Abstract
Background: Fasciola hepatica causes important economic losses in ruminants with only pharmacological treatments currently available, which produces several secondary problems. Because of this, vaccines have become an interesting alternative. Leucine aminopeptidases (LAPs) are attractive vaccine targets against fasciolosis since they play essential roles in the parasite such as host invasion and nutrient acquisition. To characterize immune responses, we produced two recombinant F. hepatica LAPs (FhLAP1 and FhLAP2), formulated with ISCOM-matrices (IMXs) nanoparticles from Quillaja brasiliensis saponins. Methods: Forty female Corriedale sheep were assigned to four groups (n = 10): FhLAP1/IMX, FhLAP1/FhLAP2/IMX, IMX (control), and FhLAP1/Adj50 (Adjuvac 50). Animals received two subcutaneous immunizations at weeks 0 and 4 and were challenged orally with 200 metacercariae at week 6. Results: FhLAP1 and FhLAP1/FhLAP2 induced specific IgG responses, with the predominance of the IgG1 response. However, these responses were lower than those generated by FhLAP1 formulated with Adj50. A qPCR analysis revealed that FhLAP1/IMX stimulated a Th1-type response profile before the challenge, but this profile was not sustained after infection. The post-infection profile of FhLAP1/FhLAP2/IMX was more congruent with expected values despite not achieving a robust IFN-γ expression. No significant differences in the fluke burden were observed. Conclusions: Further research on the optimal antigen/adjuvant combination in ruminants is encouraged. For instance, a higher concentration of adjuvant in the formulation used in this work may enhance the strength and duration of the inflammatory response and improve protective immunity against fasciolosis.
1. Introduction
Fasciolosis, an infection caused by Fasciola hepatica, leads to significant economic losses in livestock [1]. This is due to the increased susceptibility to secondary infections, reduced meat, wool, and milk production, interference with fertility, and expenses related to the application of flukicide treatment [1,2]. Furthermore, human fasciolosis is considered a re-emerging disease [3].
Controlling F. hepatica infection relies on treatment with triclabendazole (TCBZ) [4]. However, the high cost, reported cases of drug resistance, the presence of toxic residues in food, and, additionally, the impact they may have on the local environment, create significant obstacles for the marketing of these products [5,6,7]. Vaccination emerges as an alternative capable of providing greater long-term protection and sustainability, as it does not generate toxic residues for consumption and is also not detrimental to the environment [8]. However, to develop new vaccines against F. hepatica, it is crucial to enhance and deepen our understanding of the protective immune response mechanisms in ruminants [8].
During the early stages of fasciolosis, immune response is generally characterized as a mixed Th1/Th2 response, marked by an increase in certain cytokines such as IFNγ, IL4, IL10, and TGFβ in the host. As the infection advances, the Th2 response is amplified, accompanied by the suppression of Th1, which allows for a prolonged infection potentially dependent on IL4 [9].
Helminth peptidase enzymes are crucial factors for parasite establishment within their hosts. Leucine aminopeptidases (LAPs), belonging to the Zn-metalloproteases of the M17 family, have been reported to be involved in tissue invasion and parasite nutrition [10,11,12,13,14,15,16]. For this reason, LAPs have been proposed as potential vaccination antigens to control helminth infections. Previous studies demonstrated the protective potential of the recombinant leucine aminopeptidase 1 (FhLAP1) against fasciolosis in male sheep (74–86%) [17]. Moreover, a second F. hepatica leucine aminopeptidase (FhLAP2), expressed in earlier stages of the parasite [18,19], showed a 70% protection against F. hepatica infection in a murine model [20].
Another essential component in vaccine development is adjuvant selection, primarily aiming to enhance potency by activating innate immunity and promoting controlled inflammation [21]. Nanoparticle adjuvants offer advantages over traditional formulations by inducing rapid and long-lasting cellular and humoral immunity, enabling multiple routes of administration, providing thermal stability at room temperature, and preserving antigen functionality. These features reduce storage costs, facilitate global distribution, and highlight their potential to improve vaccine efficacy in agricultural species [22]. Recently, nanoparticle-based formulations containing ferritin, chitosan, or poly(lactic-co-glycolic) acid (PLGA) showed the induction of a strong immune response that could protect against Echinococcus granulosus, Schistosoma japonicum, and Haemonchus concortus infection [23,24,25]. Other studies report ISCOMs, nanoparticles formed by Quil A saponin extracted from the bark of Quillaja saponaria. These nanoparticles can encapsulate antigens or be delivered as ISCOMATRIXs, and their particulate and hydrophobic nature enhance the efficient antigen uptake by APCs [26]. Our group had previously reported that the saponin fraction (QB) extracted from the leaves of Quillaja brasiliensis, a plant native to Uruguay, can stimulate strong immune responses when formulated with viral antigens [21]. This fraction possesses effective adjuvant properties that make it competitive with market products. To reduce the inherent toxic effects of QB, it is included in micellar formulations called ISCOM-matrices (immunostimulatory complexes) (IMXs). This results in a formulation of nanoparticles which has been reported to successfully work as an early immune response activator, triggering both systemic and mucosal responses [27,28]. More importantly, tests in mice showed that IMX formulations promote high levels of IgG1/IgG2a/IgG2b antibody titers and a Th1-type response (IL2 and INFγ), which is the required immune phenotype for effective control against fasciolosis [21].
Vaccination studies in ruminant livestock species such as cattle, sheep, and goats showed a Th1-type protective response along with high levels of IgG2 [29]. Protection has also been observed, associated with high levels of IgG2 and IgG1 antibodies, indicating a mixed Th1/Th2-type response [17,30,31]. Although no data on the cytokine profile generated in a protective response has been published, it has been suggested that an effective vaccine against fasciolosis should reduce the regulatory effects induced by the parasite and generate a Th1-type or a mixed Th1/Th2-type response throughout the F. hepatica infection. This has been explored in previous studies on vaccination against fasciolosis in ruminants, facilitating advances in understanding the mechanisms of immune protection. For example, the recombinant FhLAP1 produced in our laboratory, combined with different commercial adjuvants, has shown relevant protection results in male sheep (74–86%) [17]. Other antigens with encouraging results include CL1/CL2 mimotopes (47%) and the CL1 mimotope (51%) [31,32]. However, the persistent problem is that none of these promising results have been reproducible in female sheep or cattle, nor have they been validated in field trials [33,34].
In this study, we report the results of a vaccination trial conducted in female sheep using a novel formulation including two leucine aminopeptidases, FhLAP1 and FhLAP2, the latter being expressed in early intra-mammalian developmental stages of the parasite. The antigens were formulated with IMX nanoparticles derived from Q. brasiliensis saponins, alongside another oil-based adjuvant.
2. Materials and Methods
2.1. Production of Recombinant Proteins
Recombinant expressions of the FhLAP1 (AY644459) and FhLAP2 (ON428200) were performed according to Checa et al. [20] and Maggioli et al. [16,17], with modifications. For FhLAP1, we prepared a new expression plasmid pET-FhLAP1 inserting the FhLAP1 gene into the BamHI/SalI site of pET28a vector (Novagene). For FhLAP2, we constructed a new expression plasmid pET-FhLAP2 inserting the FhLAP2 fragment into the SacI/SalI site of pET24a vector (Novagene).
For both FhLAP1 and FhLAP2 recombinant expressions, we used the same protocol. Briefly, E. coli strain BL21 (DE3) cells were transformed with the respective plasmids, and the recombinant clone was grown in LB media with kanamycin (50 μg/mL) to an optical density (OD) 600 nm of 0.8, and recombinant protein production was induced with 0.4 mM IPTG for 24 h at 25 °C.
For FhLAP1 and FhLAP2 protein purification, we used two different protocols, as described in Checa et al. [20] and Maggioli et al. [16,17]. For FhLAP1, the bacterial culture was centrifuged, the pellet resuspended in 50 mM Tris-HCl pH 8.5, 100 mM NaCl, and 5 mM imidazole, containing 1 mg/mL lysozyme, and sonicated (10 pulses of 1 min with 1 min pauses). The lysates were centrifuged for 30 min at 20,000× g, and supernatants were applied to a nickel–nitrilotriacetic acid column (GE Healthcare, Uppsala, Sweden), washed with 50 mM Tris-HC pH 8.5, 100 mM NaCl, and 5 mM imidazole and eluted in imidazole at 20, 50, 200, and 400 mM.
To obtain recombinant FhLAP2, the bacterial culture was centrifuged, and the protein was recovered from the inclusion bodies. Pellet was resuspended in 50 mM Tris pH 8.5, 100 mM NaCl, and 1.5% N-Lauroylsarcosine sodium salt, shaken gently for 1 h, sonicated and centrifuged for 30 min at 20,000× g, and 2% Triton X-100 (AppliChem, Darmstadt, Germany) was added to the supernatant and was incubated within nickel–nitrilotriacetic acid column (GEHealthcare) for 1 h. Then, it was washed with 50 mM Tris pH 8.5, 100 mM NaCl, and 5 mM Imidazole and eluted in imidazole at 20, 50, and 200 mM.
The fractions containing both recombinant proteins were dialyzed against PBS and stored at 4 °C. The protein concentration was determined using the bicinchoninic acid assay (BCA1 Kit, Sigma-Aldrich, St. Louis, MO, USA). The purity of recombinant enzymes was examined using Coomassie-stained 10% SDS-PAGE gels, under reducing conditions. The identity of each recombinant protein was confirmed by MALDI-TOF MS/MS at the Proteomic Unit of the Pasteur Institute of Montevideo.
2.2. ISCOM-Matrices Adjuvant: Preparation and Characterization
QB (A. St.-Hil. et Tul) Mart. leaves were collected in Parque Battle, Montevideo, Uruguay (−34.89302, −56.15727) (voucher MVFQ 4321, deposited at the Herbarium of the Facultad de Química, Universidad de la República, Montevideo Uruguay y). Extraction and purification of saponin fractions were carried out as previously described [35]. IMX was prepared using the dialysis method previously described [36], and nanoparticles obtained were sterilized by filtration using a 0.22 µm syringe filter and maintained at 4 °C.
The preparation was visualized by transmission electron microscopy (TEM) with a JEMM-2100 (JEOL Ltd., Tokyo, Japan) high-resolution transmission electron microscope using a previously described methodology [36].
2.3. Immunoprotection Trial
Forty female Corriedale sheep (eight months old) were housed in the fluke-free experimental field (Campo experimental del Instituto de Higiene, Canelones, Uruguay. Animals were isolated in 4 groups of n = 10 with food and water ad libitum. Animal manipulation was performed in accordance with “Comisión Honoraria de Experimentación Animal” (CHEA) guidelines and was approved by the Uruguayan University Research Ethics Committee (approval number 1046; 22 May 2020).
The vaccinated groups were received 2 subcutaneous doses of 100 µg of both purified recombinant FhLAPs formulated with 50 µg IMX (at weeks 0 and 4) as follows: group FhLAP1 received FhLAP1/IMX; group FhLAP1/FhLAP2 received FhLAP1/FhLAP2/IMX; and control group IMX received IMX alone. Additionally, we included group FhLAP1/Adj50, which received 100 µg of FhLAP1 adjvanted with Adjuvac 50 (Laboratorio VIRBAC Uruguay), with which we had previously achieved a high level of protection in male sheep (Figure 1). At week 6, sheep from the groups FhLAP1, FhLAP1/FhLAP2, and IMX were orally challenged with 200 metacercariae. Blood was collected from all animals before the first immunization and then biweekly until the time of euthanasia. The serum was obtained and stored at −80 °C until the ELISA analysis. At 14 weeks after infection, animals were euthanized. The livers were recovered, and lesions were scored based on the criteria described by Ramamoorthi et al. [37], with modifications: Score 0, no signs of damage observed on the liver; Score 1, mild or minor lesions confined to under 15% of the liver surface; Score 2, moderate to severe damage involving 30–50% of the liver surface; and Score 3, extensive necrosis of more than 50% of the liver surface. Following scoring, flukes were recovered from the liver. To determine the viability of the F. hepatica eggs, they were obtained using the protocols described by Gayo et al. [38]. Briefly, the gallbladders were separated from the livers, and the bile was decanted into a conical vessel. The deposit containing the F. hepatica eggs was extensively washed with water. The eggs were then incubated in the dark at 25 °C for 15 days, and egg hatching was induced by exposure to light. Viability was evaluated by estimating the percentage of eggs that hatched into miracidia [39].
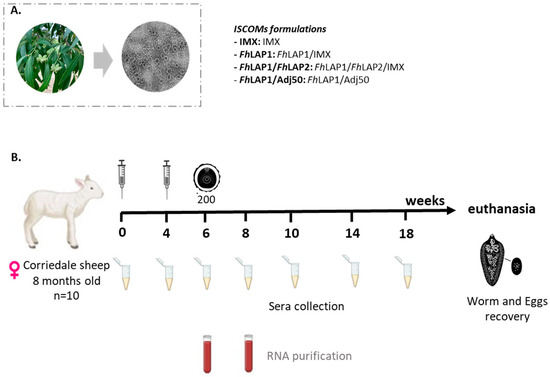
Figure 1.
(A) IMX: nanoparticles (40 nm) formulated with Q. brasilliensis (QB) purified saponin fraction (without antigen) by the dialysis method and visualized by HR-TEM. Negative staining preparation was visualized by TEM with Joel JEM 2100, LaB6-HRSTEM. (B) Scheme of the vaccination and challenge experiments. Female Corriedale sheep were immunized s.c on weeks 0 and 4 with FhLAP1/IMX; FhLAP1/FhLAP2/IMX; IMX; or FhLAP1/Adj50. Two weeks later, the first three groups were orally challenged with 200 metacercariae of F. hepatica. The blood samples were collected every two weeks. At week 20, animals were euthanized.
2.4. ELISA
2.4.1. Total IgG
Anti-FhLAP1 or anti-FhLAP2 total IgG were determined for each serum sample by ELISA, as described in Maggioli et al. [17]. Microplates (Greiner Bio-One, Darmstadt, Germany) were coated with FhLAP1 (2 μg/mL) or FhLAP2 (2 μg/mL) in PBS and incubated overnight at 4 °C. Then, plates were washed three times with PBS containing 0.05% Tween20 (PBS-T) and blocked with PBS-T and 1% BSA overnight at 4 °C. Sera collected from groups FhLAP1, FhLAP1/FhLAP2, IMX, and FhLAP1/Adj50 were diluted in PBS-T and 0.5% BSA and incubated for 1 h at 37 °C. After washing with PBS-T, plates were incubated with HRP-conjugated anti-ovine IgG (Sigma-Aldrich) for 1 h at 37 °C. Following washes, the substrate solution (3,3′,5,5′-Tetramethylbenzidine (TMB) (Sigma-Aldrich) and H2O2 were added, and the reaction was stopped by adding 50 μL/well of HCl 1 N.
A standard curve was built using a pool of sera from immunized groups, and the antibody concentration of each serum sample was obtained in terms of arbitrary units (AU/mL) by interpolating the optical density readings and multiplying them by the dilution factor.
2.4.2. IgG1 and IgG2 Subclasses
To determine specific IgG subclasses, microplates (Greiner Bio-One, Darmstadt, Germany) were coated with FhLAP1 (2 μg/mL) or FhLAP2 (2 μg/mL) in PBS incubated overnight at 4 °C. Then, plates were washed and blocked as described above.
All sera from groups FhLAP1, FhLAP1/FhLAP2, and IMX were diluted 1:100, and sera FhLAP1/Adj50 were diluted 1:500 in PBS-T and 0.5% BSA. Plates were incubated with anti-ovine IgG1 (1:1000; clone McM1; Pentlands Immunologics, Penicuik, UK) or anti-ovine IgG2 (1:500, clone McM3; Pentlands Immunologics) for 1 h at 37 °C. After washing with PBS-T, plates were incubated with HRP-conjugated anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37 °C. Following washes, the substrate TMB and H2O2 were added, and the reaction was stopped by adding 50 μL/well of HCl 1 N.
IgG subclass titers were expressed in OD 450 nm because they were much lower than those for total IgG levels.
2.5. Stimulation and Isolation of Peripheral Blood Leukocytes (PBL)
To study the immune response elicited by our formulations in sheep, changes in cytokine gene expression were assessed in blood samples stimulated in vitro with FhLAP1 or FhLAP1/FhLAP2 antigens, collected in weeks 6 (pre-challenge) and 8 (post-challenge) (Figure 1B). Four animals per group were used to collect blood in a tube containing 1% EDTA. Whole blood was stimulated for 6 h at 37 °C in 5% CO2 with 20 µg/mL of FhLAP1 (FhLAP1 group) or 20 µg/mL total FhLAP1/FhLAP2 (FhLAP1/FhLAP2 group), while the control group (IMX group) was stimulated with 20 µg/mL total of FhLAP1 and FhLAP1/FhLAP2 proteins. As a positive control, 1 µg/mL of concanavalin A (Con A, Sigma Aldrich) was used for each blood sample. Then, blood was incubated with red blood cell lysis buffer [40]. The obtained PBLs were thoroughly washed with PBS. Finally, the PBL pellets were conserved in TRIzol reagent at −80 °C.
2.6. Gene Expression Analysis
Total RNA from the pre- and post-challenge PBLs obtained above was extracted using the TRIzol protocol (InvitrogenTM) according to the manufacturer’s instructions. RNA concentration was measured using a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only RNA samples with an A280/A260 ratio in the range of 1.8–2.0 and an A260/A230 ratio in the range of 2.0–2.2 were used for cDNA synthesis. Total RNA (1 μg) was treated with 0.4 U of DNase I (Invitrogen, Carlsbad, CA, USA) to remove residual DNA and then reverse transcribed using the SensiFAST cDNA Synthesis Kit (BIO-65053, Meridian Bioscience, Cincinnati, OH, USA), following the manufacturer’s instructions.
Following cDNA synthesis, quantitative PCR (qPCR) was conducted using QuantiTect® SYBR® Green PCR Kit (Qiagen, Hilden, Germany) in an ABI 7900 HT (Applied Biosystems, Foster City, CA, USA) thermocycler. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene, as it has been previously validated by [41] and is widely used in whole blood samples [42,43,44,45]. Prior to relative quantification, we confirmed that its expression remained stable across the different experimental conditions by comparing the Ct value for GAPDH among the groups and times. No significant differences were observed, confirming its suitability as a reference gene.
The primer pairs for IL1β, IL2, IL4, IL5, IL10, IL17, IFNγ, FoxP3, TGFβ, and TNFα mRNA were designed by our group and produced amplicons of the predicted size are showed in Table 1. Cycle program was as follows: initial incubation of 15 min at 95 °C; followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C with data acquisition; and finally, a melt curve with a ramp from 60 to 95 °C at 1 °C/s. Melt curve analysis was performed to identify and exclude reactions with alternative amplicons.

Table 1.
Description and sequences of the primers designed to quantify specific ovine genes using qPCR.
The relative mRNA amount in each sample was calculated using the 2−ΔΔCt method, as previously described by Livak and Schmittgen [46], where ΔCt = Ct gene of interest—Ct GAPDH. For each cytokine, the mRNA levels in the FhLAP1 and FhLAP1/FhLAP2 groups were expressed relative to the average of the control group (IMX group) stimulated with FhLAP1 or FhLAP1/FhLAP2 proteins, respectively, at pre- and post-challenge (calibration condition).
2.7. Exploratory Analysis of Correlations Pre- and Post-Challenge
To analyze the relationships between the changes in cytokine gene expression (IFNγ, TNFα, IL1β, TGFβ, IL10, FoxP3, IL17, and IL2) and the worms recovered (WR) at pre- and post-challenge points within the FhLAP1 or FhLAP1/FhLAP2 groups, the Pearson correlation coefficient was used [47].
2.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 8.00) and PAST (version 4.1) [48]. A p-value of <0.05 was considered statistically significant for all analyses.
For the ELISA data, all results were presented as the mean ± standard error (SE). The Kruskal–Wallis test was employed to compare differences between groups for non-parametric data. When significant differences were detected (p < 0.05), pairwise comparisons were carried out using Dunn’s post hoc test.
For the cytokine gene expression data, statistical analysis was conducted at each time point (pre- and post-challenge) within each group (FhLAP1 or FhLAP1/FhLAP2). Most data were log-transformed to achieve a more normal distribution. The Shapiro–Wilk test was applied to assess the normality of distributions. For within-group comparisons over time, the paired Student’s t-test [49] was used for parametric data after verifying the assumptions of normality [49] and homogeneity of variances [50]. If these assumptions were not met, a non-parametric paired Wilcoxon test was used [47] (Table 2).

Table 2.
Statistical analysis summary for evaluating changes in cytokine gene expression levels. Student test (t), Wilcoxon test (W). Significant * p < 0.05 and ** p-values = 0.023; ns: not significant.
The statistical relationship among variables, specifically worm recovery (WR) and expression of various cytokine genes, was investigated. A Pearson’s correlation test was used to analyze the data after it had been log-transformed to meet the assumption of a normal distribution. p-values of 0.05 or lower were considered statistically significant.
3. Results
3.1. Obtaining Recombinant FhLAP1, FhLAP2, and IMX
Recombinant FhLAP1 was purified by nickel-chelate affinity chromatography and resulted in a protein of high yield (22.4 mg/L). However, for FhLAP2, the protein refolding procedure resulted in a substantial loss of the protein probable by precipitation. Nevertheless, this procedure improved protein recovery compared to the one described by Checa et al. [20], yielding 1 mg of recombinant FhLAP2/liter of cell culture.
Both recombinant LAPs analyzed by SDS-PAGE showed a high purity level (>90%). The identity of each recombinant protein was confirmed by MALDI-TOF MS/MS. In addition, we obtained the IMX nanoparticles (40 nm) formulated with Q. brasiliensis purified saponin fraction by the dialysis method and visualized by HR-TEM (Figure 1A).
3.2. Vaccination Efficacy Based on Worm Recovery and Egg Viability
We carried out the total adult fluke count for each group in the trial (Figure 1B). The fluke burdens were 27.8% lower in the FhLAP1/FhLAP2 group compared to the control IMX vaccinated group (Figure 2B). There is no significant difference compared to the control group IMX. In addition, FhLAP1 and IMX groups showed greater variability compared with the FhLAP1/FhLAP2 group (Figure 2A). Furthermore, the FhLAP1 group displayed a reduction in egg viability (20%) compared to the FhLAP1/FhLAP2 and IMX groups (Figure 2B).
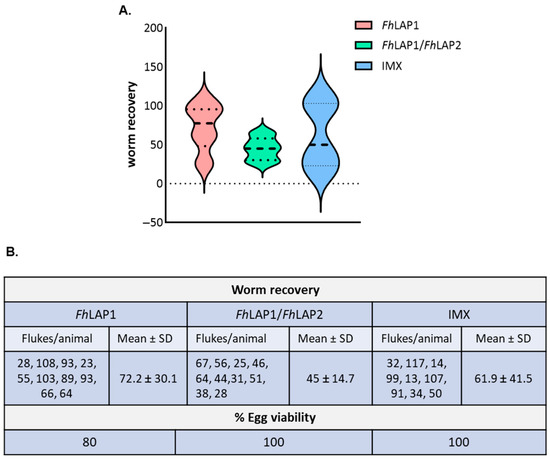
Figure 2.
(A) Analysis of liver flukes and egg viability at necropsy within FhLAP1, FhLAP1/FhLAP2, and IMX groups. Violin plots illustrate worm recovery variability. Median (striped line), minimum, and maximum (dotted line). (B) The data is shown in the table as the mean value ± SD (n = 10) and was analyzed by Kruskal Wallis test followed by Dunns’ test. No significant difference was detected.
3.3. Humoral Response Induced by Vaccination with FhLAP1, FhLAP2, and IMX
To assess the humoral immune response induced by the formulations, serum samples collected from sheep were tested for the FhLAP1 or FhLAP2 specific total IgG and the isotypes IgG1 and IgG2 by ELISA. Figure 3 shows the reactivity of sheep sera towards the FhLAP1 in the immunized groups (FhLAP1, FhLAP1/FhLAP2, and FhLAP1/Adj50). As expected, specific total IgG levels were higher in the serum of sheep vaccinated than in the control IMX group.
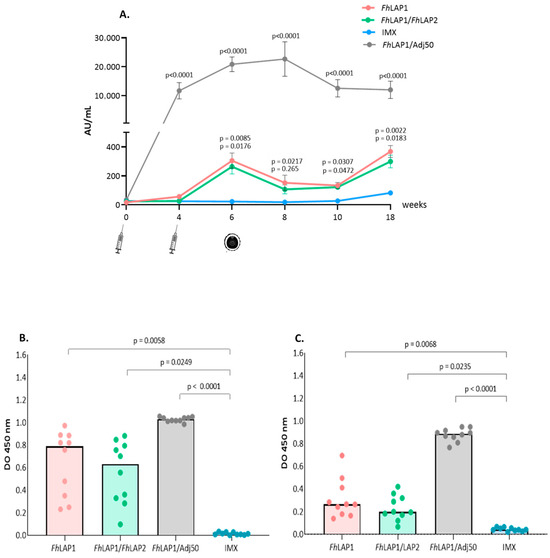
Figure 3.
FhLAP1-specific IgG antibodies detection by ELISA. (A) Total IgG antibodies were measured in sheep sera (weeks 0, 4, 6, 8, 10, and 18). (B) IgG1 and (C) IgG2 subclass antibodies were measured in sheep sera at week 6 post-immunization. Serum samples were collected from the sheep every two weeks; syringe icon = immunizations and metacercaria icon = infection. Results have been expressed as the mean value ± SE (n = 10). The data were analyzed by Kruskal–Wallis test followed by Dunns’ test.
A significant antibody response was elicited in the FhLAP1 and FhLAP1/FhLAP2 groups two weeks after the second immunization. Then, a decrease in the absorbance of these groups was observed during the 4 weeks after the challenge. In week 10, antibody levels increased, possibly due to liver fluke infection. Nevertheless, the FhLAP1/Adj50 formulation (non-infected group) showed the highest total IgG levels compared to the other three groups (>60-fold). Moreover, this high titer was maintained as high for a more extended period (Figure 3A). Furthermore, significant differences can already be observed with a single dose by week 4.
Levels of IgG1 and IgG2 reacting to the FhLAP1 in sheep from all four groups were measured at week 6 (Figure 3B,C). IgG1 and IgG2 levels were significantly higher in the immunized groups compared with the IMX group. Also, we observed that FhLAP1/Adj50 showed the highest IgG1 and IgG2 levels.
The reactivity of sera towards FhLAP2 in the immunized groups shows that the specific total IgG levels were higher in the serum of FhLAP1/FhLAP2 and FhLAP1/Adj50 groups than in the FhLAP1 and IMX groups (Figure 4). A significant antibody response was elicited in the FhLAP1/FhLAP2 and FhLAP1/Adj50 groups two weeks after the second immunization. A decrease in the absorbance of the FhLAP1/FhLAP2 group was observed during the two weeks after the challenge, then antibody levels remained until the end of the trial.
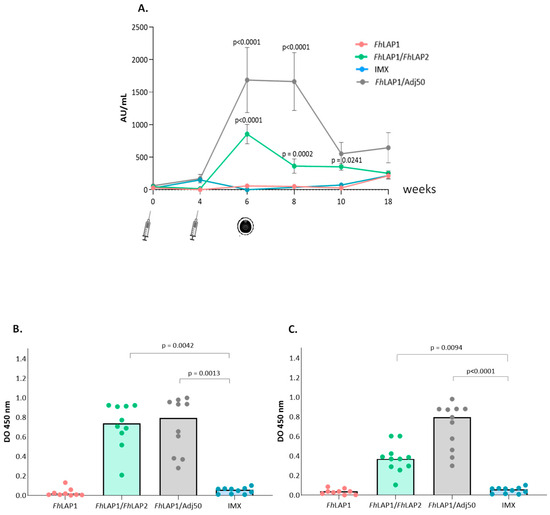
Figure 4.
FhLAP2-specific IgG antibodies detection by ELISA. (A) Total IgG antibodies were measured in sheep sera (weeks 0, 4, 6, 8, 10, and 18). (B) IgG1 and (C) IgG2 subclass antibodies were measured in sheep sera at week 6 post-immunization. Serum samples were collected from the sheep every two weeks (syringe) for immunizations and (metacercaria) infection. Results have been expressed as the mean value ± SE (n = 10). The data were analyzed by Kruskal–Wallis test followed by Dunns’ test.
On week 6, IgG1 and IgG2 levels against FhLAP2 were significantly higher in the FhLAP1/FhLAP2 and FhLAP1/Adj50 groups compared with the FhLAP1 and IMX groups (Figure 4B,C).
For the FhLAP1/Adj50 group, we observed the highest total IgG and isotype IgG2 levels compared to the other three groups (Figure 4). This reactivity may be due to the cross-reaction with specific antibodies against the FhLAP1 present in the FhLAP1/Adj50 group; however, this was not observed in the FhLAP1 group.
3.4. Gene Expression Levels Induced by Vaccination with FhLAP1 or FhLAP1/FhLAP2
To evaluate the immune response induced by our formulations in sheep, cytokine gene expression changes were assessed in in vitro-stimulated blood samples using FhLAP1 or FhLAP1/FhLAP2 antigens at pre- and post-challenge time points.
In the FhLAP1 group, we detected a significant increase in the pro-inflammatory and regulatory cytokine profile (TNFα, IL10, and IL17) before the challenge. Additionally, we observed an upward trend in IFNγ, IL1β, and IL2 expression at the pre-challenge. After the challenge, the expression levels of cytokines decreased (Figure 5A and Table 2).
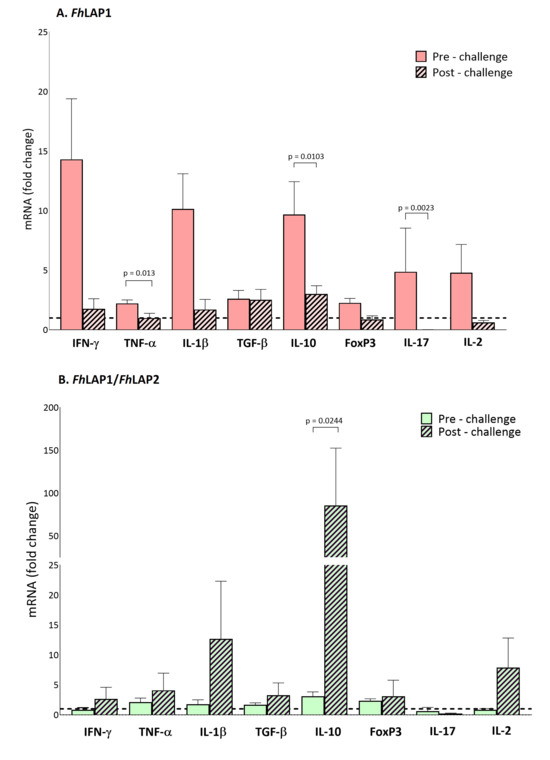
Figure 5.
Gene expression levels in PBLs of cytokines (INFγ, TNFα, IL1β, TGFβ, IL10, FoxP3, IL17, and IL2) induced by the (A) FhLAP1 and (B) FhLAP1/FhLAP2 formulations pre- and post-challenge. Gene expression was analyzed using ΔΔCT [46], normalized with reference gene (GAPDH) and relative to IMX group. Each bar represents the mean ± SEM (n = 4). Statistical analysis was performed on log-transformed data using either a parametric paired t-test or a non-parametric paired Wilcoxon test (Table 2). The dotted line indicates a log2 fold-change threshold of 1.0 relative to the IMX group.
In contrast, the FhLAP1/FhLAP2 vaccinated group exhibited a different cytokine profile at both the pre- and post-challenge time points compared to the FhLAP1 group. Before the challenge, no changes in cytokine expression levels were detected relative to the control IMX group. However, after the challenge, a significant shift toward both regulatory (IL10) and pro-inflammatory (TNFα, IL1β, and IL2) responses was observed (Figure 5B and Table 2).
Notably, we were unable to measure Th2-associated cytokines (IL4, IL5) in any experimental group.
3.5. Correlation Between Immunological Parameters and Recovery Worm
We then performed a correlation analysis to investigate whether WR might be associated with the immunological parameters analyzed in this study. To this end, pre- and post-challenge cytokine expression levels were assessed within the FhLAP1 and FhLAP1/FhLAP2 groups
Figure 6 and Figure 7 show correlation matrices based on Pearson’s correlation coefficient, generated from an exploratory analysis. For the FhLAP1 group, we observed a significant positive correlation between FOXP3 and IFNγ and significant negative correlations between IL17 with FOXP3, IFNγ, and IL10 expression levels at the pre-challenge point (Figure 6A). After the challenge, a shift in the correlation profile was observed, with a significant positive correlation between the TNFα and IL1β (Figure 6B), indicating the potential modulation of the immune response during infection.
In the FhLAP1/FhLAP2 group, the pre-challenge analysis revealed a cytokine expression profile distinct from that observed in the FhLAP1 group. IFNγ expression levels showed a positive correlation with both IL2 and TGFβ. Additionally, negative correlations were observed between TGFβ and IL2, as well as between FOXP3 and IL17 (Figure 7A). WR was positively correlated with TGFβ and negatively correlated with IL2.
After the challenge, the expression profile changed, with significant positive correlations observed among several cytokines: IL1β with TNFα; TGFβ with IFNγ, TNFα, and IL1β; IL10 with IFNγ, TNFα, and TGFβ; and FOXP3 with IFNγ, IL1β, and TGFβ. No correlation was found between the WR and changes in cytokine expression (Figure 7B).
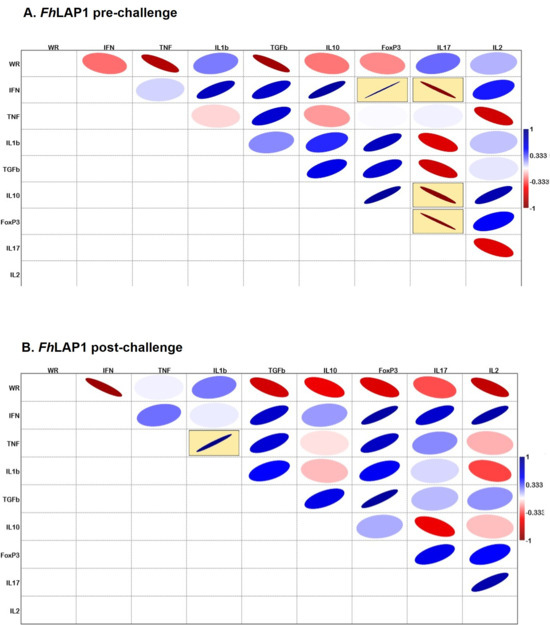
Figure 6.
Correlation matrix using Pearson’s correlation coefficient between worm recovery (WR) and gene expression levels of cytokines (IFNγ, TNFα, IL1β, TGFβ, IL10, FoxP3, IL17, and IL2) within the FhLAP1 group. (A) Pre-challenge and (B) post-challenge points. Blue line indicates positive correlation, and red line indicates negative correlation. The line thickness and color intensity reflect the magnitude of the correlation. Yellow boxes indicate a statistically significant correlation (p < 0.05).
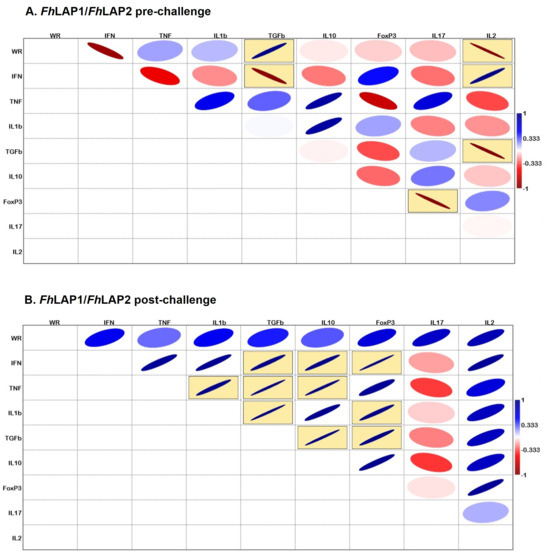
Figure 7.
Correlation matrix using Pearson’s correlation coefficient between worm recovery (WR) and gene expression levels of cytokines (IFNγ, TNFα, IL1β, TGFβ, IL10, FoxP3, IL17, and IL2) within the FhLAP1/FhLAP2 group. (A) Pre-challenge and (B) post-challenge points. Blue line indicates positive correlation, and red line indicates negative correlation. The line thickness and color intensity reflect the magnitude of the correlation. Yellow boxes indicate a statistically significant correlation (p < 0.05).
4. Discussion
Fasciolosis remains a public health and economical problem in many countries, and the identification of vaccine candidates with antiparasitic effects is a challenge in this multicellular parasite. Given the key roles that proteases such as LAPs play in the biological processes of helminths, including digestion, invasion, and migration through the host’s tissues [12,19,51,52], we expected that LAPs-formulated vaccines can reduce the parasite burden. Previous data showed LAPs as the main candidates for vaccine development against fasciolosis, specifically the use of recombinant FhLAP1 combined with different adjuvants, including FhLAP1/Adj50, which has shown relevant protection results in male sheep [17].
Previous studies using recombinant FhLAP1 resulted in fluke burden reductions of 29% and 89%, depending on the vaccine candidate and/or adjuvant used, with highly diverse results [17,20,53,54,55]. Regarding helminths, the use of a recombinant protein cocktail vaccine in ruminants may be more effective than vaccines based on individual antigens [56,57]. We therefore postulate FhLAP2 as an antigen to be used together with FhLAP1 in a bivalent vaccine formulation. FhLAP2 has shown a higher expression in metacercariae and NEJ than FhLAP1 [18,19], and the immunization of mice with FhLAP2/FIA resulted in significant protection against F. hepatica infection [20].
Until the present work, these vaccines’ effectiveness in female sheep have not been evaluated. In the female sheep vaccination trial, the mean number of flukes recovered in the IMX group was 61.9 ± 41.5 (range 13–117) and 45 ± 14.7 (range 25–67) in the FhLAP1/FhLAP2 group. Statistical tests showed no significant differences between vaccinated and non-vaccinated female animals, likely due to the high individual variability in the IMX groups (Figure 2). It is noteworthy that two sheep in the IMX group presented low parasite burdens of 13 and 14 flukes. Previous studies have suggested that individual differences in immune responsiveness contribute to the variability of fluke burdens following experimental infection [58,59]. Although evidence on sex-related differences in immune responses remains limited, a vaccination study against fasciolosis in rats suggested potential sex-specific differences, where males exhibited more protective responses than females [60]. In our studies, it is also possible that the variability observed may reflect underlying differences in the type of immune response between the sexes in sheep. There are some reports showing a higher prevalence and intensity of helminth infections in males compared to females, evidencing sex-related differences in the immune response [61,62]. The potential implications of these studies warrant further investigation.
In this work, FhLAP1/Adj50 was used as a reference for IMX-based formulations. These nanoparticles based on Q. brasiliensis saponins have been tested in mice with viral antigens polarizing toward a strong Th1 profile [21]. Herein, two fluke antigens, FhLAP1 and FhLAP1/FhLAP2, formulated with IMX, were able to induce a specific total IgG antibody response compared with the IMX control group in ruminants. However, the IgG levels were not as high as those generated by FhLAP1 formulated with Adj50. In accordance with this, other reports using QuilA as an adjuvant in ruminants [58,63,64,65] also showed that using 1 mg/mL QuilA achieved higher levels of specific antibodies compared to those induced by other adjuvants. QuilA injections drive the transition to Th1-type cells, leading to IFN-γ secretion and triggering IgG2 production by B cells. QuilA strongly skews immune responses towards the Th1 type [65], a response aimed to be stimulated to induce protection. In this work, twenty times less saponin was used to prepare the IMX. Although more experimental studies are required, we suggest that it may be necessary to increase the IMX concentration to achieve more robust humoral and inflammatory responses and to sustain them during the infection. The FhLAP1/Adj50 formulation was effective at inducing a strong specific IgG antibody response composed of both IgG1 and IgG2 subtypes, indicating a mixed Th1/Th2 response in female sheep. This result could be in line with evidence associating high antibody levels with protection against F. hepatica in ruminants [57,63,66,67]. Previous data showed that male sheep immunized with FhLAP1/Adj50 had a significant fluke burden reduction and induced a mixed Th1/Th2 immune response [17]. So, it would be reasonable to assume that this formulation would also reduce the worm burden in female sheep.
Several studies have demonstrated that oil-based adjuvants promote high and durable antibody titers but induce intolerable reactogenicity, such as abscess and cyst formation at the injection site [68,69], which negatively impacts the meat and hides of livestock [70]. An alternative is the use of saponins extracted from the leaves of Q. brasiliensis without the need to cut down the tree, formulated as nanoparticles (IMX). In addition, they exhibit greater biodegradability compared with oil-based adjuvants. The present work demonstrated the induction of mixed Th1/Th2 responses using FhLAPs in female sheep that potentially protect against fasciolosis. Additional challenge trials using a high number of females are needed to confirm the antiparasitic effect of the FhLAPs/IMX formulation.
When the immune profile through the gene expression of Th1/Th2/Th17/Treg cytokines was analyzed, the FhLAP1/FhLAP2 group showed a trend toward a pro-inflammatory and regulatory profile compared with the FhLAP1 group after the challenge. We observed that infection with F. hepatica might change the cellular immune profile initially generated by the formulations, which could be beneficial for worm establishment [71,72]. Protective immunity requires a dominant Th1 response [29]. Chronic infection promotes an immunoregulatory environment with the suppression of parasite-specific Th1/Th2 responses and increased IL-10 [73,74,75]. In this study, cytokines associated with a Th2-type response (IL-4, IL-5, or IL-13) were not evaluated, and therefore, their impact could not be assessed. However, we observed that FhLAP1/IMX formulations stimulated a Th1-type response profile before the challenge, but this profile was not sustained after infection. With the FhLAP1/FhLAP2 formulation, the post-infection profile appeared more appropriate; however, a robust IFN-γ expression, indicative of protective immunity, was not achieved. The presence of IL-10 was observed, suggesting a suppressive Th1/Th2 response more consistent with that developed during a chronic infection [66].
To date, it remains unclear which specific immune mechanisms need to be induced by vaccination. Taking everything into account, we suggest that a strong humoral immune response, coupled with the selection of an appropriate adjuvant, is required for the specific inhibition of these enzymes, which may compromise the survival of the parasite by impeding vital processes. Strategic adjuvant selection is essential to enhance vaccine immunogenicity and stimulate the production of immunologically active molecules, thereby inducing stronger and longer-lasting protective immunity [76,77,78]. In this sense, it would be necessary to generate a robust humoral immune response in addition to stimulating greater production of IFN-γ, indicating a Th1 profile immune stimulus. Our data suggests that future trials should consider increasing the IMX concentration or using an oil-based adjuvant such as Adj50 to generate a more robust humoral immune response in addition to stimulating a Th1 profile immune response.
5. Conclusions
In this study, we evaluated FhLAP-based formulations using IMX as the adjuvant that was capable of inducing a regulatory Th1/Th2 immune environment in ruminants. No significant differences in the worm burden were observed, probably due to high variability in the IMX control group inherent to characteristics of this natural host such as sexor breed. These findings provide valuable insights into the design of vaccination trials in ruminants. They will guide future studies aimed at refining candidate selection, optimizing formulations, evaluating novel adjuvants and delivery systems, and ensuring statistically robust group sizes for F. hepatica vaccination. Additional studies are warranted to assess this strategy in ruminants.
Author Contributions
Conceptualization and funding acquisition: G.M. Literature/data curation: J.C., W.N., M.C., F.S., and G.M. Formal analysis and visualization: J.C., R.V., W.N., A.R., F.S., M.C., and G.M. Writing—original draft preparation: J.C. and G.M. Writing—review and editing: J.C., A.G., R.V., W.N., P.A., F.S., M.C., and G.M. Methodology and software: J.C., A.G., R.V., P.A., F.S., E.C., O.C., W.N., A.R., M.C., and G.M. Final validation and supervision: F.S., A.R., and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Agencia Nacional de Investigación e Innovación—Fondo María Viñas (ANII-FMV_1_2019_1_155469), Comisión Academica de Posgrado (CAP) and PEDEClBA—Program for the Development of Basic Sciences, Uruguay.
Conflicts of Interest
There are no conflicts of interest.
References
- Mehmood, K.; Zhang, H.; Sabir, A.J.; Abbas, R.Z.; Ijaz, M.; Durrani, A.Z.; Saleem, M.H.; Ur Rehman, M.; Iqbal, M.K.; Wang, Y.; et al. A Review on Epidemiology, Global Prevalence and Economical Losses of Fasciolosis in Ruminants. Microb. Pathog. 2017, 109, 253–262. [Google Scholar] [CrossRef]
- Sinclair, K.B. The Pathogenicity of Fasciola hepatica in Pregnant Sheep. Br. Vet. J. 1972, 128, 249–259. [Google Scholar] [CrossRef] [PubMed]
- WHO. Integrating Neglected Tropical Diseases into Global Health and Development: Fourth WHO Report on Neglected Tropical Diseases; WHO: Geneva, Switzerland, 2017; 270p. [Google Scholar]
- Fairweather, I. Liver Fluke Isolates: A Question of Provenance. Vet. Parasitol. 2011, 176, 1–8. [Google Scholar] [CrossRef]
- Stuen, S.; Ersdal, C. Fasciolosis—An Increasing Challenge in the Sheep Industry. Animals 2022, 12, 1491. [Google Scholar] [CrossRef]
- Canevari, J.; Ceballos, L.; Sanabria, R.; Romero, J.; Olaechea, F.; Ortiz, P.; Cabrera, M.; Gayo, V.; Fairweather, I.; Lanusse, C.; et al. Testing Albendazole Resistance in Fasciola hepatica: Validation of an Egg Hatch Test with Isolates from South America and the United Kingdom. J. Helminthol. 2014, 88, 286–292. [Google Scholar] [CrossRef]
- Imperiale, F.; Ortiz, P.; Cabrera, M.; Farias, C.; Sallovitz, J.M.; Iezzi, S.; Pérez, J.; Alvarez, L.; Lanusse, C. Residual Concentrations of the Flukicidal Compound Triclabendazole in Dairy Cows’ Milk and Cheese. Food Addit. Contam.—Part A 2011, 28, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernández, V.; Mulcahy, G.; Pérez, J.; Martínez-Moreno, Á.; Donnelly, S.; O’Neill, S.M.; Dalton, J.P.; Cwiklinski, K. Fasciola hepatica Vaccine: We May Not Be There yet but We’re on the Right Road. Vet. Parasitol. 2015, 208, 101–111. [Google Scholar] [CrossRef]
- Flores-Velázquez, L.M.; Ruiz-Campillo, M.T.; Herrera-Torres, G.; Martínez-Moreno, Á.; Martínez-Moreno, F.J.; Zafra, R.; Buffoni, L.; Rufino-Moya, P.J.; Molina-Hernández, V.; Pérez, J. Fasciolosis: Pathogenesis, Host-Parasite Interactions, and Implication in Vaccine Development. Front. Vet. Sci. 2023, 10, 1270064. [Google Scholar] [CrossRef] [PubMed]
- Acosta, D.; Goni, F.; Carmona, C. Characterization and Partial Purification of a Leucine Aminopeptidase from Fasciola hepatica. J. Parasitol. 1998, 84, 1–7. [Google Scholar] [CrossRef]
- Acosta, D.; Cancela, M.; Piacenza, L.; Roche, L.; Carmona, C.; Tort, J.F. Fasciola hepatica Leucine Aminopeptidase, a Promising Candidate for Vaccination against Ruminant Fasciolosis. Mol. Biochem. Parasitol. 2008, 158, 52–64. [Google Scholar] [CrossRef]
- Williamson, A.L.; Lecchi, P.; Turk, B.E.; Choe, Y.; Hotez, P.J.; McKerrow, J.H.; Cantley, L.C.; Sajid, M.; Craik, C.S.; Loukas, A. A Multi-Enzyme Cascade of Hemoglobin Proteolysis in the Intestine of Blood-Feeding Hookworms. J. Biol. Chem. 2004, 279, 35950–35957. [Google Scholar] [CrossRef]
- McCarthy, E.; Stack, C.; Donnelly, S.M.; Doyle, S.; Mann, V.H.; Brindley, P.J.; Stewart, M.; Day, T.A.; Maule, A.G.; Dalton, J.P. Leucine Aminopeptidase of the Human Blood Flukes, Schistosoma mansoni and Schistosoma japonicum. Int. J. Parasitol. 2004, 34, 703–714. [Google Scholar] [CrossRef]
- Rinaldi, G.; Morales, M.E.; Alrefaei, Y.N.; Cancela, M.; Castillo, E.; Dalton, J.P.; Tort, J.F.; Brindley, P.J. RNA Interference Targeting Leucine Aminopeptidase Blocks Hatching of Schistosoma mansoni Eggs. Mol. Biochem. Parasitol. 2009, 167, 118–126. [Google Scholar] [CrossRef]
- Kang, J.M.; Ju, H.L.; Ju, J.W.; Sohn, W.M.; Kim, T.S.; Bahk, Y.Y.; Hong, S.J.; Na, B.K. Comparative Biochemical and Functional Properties of Two Leucine Aminopeptidases of Clonorchis Sinensis. Mol. Biochem. Parasitol. 2012, 182, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Maggioli, G.; Rinaldi, G.; Giaudrone, I.; Berasain, P.; Tort, J.F.; Brindley, P.J.; Carmona, C. Expression, Purification and Characterization of Two Leucine Aminopeptidases of the Blood Fluke, Schistosoma mansoni. Mol. Biochem. Parasitol. 2018, 219, 17–23. [Google Scholar] [CrossRef]
- Maggioli, G.; Acosta, D.; Silveira, F.; Rossi, S.; Giacaman, S.; Basika, T.; Gayo, V.; Rosadilla, D.; Roche, L.; Tort, J.; et al. The Recombinant Gut-Associated M17 Leucine Aminopeptidase in Combination with Different Adjuvants Confers a High Level of Protection against Fasciola hepatica Infection in Sheep. Vaccine 2011, 29, 9057–9063. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Jewhurst, H.; McVeigh, P.; Barbour, T.; Maule, A.G.; Tort, J.; O’Neill, S.M.; Robinson, M.W.; Donnelly, S.; Dalton, J.P. Infection by the Helminth Parasite Fasciola hepatica Requires Rapid Regulation of Metabolic, Virulence, and Invasive Factors to Adjust to Its Mammalian Host. Mol. Cell. Proteom. 2018, 17, 792–809. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; Robinson, M.W.; Donnelly, S.; Dalton, J.P. Complementary Transcriptomic and Proteomic Analyses Reveal the Cellular and Molecular Processes That Drive Growth and Development of Fasciola hepatica in the Host Liver. BMC Genom. 2021, 22, 46. [Google Scholar] [CrossRef]
- Checa, J.; Salazar, C.; Goyeche, A.; Rivera, M.; Silveira, F.; Maggioli, G. A Promising New Target to Control Fasciolosis: Fasciola hepatica Leucine Aminopeptidase 2. Vet. Parasitol. 2023, 320, 109959. [Google Scholar] [CrossRef]
- Morais, V.; Suarez, N.; Cibulski, S.; Silveira, F. Leaf Saponins of Quillaja brasiliensis as Powerful Vaccine Adjuvants. Pharmaceutics 2025, 17, 966. [Google Scholar] [CrossRef]
- Maina, T.W.; Grego, E.A.; Boggiatto, P.M.; Sacco, R.E.; Narasimhan, B.; McGill, J.L. Applications of Nanovaccines for Disease Prevention in Cattle. Front. Bioeng. Biotechnol. 2020, 8, 608050. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, X.; Liu, X.; Zhou, C.; Shang, Y.; Wu, T.; Jia, H.; Zhang, Z.; Li, Y.; Xin, T. A Ferritin-Based Eg95 Nanoparticle Vaccine Adjuvanted with PCpG Eliciting Robust Immune Responses Against Cystic Echinococcosis in Mice Model. Int. J. Nanomed. 2025, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Muhammad, T.A.; Muhammad, W.H.; Muhammad, A.M.; Muhammad, H.; Yan, R.F.; Xu, L.X.; Song, X.K.; Li, X.R. Hepatocellular Carcinoma-Associated Antigen 59 and ADP-Ribosylation Factor 1 with Poly (Lactic-Co-Glycolic Acid): A Promising Candidate as Nanovaccine against Haemonchosis. Microb. Pathog. 2022, 168, 105614. [Google Scholar] [CrossRef]
- Xinxin, Z.; Xianzhou, L.; Dandan, P.; Yan, W.; Zhenyu, L. Immunization with the Glutathione S-Transferase Sj26GST with Chi-CpG NP against Schistosoma japonicum in Mice. Microb. Pathog. 2024, 195, 106847. [Google Scholar] [CrossRef]
- Solano-Parada, J.; Gonzalez-Gonzalez, G.; de Pablos Torró, L.M.; Brazil dos Santos, M.F.; Espino, A.M.; Burgos, M.; Osuna, A. Effectiveness of Intranasal Vaccination against Angiostrongylus costaricensis Using a Serine/Threonine Phosphatase 2 A Synthetic Peptide and Recombinant Antigens. Vaccine 2010, 28, 5185–5196. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis Saponins Induce Mucosal and Systemic Antibody Production, T-Cell Responses and Improved Antigen Uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Rivera-Patron, M.; Mourglia-Ettlin, G.; Casaravilla, C.; Yendo, A.C.A.; Fett-Neto, A.G.; Chabalgoity, J.A.; Moreno, M.; Roehe, P.M.; Silveira, F. Quillaja brasiliensis Saponin-Based Nanoparticulate Adjuvants Are Capable of Triggering Early Immune Responses. Sci. Rep. 2018, 8, 13582. [Google Scholar] [CrossRef]
- Mulcahy, G.; O’Connor, F.; Clery, D.; Hogan, S.F.; Dowd, A.J.; Andrews, S.J.; Dalton, J.P. Immune Responses of Cattle to Experimental Anti-Fasciola hepatica Vaccines. Res. Vet. Sci. 1999, 67, 27–33. [Google Scholar] [CrossRef]
- Golden, O.; Flynn, R.J.; Read, C.; Sekiya, M.; Donnelly, S.M.; Stack, C.; Dalton, J.P.; Mulcahy, G. Protection of Cattle against a Natural Infection of Fasciola hepatica by Vaccination with Recombinant Cathepsin L1 (RFhCL1). Vaccine 2010, 28, 5551–5557. [Google Scholar] [CrossRef]
- Villa-Mancera, A.; Méndez-Mendoza, M. Protection and Antibody Isotype Responses against Fasciola hepatica with Specific Antibody to PIII-Displayed Peptide Mimotopes of Cathepsin L1 in Sheep. Vet. J. 2012, 194, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Villa-Mancera, A.; Quiroz-Romero, H.; Correa, D.; Ibarra, F.; Reyes-Pérez, M.; Reyes-Vivas, H.; López-Velázquez, G.; Gazarian, K.; Gazarian, T.; Alonso, R.A. Induction of Immunity in Sheep to Fasciola hepatica with Mimotopes of Cathepsin L Selected from a Phage Display Library. Parasitology 2008, 135, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Beesley, N.J.; Caminade, C.; Charlier, J.; Flynn, R.J.; Hodgkinson, J.E.; Martinez-Moreno, A.; Martinez-Valladares, M.; Perez, J.; Rinaldi, L.; Williams, D.J.L. Fasciola and Fasciolosis in Ruminants in Europe: Identifying Research Needs. Transbound. Emerg. Dis. 2018, 65, 199–216. [Google Scholar] [CrossRef]
- Spithill, T.W.; Toet, H.; Rathinasamy, V.; Zerna, G.; Swan, J.; Cameron, T.; Smooker, P.M.; Piedrafita, D.M.; Dempster, R.; Beddoe, T. Vaccines for Fasciola: New Thinking for an Old Problem. Fasciolosis II 2021, 379–422. [Google Scholar] [CrossRef]
- Yendo, A.C.A.; de Costa, F.; Kauffmann, C.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A.G. Purification of an Immunoadjuvant Saponin Fraction from Quillaja brasiliensis Leaves by Reversed-Phase Silica Gel Chromatography. Methods Mol. Biol. 2017, 1494, 87–93. [Google Scholar] [CrossRef]
- Rivera-Patron, M.; Cibulski, S.P.; Miraballes, I.; Silveira, F. Formulation of IMXQB: Nanoparticles Based on Quillaja brasiliensis Saponins to Be Used as Vaccine Adjuvants. Methods Mol. Biol. 2022, 2469, 183–191. [Google Scholar] [CrossRef]
- Jayaraj, R.; Piedrafita, D.; Dynon, K.; Grams, R.; Spithill, T.W.; Smooker, P.M. Vaccination against Fasciolosis by a Multivalent Vaccine of Stage-Specific Antigens. Vet. Parasitol. 2009, 160, 230–236. [Google Scholar] [CrossRef]
- Gayo, V.; Cancela, M.; Acosta, D. Maintenance of Life Cycle Stages of Fasciola hepatica in the Laboratory. Methods Mol. Biol. 2020, 2137, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, I.; McShane, D.D.; Shaw, L.; Ellison, S.E.; O’Hagan, N.T.; York, E.A.; Trudgett, A.; Brennan, G.P. Development of an Egg Hatch Assay for the Diagnosis of Triclabendazole Resistance in Fasciola hepatica: Proof of Concept. Vet. Parasitol. 2012, 183, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Guarnaschelli, J.; Rial, A.; Moreno, M.; Rivera-Patron, M.; Iriarte, A.; Chabalgoity, J.A. Humoral and Cellular Immune Responses in Cattle upon Clostridium Chauvoei Vaccination and Challenge. Front. Immunol. 2025, 16, 1584168. [Google Scholar] [CrossRef]
- Budhia, S.; Haring, L.F.; McConnell, I.; Blacklaws, B.A. Quantitation of Ovine Cytokine MRNA by Real-Time RT–PCR. J. Immunol. Methods 2006, 309, 160–172. [Google Scholar] [CrossRef]
- Wattegedera, S.R.; Watson, D.M.; Hope, J.C.; Kaiser, P.; Sales, J.; McInnes, C.J.; Entrican, G. Relative Quantitative Kinetics of Interferon-Gamma and Interleukin-10 MRNA and Protein Production by Activated Ovine Peripheral Blood Mononuclear Cells. Vet. Immunol. Immunopathol. 2010, 136, 34–42. [Google Scholar] [CrossRef]
- Montagne, A.; Grépinet, O.; Peloille, M.; Lantier, F.; Lalmanach, A.C. Quantification of Ovine Cytokine Gene Expression by a Competitive RT-PCR Method. J. Immunol. Methods 2001, 253, 83–93. [Google Scholar] [CrossRef]
- Peletto, S.; Bertuzzi, S.; Campanella, C.; Modesto, P.; Maniaci, M.G.; Bellino, C.; Ariello, D.; Quasso, A.; Caramelli, M.; Acutis, P.L. Evaluation of Internal Reference Genes for Quantitative Expression Analysis by Real-Time PCR in Ovine Whole Blood. Int. J. Mol. Sci. 2011, 12, 7732–7747. [Google Scholar] [CrossRef]
- Mahakapuge, T.A.N.; Scheerlinck, J.P.Y.; Rojas, C.A.A.; Every, A.L.; Hagen, J. Assessment of Reference Genes for Reliable Analysis of Gene Transcription by RT-QPCR in Ovine Leukocytes. Vet. Immunol. Immunopathol. 2016, 171, 1–6. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010; 949p. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package For Education And Data Analysis. Curr. Sci. 2001, 105, 1352–1357. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Dalton, J.P.; Brindley, P.J. Proteases of Trematodes. Advances in Tremadote Biology; Fried, B., Graczyk, T., Eds.; CRC Press: Boca Raton, FL, USA, 1996; ISBN 9781003574118. [Google Scholar]
- Tort, J.; Brindley, P.J.; Knox, D.; Wolfe, K.H.; Dalton, J.P. Proteinases and Associated Genes of Parasitic Helminths. Adv. Parasitol. 1999, 43, 161–266. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, L.; Acosta, D.; Basmadjian, I.; Dalton, J.P.; Carmona, C. Vaccination with Cathepsin L Proteinases and with Leucine Aminopeptidase Induces High Levels of Protection against Fascioliasis in Sheep. Infect. Immun. 1999, 67, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Changklungmoa, N.; Cheukamud, W.; Jaikua, W.; Meemon, K.; Sobhon, P.; Kueakhai, P. Combination Vaccines of Fasciola gigantica Saposin-like Protein-2 and Leucine Aminopeptidase. Trop. Med. Infect. Dis. 2023, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Molina-hernández, V.; Ruiz-campillo, M.T.; Martínez-moreno, F.J.; Buffoni, L.; Martínez-moreno, Á.; Zafra, R.; Bautista, M.J.; Escamilla, A.; Pérez-caballero, R.; Pérez, J. A Partially Protective Vaccine for Fasciola hepatica Induced Degeneration of Adult Flukes Associated to a Severe Granulomatous Reaction in Sheep. Animals 2021, 11, 2869. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.J.; McNeilly, T.N.; Wildblood, L.A.; Morrison, A.A.; Bartley, D.J.; Bartley, Y.; Longhi, C.; McKendrick, I.J.; Palarea-Albaladejo, J.; Matthews, J.B. Successful Immunization against a Parasitic Nematode by Vaccination with Recombinant Proteins. Vaccine 2013, 31, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Zafra, R.; Buffoni, L.; Pérez-Caballero, R.; Molina-Hernández, V.; Ruiz-Campillo, M.T.; Pérez, J.; Martínez-Moreno, Á.; Martínez Moreno, F.J. Efficacy of a Multivalent Vaccine against Fasciola hepatica Infection in Sheep. Vet. Res. 2021, 52, 13. [Google Scholar] [CrossRef]
- Mendes, R.E.; Pérez-Écija, R.A.; Zafra, R.; Buffoni, L.; Martínez-Moreno, Á.; Dalton, J.P.; Mulcahy, G.; Pérez, J. Evaluation of Hepatic Changes and Local and Systemic Immune Responses in Goats Immunized with Recombinant Peroxiredoxin (Prx) and Challenged with Fasciola hepatica. Vaccine 2010, 28, 2832–2840. [Google Scholar] [CrossRef]
- Ingale, S.L.; Singh, P.; Raina, O.K.; Mehra, U.R.; Verma, A.K.; Gupta, S.C.; Mulik, S.V. Interferon-Gamma and Interleukin-4 Expression during Fasciola gigantica Primary Infection in Crossbred Bovine Calves as Determined by Real-Time PCR. Vet. Parasitol. 2008, 152, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A.; Basałaj, K.; Norbury, L.J.; Sielicka, A.; Wędrychowicz, H.; Zawistowska-Deniziak, A. Sex and Vaccination: Insights from Female Rats Vaccinated with Juvenile-Specific Proteases from Fasciola hepatica. Vet. Parasitol. 2018, 255, 91–96. [Google Scholar] [CrossRef]
- Wesołowska, A. Sex-the Most Underappreciated Variable in Research: Insights from Helminth-Infected Hosts. Vet. Res. 2022, 53, 94. [Google Scholar] [CrossRef] [PubMed]
- Okino, C.H.; Niciura, S.C.M.; Minho, A.P.; Esteves, S.N.; Melito, G.R.; Montassier, H.J.; Chagas, A.C.d.S. Divergent Humoral Responses between Males and Females against 24 KDa Excretory-Secretory Protein of Haemonchus Contortus and Influence of Ovine β-Globin Polymorphism. Dev. Comp. Immunol. 2024, 159, 105216. [Google Scholar] [CrossRef]
- Ortega-Vargas, S.; Espitia, C.; Sahagún-Ruiz, A.; Parada, C.; Balderas-Loaeza, A.; Villa-Mancera, A.; Quiroz-Romero, H. Moderate Protection Is Induced by a Chimeric Protein Composed of Leucine Aminopeptidase and Cathepsin L1 against Fasciola hepatica Challenge in Sheep. Vaccine 2019, 37, 3234–3240. [Google Scholar] [CrossRef]
- Lee, R.P.; Jackson, L.A.; Opdebeeck, J.P. Immune Responses of Cattle to Biochemically Modified Antigens from the Midgut of the Cattle Tick, Boophilus Microplus. Parasite Immunol. 1991, 13, 661–672. [Google Scholar] [CrossRef]
- Haçariz, O.; Sayers, G.; McCullough, M.; Garrett, M.; O’Donovan, J.; Mulcahy, G. The Effect of Quil A Adjuvant on the Course of Experimental Fasciola hepatica Infection in Sheep. Vaccine 2009, 27, 45–50. [Google Scholar] [CrossRef]
- Viana, K.F.; Sperandio, N.d.C.; Neto, F.B.; Donatele, D.M.; de Souza, A.B.; dos Santos, A.G.V.; Rivas, A.V.; Barcellos, E.C.d.A.; Martins, I.V.F. Safety and Immunogenicity of an FhSAMS Vaccine Against Fasciola hepatica in Dairy Cattle. Parasite Immunol. 2024, 46, e13074. [Google Scholar] [CrossRef]
- Garza-Cuartero, L.; Geurden, T.; Mahan, S.M.; Hardham, J.M.; Dalton, J.P.; Mulcahy, G. Antibody Recognition of Cathepsin L1-Derived Peptides in Fasciola hepatica-Infected and/or Vaccinated Cattle and Identification of Protective Linear B-Cell Epitopes. Vaccine 2018, 36, 958–968. [Google Scholar] [CrossRef]
- Buonavoglia, D.; Fasanella, A.; Sagazio, P.; Tempesta, M.; Lovane, G.; Buonavoglia, C. Persistence of Antibodies to Mycoplasma agalactiae in Vaccinated Sheep. New Microbiol. 1998, 21, 209–212. [Google Scholar] [PubMed]
- Greco, G.; Corrente, M.; Buonavoglia, D.; Aliberti, A.; Fasanella, A. Inactivated Vaccine Induces Protection against Mycoplasma agalactiae Infection in Sheep. New Microbiol. 2002, 25, 17–20. [Google Scholar]
- Buonavoglia, D.; Greco, G.; Corrente, M.; Greco, M.F.; D’Abramo, M.; Latronico, F.; Fasanella, A.; Decaro, N. Long-Term Immunogenicity and Protection against Mycoplasma agalactiae Induced by an Oil Adjuvant Vaccine in Sheep. Res. Vet. Sci. 2010, 88, 16–19. [Google Scholar] [CrossRef]
- Dalton, J.P.; Robinson, M.W.; Mulcahy, G.; O’Neill, S.M.; Donnelly, S. Immunomodulatory Molecules of Fasciola hepatica: Candidates for Both Vaccine and Immunotherapeutic Development. Vet. Parasitol. 2013, 195, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chryssafidis, A.L.; Browne, J.A.; O’Sullivan, J.; McGettigan, P.A.; Mulcahy, G. Transcriptomic Study on Ovine Immune Responses to Fasciola hepatica Infection. PLoS Negl. Trop. Dis. 2016, 10, e0005015. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.J.; Mulcahy, G.; Elsheikha, H.M. Coordinating Innate and Adaptive Immunity in Fasciola hepatica Infection: Implications for Control. Vet. Parasitol. 2010, 169, 235–240. [Google Scholar] [CrossRef]
- Pacheco, I.L.; Abril, N.; Zafra, R.; Molina-Hernández, V.; Morales-Prieto, N.; Bautista, M.J.; Ruiz-Campillo, M.T.; Pérez-Caballero, R.; Martínez-Moreno, A.; Pérez, J. Fasciola hepatica Induces Foxp3 T Cell, Proinflammatory and Regulatory Cytokine Overexpression in Liver from Infected Sheep during Early Stages of Infection. Vet. Res. 2018, 49, 56. [Google Scholar] [CrossRef]
- Ruiz-Campillo, M.T.; Barrero-Torres, D.M.; Abril, N.; Pérez, J.; Zafra, R.; Buffoni, L.; Martínez-Moreno, Á.; Martínez-Moreno, F.J.; Molina-Hernández, V. Fasciola hepatica Primoinfections and Reinfections in Sheep Drive Distinct Th1/Th2/Treg Immune Responses in Liver and Hepatic Lymph Node at Early and Late Stages. Vet. Res. 2023, 54, 2. [Google Scholar] [CrossRef] [PubMed]
- Preyavichyapugdee, N.; Sahaphong, S.; Riengrojpitak, S.; Grams, R.; Viyanant, V.; Sobhon, P. Fasciola gigantica and Schistosoma mansoni: Vaccine Potential of Recombinant Glutathione S-Transferase (RFgGST26) against Infections in Mice. Exp. Parasitol. 2008, 119, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Changklungmoa, N.; Kueakhai, P.; Riengrojpitak, S.; Chaithirayanon, K.; Chaichanasak, P.; Preyavichyapugdee, N.; Chantree, P.; Sansri, V.; Itagaki, T.; Sobhon, P. Immunization with Recombinant Leucine Aminopeptidase Showed Protection against Fasciola gigantica in Mice. Parasitol. Res. 2013, 112, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Buffoni, L.; Garza-Cuartero, L.; Pérez-Caballero, R.; Zafra, R.; Javier Martínez-Moreno, F.; Molina-Hernández, V.; Pérez, J.; Martínez-Moreno, Á.; Mulcahy, G. Identification of Protective Peptides of Fasciola hepatica-Derived Cathepsin L1 (FhCL1) in Vaccinated Sheep by a Linear B-Cell Epitope Mapping Approach. Parasit. Vectors 2020, 13, 390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).