 819 | 100.0% | 1062 | 100.0% |
| Others * | 659 | 33.1% | 52 | 46.4% | 278 | 33.9% | 329 | 31.0% | |
| 8 | 353 | 17.7% | 5 | 4.5% | 188 | 23.0% | 160 | 15.1% | |
| 3 | 293 | 14.7% | 15 | 13.4% | 99 | 12.1% | 179 | 16.9% | |
| 22F | 99 | 5.0% | 5 | 4.5% | 40 | 4.9% | 54 | 5.1% | |
| 9N | 79 | 4.0% | 0 | 0.0% | 35 | 4.3% | 44 | 4.1% | |
| 14 | 71 | 3.6% | 5 | 4.5% | 21 | 2.6% | 45 | 4.2% | |
| 19A | 70 | 3.5% | 5 | 4.5% | 22 | 2.7% | 43 | 4.0% | |
| 6C | 64 | 3.2% | 1 | 0.9% | 21 | 2.6% | 42 | 4.0% | |
| 23A | 61 | 3.1% | 3 | 2.7% | 19 | 2.3% | 39 | 3.7% | |
| 10A | 56 | 2.8% | 10 | 8.9% | 24 | 2.9% | 22 | 2.1% | |
| 15A | 46 | 2.3% | 3 | 2.7% | 18 | 2.2% | 25 | 2.4% | |
| 31 | 44 | 2.2% | 0 | 0.0% | 15 | 1.8% | 29 | 2.7% | |
| 11A | 43 | 2.2% | 2 | 1.8% | 13 | 1.6% | 28 | 2.6% | |
| 23B | 40 | 2.0% | 6 | 5.4% | 17 | 2.1% | 17 | 1.6% | |
| Invalid | 15 | 0.8% | 0 | 0.0% | 9 | 1.1% | 6 | 0.6% | |
3.3. Serotypes and Vaccination
3.4. Serotype Trends over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Pneumococcus. In Vaccine-Preventable Diseases Surveillance Standards; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-pneumococcus (accessed on 3 June 2025).
- Drijkoningen, J.J.; Rohde, G.G. Pneumococcal infection in adults: Burden of disease. Clin. Microbiol. Infect. 2014, 20 (Suppl. 5), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Grabenstein, J.D.; Klugman, K.P. A century of pneumococcal vaccination research in humans. Clin. Microbiol. Infect. 2012, 18 (Suppl. 5), 15–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Pneumococcal Disease: Vaccine Standardization; World Health Organization: Geneva, Switzerland, 2025; Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/pneumococcal-disease (accessed on 3 June 2025).
- Scelfo, C.; Menzella, F.; Fontana, M.; Ghidoni, G.; Galeone, C.; Facciolongo, N.C. Pneumonia and Invasive Pneumococcal Diseases: The Role of Pneumococcal Conjugate Vaccine in the Era of Multi-Drug Resistance. Vaccines 2021, 9, 420. [Google Scholar] [CrossRef]
- Davies, L.R.L.; Cizmeci, D.; Guo, W.; Luedemann, C.; Alexander-Parrish, R.; Grant, L.; Isturiz, R.; Theilacker, C.; Jodar, L.; Gessner, B.D.; et al. Polysaccharide and conjugate vaccines to Streptococcus pneumoniae generate distinct humoral responses. Sci. Transl. Med. 2022, 14, eabm4065. [Google Scholar] [CrossRef]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Lynfield, R.; Reingold, A.; Cieslak, P.R.; Pilishvili, T.; Jackson, D.; et al. Decline in invasive pneumococcal disease after the introduction of protein–polysaccharide conjugate vaccine. N. Engl. J. Med. 2003, 348, 1737–1746. [Google Scholar] [CrossRef]
- Lupinacci, R.; Rupp, R.; Wittawatmongkol, O.; Jones, J.; Quinones, J.; Ulukol, B.; Dagan, R.; Richmond, P.; Stek, J.E.; Romero, L.; et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine. 2023, 41, 1142–1152. [Google Scholar] [CrossRef]
- Essink, B.; Sabharwal, C.; Cannon, K.; Frenck, R.W.; Lal, H.; Xu, X.; Sundaraiyer, V.; Peng, Y.; Moyer, L.; Pride, M.W.; et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18 Years. Clin. Infect. Dis. 2022, 75, 390–398. [Google Scholar] [CrossRef]
- Scott, P.; Haranaka, M.; Choi, J.H.; Stacey, H.; Dionne, M.; Greenberg, D.; Grijalva, C.G.; Orenstein, W.A.; Fernsler, D.; Gallagher, N.; et al. A phase 3 clinical study to evaluate the safety, tolerability, and immunogenicity of V116 in pneumococcal vaccine–experienced adults 50 years of age or older (STRIDE-6). Clin. Infect. Dis. 2024, 79, 1366–1374. [Google Scholar] [CrossRef]
- Pfizer Inc. U.S. Food and Drug Administration. PREVNAR 20 (Pneumococcal 20-valent Conjugate Vaccine). Prescribing Information. 2023. Available online: https://www.fda.gov/media/150386/download (accessed on 8 June 2025).
- Merck & Co., Inc. CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine): FDA Approval Press Release. 2023. Available online: https://www.merck.com/news/u-s-fda-approves-capvaxive-pneumococcal-21-valent-conjugate-vaccine-for-prevention-of-invasive-pneumococcal-disease-and-pneumococcal-pneumonia-in-adults/ (accessed on 8 June 2025).
- European Medicines Agency. Prevenar 20 (Pneumococcal Polysaccharide Conjugate Vaccine—20 Valent); EMA/80599/2024; EMA: Amsterdam, The Netherlands, 2022; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-20 (accessed on 8 June 2025).
- Generalitat Valenciana. El Consell Adquiere 500,000 Dosis de Vacuna Frente al Neumococo Para Reforzar la Protección Infantil y de Personas Adultas. Comunica GVA. 2024. Available online: https://comunica.gva.es/es/detalle?id=384988364&site=373422400 (accessed on 8 June 2025).
- Comité Asesor de Vacunas de la AEP. Calendario de Vacunaciones de la Comunidad Valenciana. Vacunas AEP. 2025. Available online: https://vacunasaep.org/profesionales/calendario-vacunas/comunidad-valenciana (accessed on 8 June 2025).
- Muñoz, I.; Vanaclocha, H.; Martín-Sierra, M.; González, F. Red de Vigilancia Microbiológica de la Comunidad Valenciana (RedMIVA) [Microbiological Surveillance Network in the Valencian community]. Enferm. Infecc. Microbiol. Clin. 2008, 26, 77–81. [Google Scholar] [CrossRef]
- Chan, K.-P.F.; Ma, T.-F.; Fang, H.; Tsui, W.-K.; Ho, J.C.-M.; Ip, M.S.-M.; Ho, P.-L. Changes in the incidence, viral coinfectionpattern and outcomes of pneumococcal hospitalizations during and after the COVID-19 pandemic. Pneumonia. 2025, 17, 10. [Google Scholar] [CrossRef]
- Amin-Chowdhury, Z.; Aiano, F.; Mensah, A.; Sheppard, C.L.; Litt, D.; Fry, N.K.; Andrews, N.; Ramsay, M.E.; Ladhani, S.N. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Prospective national cohort study, England. Clin. Infect. Dis. 2021, 72, e65–e75. [Google Scholar] [CrossRef]
- Diehl, J.M.; Kohr, H.U. Deskriptive Statistik, 12th ed.; Klotz Eschborn: Eschborn, Germany, 1999; p. 161. [Google Scholar]
- Pilishvili, T.; Lexau, C.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Reingold, A.; Thomas, A.; Schaffner, W.; Craig, A.S.; et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 2010, 201, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Wiese, A.D.; Griffin, M.R.; Grijalva, C.G. Impact of pneumococcal conjugate vaccines on hospitalizations for pneumonia in the United States. Expert. Rev. Vaccines 2019, 18, 327–341. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Invasive Pneumococcal Disease—Annual Epidemiological Report for 2018. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2018 (accessed on 1 June 2023).
- Conselleria de Sanitat. Orden 3 Abr 2015, de Inclusión de la Vacuna Frente al Neumococo en los Niños Nacidos a Partir del 1 de enero 2015. Diari Oficial de la Generalitat Valenciana. 2015. Available online: https://dogv.gva.es/datos/2015/04/13/pdf/2015_3241.pdf (accessed on 12 June 2025).
- Perniciaro, S.; van der Linden, M. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: A retrospective cohort study. Lancet Reg. Health Eur. 2021, 7, 100126. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.A.; Mendes, R.E.; Sings, H.L.; Arguedas, A.; Reinert, R.-R.; Jodar, L.; Isturiz, R.E.; Gessner, B.D. Streptococcus pneumoniae serotype distribution and antimicrobial nonsusceptibility trends among adults with pneumonia in the United States, 2009–2017. J. Infect. 2020, 81, 557–566. [Google Scholar] [CrossRef]
- Diab-Casares, L.; Tormo-Palop, N.; Hernández-Felices, F.J.; Artal-Muñoz, V.; Floría-Baquero, P.; Martin-Rodríguez, J.L.; Medina-González, R.; Cortés-Badenes, S.; Fuster-Escrivá, B.; Gil-Bruixola, A.; et al. Predominant pneumococcal serotypes in isolates causing invasive disease in a Spanish region: An examination of their association with clinical factors, antimicrobial resistance, and vaccination coverage. J. Clin. Med. 2025, 14, 1612. [Google Scholar] [CrossRef]
- Ciruela, P.; Broner, S.; Izquierdo, C.; Pallarés, R.; Muñoz-Almagro, C.; Hernández, S.; Grau, I.; Domínguez, A.; Jané, M.; Catalan Working Group on Invasive Pneumococcal Disease. Indirect effects of paediatric conjugate vaccines on invasive pneumococcal disease in older adults. Int. J. Infect. Dis. 2019, 86, 122–130. [Google Scholar] [CrossRef]
- Domínguez, Á.; Ciruela, P.; Hernández, S.; García-García, J.J.; Soldevila, N.; Izquierdo, C.; Moraga-Llop, F.; Díaz, A.; de Sevilla, M.F.; González-Peris, S.; et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7–59 months: A matched case-control study. PLoS ONE 2017, 12, e0183191. [Google Scholar] [CrossRef]
- Luck, J.N.; Tettelin, H.; Orihuela, C.J. Sugar-Coated Killer: Serotype 3 Pneumococcal Disease. Front. Cell. Infect. Microbiol. 2020, 10, 613287. [Google Scholar] [CrossRef]
- Platt, H.L.; Bruno, C.; Buntinx, E.; Pelayo, E.; Garcia-Huidobro, D.; Sjoberg, F.; Song, J.Y.; Grijalva, C.G.; A Orenstein, W.; Morgan, L.; et al. Safety, tolerability, and immunogenicity of an adult pneumococcal conjugate vaccine, V116 (PCV21), in healthy adults: Phase 1/2, randomised, double-blind, active comparator-controlled, multicentre, US-based trial. Lancet Infect. Dis. 2023, 23, 233–246. [Google Scholar] [CrossRef]
- Løchen, A.; Croucher, N.J.; Anderson, R.M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 2020, 10, 18977. [Google Scholar] [CrossRef]
- Soler Soneira, M.; Del-Águila-Mejía, J.; Acosta-Gutiérrez, M.; Sastre-García, M.; Amillategui-Dos-Santos, R.; Cano Portero, R. Enfermedad neumocócica invasiva en España en 2023. BES 2024, 32, 74–93. [Google Scholar] [CrossRef]
- Kitowska, W.; Gonzalez-Perez, A.C.; Neto, J.S.; Kanerva, M.; Kaukavuori, H.; Lindström, I.; Frilander, H.; Dub, T.; Siira, L.; Team, P.S.O. Second reported outbreak of pneumococcal pneumonia among shipyard employees in Turku, Finland, Aug–Oct 2023: A case–control study. Epidemiol. Infect. 2025, 153, e32. [Google Scholar] [CrossRef]
| Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total | <10 | 10–64 | >64 | |||||
| Count | N % | Count | N % | Count | N % | Count | N % | |
| Total * | 1775 | 100.0% | 75 | 100.0% | 729 | 100.0% | 971 | 100.0% |
| Not vaccinated | 1397 | 78.7% | 18 | 24.0% | 625 | 85.7% | 754 | 77.7% |
| Vaccinated with PCV13 | 125 | 7.0% | 53 | 70.7% | 51 | 7.0% | 21 | 2.2% |
| Vaccinated with PPV23 | 226 | 12.7% | 3 | 4.0% | 45 | 6.2% | 178 | 18.3% |
| Vaccinated with both PCV13 and PPV23 | 27 | 1.5% | 1 | 1.3% | 8 | 1.1% | 18 | 1.9% |
| Vaccination Status | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Not Vaccinated | Vaccinated with PCV13 | Vaccinated with PPV2323 | Vaccinated with PCV13 and PPV23 | |||||||
| Count | N % | Count | N % | Count | N % | Count | N % | Count | N % | ||
| Serotypes | Total | 1775 | 100.0% | 1397 | 100.0% | 125 | 100.0% | 226 | 100.0% | 27 | 100.0% |
| Others * | 575 | 32.4% | 413 | 29.6% | 61 | 48.8% | 87 | 38.5% | 14 | 51.9% | |
| 8 | 325 | 18.3% | 286 | 20.5% | 11 | 8.8% | 23 | 10.2% | 5 | 18.5% | |
| 3 | 265 | 14.9% | 218 | 15.6% | 12 | 9.6% | 32 | 14.2% | 3 | 11.1% | |
| 22F | 89 | 5.0% | 74 | 5.3% | 5 | 4.0% | 8 | 3.5% | 2 | 7.4% | |
| 9N | 74 | 4.2% | 66 | 4.7% | 0 | 0.0% | 8 | 3.5% | 0 | 0.0% | |
| 14 | 63 | 3.5% | 53 | 3.8% | 3 | 2.4% | 7 | 3.1% | 0 | 0.0% | |
| 19A | 59 | 3.3% | 48 | 3.4% | 5 | 4.0% | 6 | 2.7% | 0 | 0.0% | |
| 6C | 59 | 3.3% | 44 | 3.1% | 2 | 1.6% | 13 | 5.8% | 0 | 0.0% | |
| 23A | 50 | 2.8% | 39 | 2.8% | 1 | 0.8% | 9 | 4.0% | 1 | 3.7% | |
| 10A | 50 | 2.8% | 32 | 2.3% | 12 | 9.6% | 5 | 2.2% | 1 | 3.7% | |
| 15A | 41 | 2.3% | 29 | 2.1% | 6 | 4.8% | 6 | 2.7% | 0 | 0.0% | |
| 31 | 41 | 2.3% | 34 | 2.4% | 0 | 0.0% | 7 | 3.1% | 0 | 0.0% | |
| 11A | 39 | 2.2% | 26 | 1.9% | 4 | 3.2% | 9 | 4.0% | 0 | 0.0% | |
| 23B | 35 | 2.0% | 27 | 1.9% | 3 | 2.4% | 5 | 2.2% | 0 | 0.0% | |
| Invalidated | 10 | 0.6% | 8 | 0.6% | 0 | 0.0% | 1 | 0.4% | 1 | 3.7% | |
| Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2014–2019 | 2020–2022 | 2023–2024 | ||||||
| Count | N % | Count | N % | Count | N % | Count | N % | ||
| Serotype | Total | 2014 | 100.0% | 1184 | 100.0% | 174 | 100.0% | 656 | 100.0% |
| Others * | 669 | 33.2% | 374 | 31.6% | 63 | 36.2% | 232 | 35.4% | |
| 8 | 354 | 17.6% | 185 | 15.6% | 43 | 24.7% | 126 | 19.2% | |
| 3 | 297 | 14.7% | 164 | 13.9% | 28 | 16.1% | 105 | 16.0% | |
| 22F | 101 | 5.0% | 66 | 5.6% | 4 | 2.3% | 31 | 4.7% | |
| 9N | 80 | 4.0% | 60 | 5.1% | 2 | 1.1% | 18 | 2.7% | |
| 14 | 71 | 3.5% | 46 | 3.9% | 1 | 0.6% | 24 | 3.7% | |
| 19A | 70 | 3.5% | 53 | 4.5% | 9 | 5.2% | 8 | 1.2% | |
| 6C | 66 | 3.3% | 43 | 3.6% | 1 | 0.6% | 22 | 3.4% | |
| 23A | 61 | 3.0% | 30 | 2.5% | 5 | 2.9% | 26 | 4.0% | |
| 10A | 56 | 2.8% | 28 | 2.4% | 9 | 5.2% | 19 | 2.9% | |
| 15A | 46 | 2.3% | 37 | 3.1% | 2 | 1.1% | 7 | 1.1% | |
| 31 | 45 | 2.2% | 32 | 2.7% | 3 | 1.7% | 10 | 1.5% | |
| 11A | 43 | 2.1% | 37 | 3.1% | 2 | 1.1% | 4 | 0.6% | |
| 23B | 40 | 2.0% | 29 | 2.4% | 2 | 1.1% | 9 | 1.4% | |
| Invalid | 15 | 0.7% | 0 | 0.0% | 0 | 0.0% | 15 | 2.3% | |
| Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 2014–2019 | 2020–2022 | 2023–2024 | ||||||
| Count | N % | Count | N % | Count | N % | Count | N % | ||
| Serotype | Total | 2014 | 100.0% | 1184 | 100.0% | 174 | 100.0% | 656 | 100.0% |
| 4 | 29 | 1.4% | 10 | 0.8% | 3 | 1.7% | 16 | 2.4% | |
| 16F | 29 | 1.4% | 29 | 2.4% | 0 | 0.0% | 0 | 0.0% | |
| 38 | 23 | 1.1% | 9 | 0.8% | 0 | 0.0% | 14 | 2.1% | |
| 12F | 22 | 1.1% | 4 | 0.3% | 5 | 2.9% | 13 | 2.0% | |
| 17F | 12 | 0.6% | 10 | 0.8% | 2 | 1.1% | 0 | 0.0% | |
| 24F | 11 | 0.5% | 1 | 0.1% | 0 | 0.0% | 10 | 1.5% | |
| 15B/C | 10 | 0.5% | 1 | 0.1% | 4 | 2.3% | 5 | 0.8% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab-Casares, L.; Tormo-Palop, N.; Medina-González, R.; Cortés-Badenes, S.; Hernández-Felices, F.J.; Artal-Muñoz, V.; Martín-Rodríguez, J.L.; Roig-Sena, F.; Marín, J.M.; Gómez-Ruiz, M.D.; et al. Comparative Analysis of Pneumococcal Serotypes for 10 Years (2014–2024) in the Comunidad Valenciana Region, Spain, and How They Are Correlated with PCV13, PCV20, and PCV21. Vaccines 2025, 13, 1018. https://doi.org/10.3390/vaccines13101018
Diab-Casares L, Tormo-Palop N, Medina-González R, Cortés-Badenes S, Hernández-Felices FJ, Artal-Muñoz V, Martín-Rodríguez JL, Roig-Sena F, Marín JM, Gómez-Ruiz MD, et al. Comparative Analysis of Pneumococcal Serotypes for 10 Years (2014–2024) in the Comunidad Valenciana Region, Spain, and How They Are Correlated with PCV13, PCV20, and PCV21. Vaccines. 2025; 13(10):1018. https://doi.org/10.3390/vaccines13101018
Chicago/Turabian StyleDiab-Casares, Laura, Nuria Tormo-Palop, Rafael Medina-González, Sonia Cortés-Badenes, Francisco Javier Hernández-Felices, Violeta Artal-Muñoz, José Luis Martín-Rodríguez, Francisco Roig-Sena, José Manuel Marín, María Dolores Gómez-Ruiz, and et al. 2025. "Comparative Analysis of Pneumococcal Serotypes for 10 Years (2014–2024) in the Comunidad Valenciana Region, Spain, and How They Are Correlated with PCV13, PCV20, and PCV21" Vaccines 13, no. 10: 1018. https://doi.org/10.3390/vaccines13101018
APA StyleDiab-Casares, L., Tormo-Palop, N., Medina-González, R., Cortés-Badenes, S., Hernández-Felices, F. J., Artal-Muñoz, V., Martín-Rodríguez, J. L., Roig-Sena, F., Marín, J. M., Gómez-Ruiz, M. D., Rodríguez-Nortes, F. J., Lamas-Santángelo, M., Gimeno-Cardona, C., & Guna-Serrano, R. (2025). Comparative Analysis of Pneumococcal Serotypes for 10 Years (2014–2024) in the Comunidad Valenciana Region, Spain, and How They Are Correlated with PCV13, PCV20, and PCV21. Vaccines, 13(10), 1018. https://doi.org/10.3390/vaccines13101018






