10-Year Effects of the 13-Valent Pneumococcal Conjugate Vaccine in Patients with Chronic Obstructive Pulmonary Disease and Stable Angina Pectoris
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.J. Risk of Coronary Heart Disease in People with Chronic Obstructive Pulmonary Disease: A Meta-Analysis. Int. J. Chron. Obs. Pulmon. Dis. 2021, 16, 2939–2944. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- National Heart Lung and Blood Institute. Overall Mortality Data; National Heart Lung and Blood Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- The Global Initiative for Chronic Obstructive Lung Disease. Global Initiative for Chronic Obstructive Lung Disease, Global Strategy for Prevention, Diagnosis, and Management of COPD: 2025 Report; The Global Initiative for Chronic Obstructive Lung Disease: Deer Park, IL, USA, 2025. [Google Scholar]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Heris, J.A.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.-A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef]
- Whittaker, H.; Rothnie, K.J.; Quint, J.K. Cause-specific mortality in COPD subpopulations: A cohort study of 339 647 people in England. Thorax 2024, 79, 202–208. [Google Scholar] [CrossRef]
- Svendsen, C.D.; Kuiper, K.K.J.; Ostridge, K.; Larsen, T.H.; Nielsen, R.; Hodneland, V.; Nordeide, E.; Bakke, P.S.; Eagan, T.M.; Torén, K. Factors associated with coronary heart disease in COPD patients and controls. PLoS ONE 2022, 17, e0265682. [Google Scholar] [CrossRef]
- Elkhapery, A.; Hammami, M.B.; Sulica, R.; Boppana, H.; Abdalla, Z.; Iyer, C.; Taifour, H.; Niu, C.; Deshwal, H. Pulmonary Vasodilator Therapy in Severe Pulmonary Hypertension Due to Chronic Obstructive Pulmonary Disease (Severe PH-COPD): A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 498. [Google Scholar] [CrossRef]
- Alter, P.; Lucke, T.; Watz, H.; Andreas, S.; Kahnert, K.; Trudzinski, F.C.; Speicher, T.; Söhler, S.; Bals, R.; Waschki, B.; et al. Cardiovascular predictors of mortality and exacerbations in patients with COPD. Sci. Rep. 2022, 12, 21882. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; Van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Bruxvoort, K.J.; Fischer, H.; Hong, V.X.; Grant, L.R.; Jódar, L.; Cané, A.; Gessner, B.D.; Tartof, S.Y. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Medically Attended Lower Respiratory Tract Infection and Pneumonia Among Older Adults. Clin. Infect. Dis. 2022, 75, 832–841. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.M.; Jiang, Q.; Isturiz, R.E.; Sings, H.L.; Swerdlow, D.L.; Gessner, B.D.; Carrico, R.M.; Peyrani, P.; Wiemken, T.L.; Mattingly, W.A.; et al. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Hospitalization for Community-Acquired Pneumonia in Older US Adults: A Test-Negative Design. Clin. Infect. Dis. 2018, 67, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, G.; Avdeev, S.N.; Antonov, V.N.; Blinova, E.V. Ten-year analysis of the efficacy of vaccination against pneumococcal infection in patients with chronic obstructive pulmonary disease. Pulmonologiya 2023, 33, 750–758. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Rodriguez-Roisin, R. The 2011 revision of the global strategy for the diagnosis, management and prevention of COPD (GOLD)—Why and what? Clin. Respir. J. 2012, 6, 208–214. [Google Scholar] [CrossRef]
- Fox, K.; Garcia, M.A.A.; Ardissino, D.; Buszman, P.; Camici, P.G.; Crea, F.; Daly, C.; De Backer, G.; Hjemdahl, P.; Lopez-Sendon, J.; et al. Guidelines on the management of stable angina pectoris: Executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur. Heart. J. 2006, 27, 1341–1381. [Google Scholar]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; Van Der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Heart. J. 2005, 26, 948–968. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; p. 176. [Google Scholar]
- Ministry of Health of the Russian Federation. Clinical Guidelines Rubricator. 2025. Available online: https://cr.minzdrav.gov.ru/ (accessed on 14 September 2025).
- Avdeev, S.N.; Alyeva, M.H.; Baranov, A.A.; Bikmieva, A.V.; Briko, N.I.; Bulgakova, V.A.; Vishneva, E.A.; Gorelov, A.V.; Demko, I.V.; Dobrynina, E.A.; et al. Federal Clinical Guidelines on Vaccination of pneumococcal infection in children and adults. Profil. Meditsina 2023, 26, 3. [Google Scholar] [CrossRef]
- Ignatova, G.L.; Avdeev, S.N.; Antonov, V.N. Comparative effectiveness of pneumococcal vaccination with PPV23 and PCV13 in COPD patients over a 5-year follow-up cohort study. Sci. Rep. 2021, 11, 15948. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, S.N.; Truschenko, N.V.; Gaynitdinova, V.V.; Soe, A.K.; Nuralieva, G.S. Treatment of exacerbations of chronic obstructive pulmonary disease. Ter. Arkhiv 2018, 90, 68–75. [Google Scholar] [CrossRef]
- Tam, A.; Morrish, D.; Wadsworth, S.; Dorscheid, D.; Man, S.P.; Sin, D.D. The role of female hormones on lung function in chronic lung diseases. BMC Women’s Health 2011, 11, 24. [Google Scholar] [CrossRef]
- Channouf, N.; Fredette, M.; MacGibbon, B. Power and sample size calculations for Poisson and zero-inflated Poisson regression models. Comput. Stat. Data Anal. 2014, 72, 241–251. [Google Scholar] [CrossRef]
- Stenton, C. The MRC breathlessness scale. Occup. Med. 2008, 58, 226–227. [Google Scholar] [CrossRef]
- Perez, T.; Burgel, P.R.; Paillasseur, J.L.; Caillaud, D.; Deslée, G.; Chanez, P.; Roche, N. Modified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 1663–1672. [Google Scholar] [CrossRef]
- Matos Casano, H.A.; Ahmed, I.; Anjum, F. Six-Minute Walk Test. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Group, B. Unified Medical Information System. 2025. Available online: https://bars.group/directions/meditsinskaya-informatsionnaya-sistema2/ (accessed on 14 September 2025).
- Hsiao, A.; Hansen, J.; Timbol, J.; Lewis, N.; Isturiz, R.; Alexander-Parrish, R.; McLaughlin, J.M.; Gessner, B.D.; Klein, N.P. Incidence and Estimated Vaccine Effectiveness Against Hospitalizations for All-Cause Pneumonia Among Older US Adults Who Were Vaccinated and Not Vaccinated With 13-Valent Pneumococcal Conjugate Vaccine. JAMA Netw. Open 2022, 5, e221111. [Google Scholar] [CrossRef]
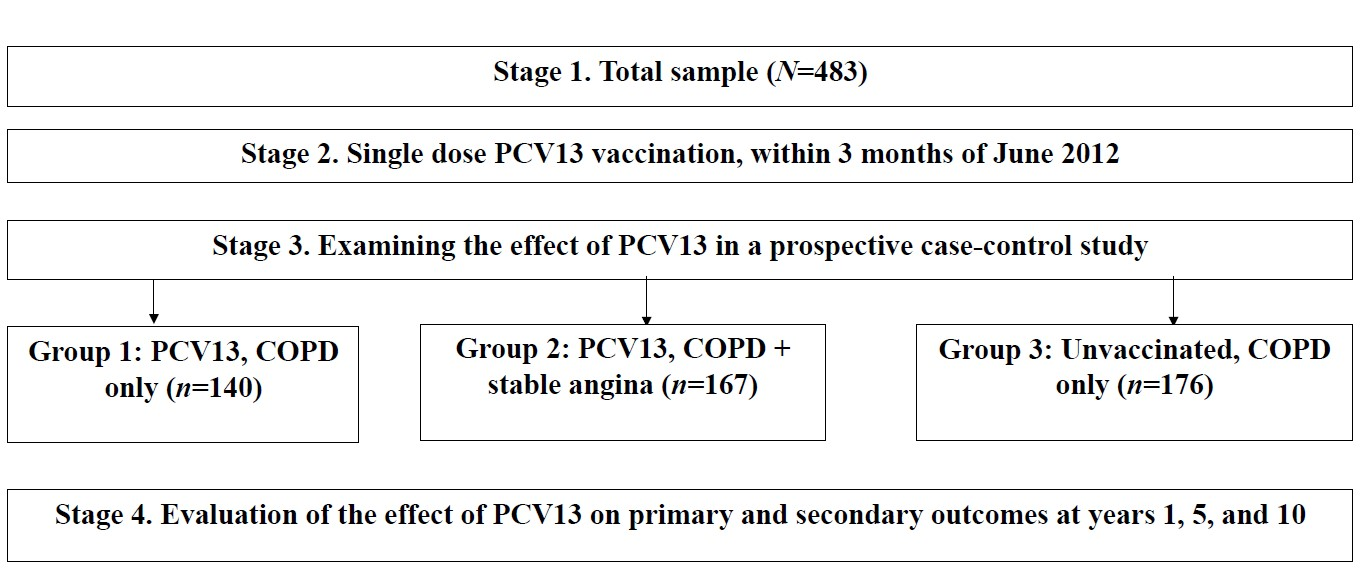
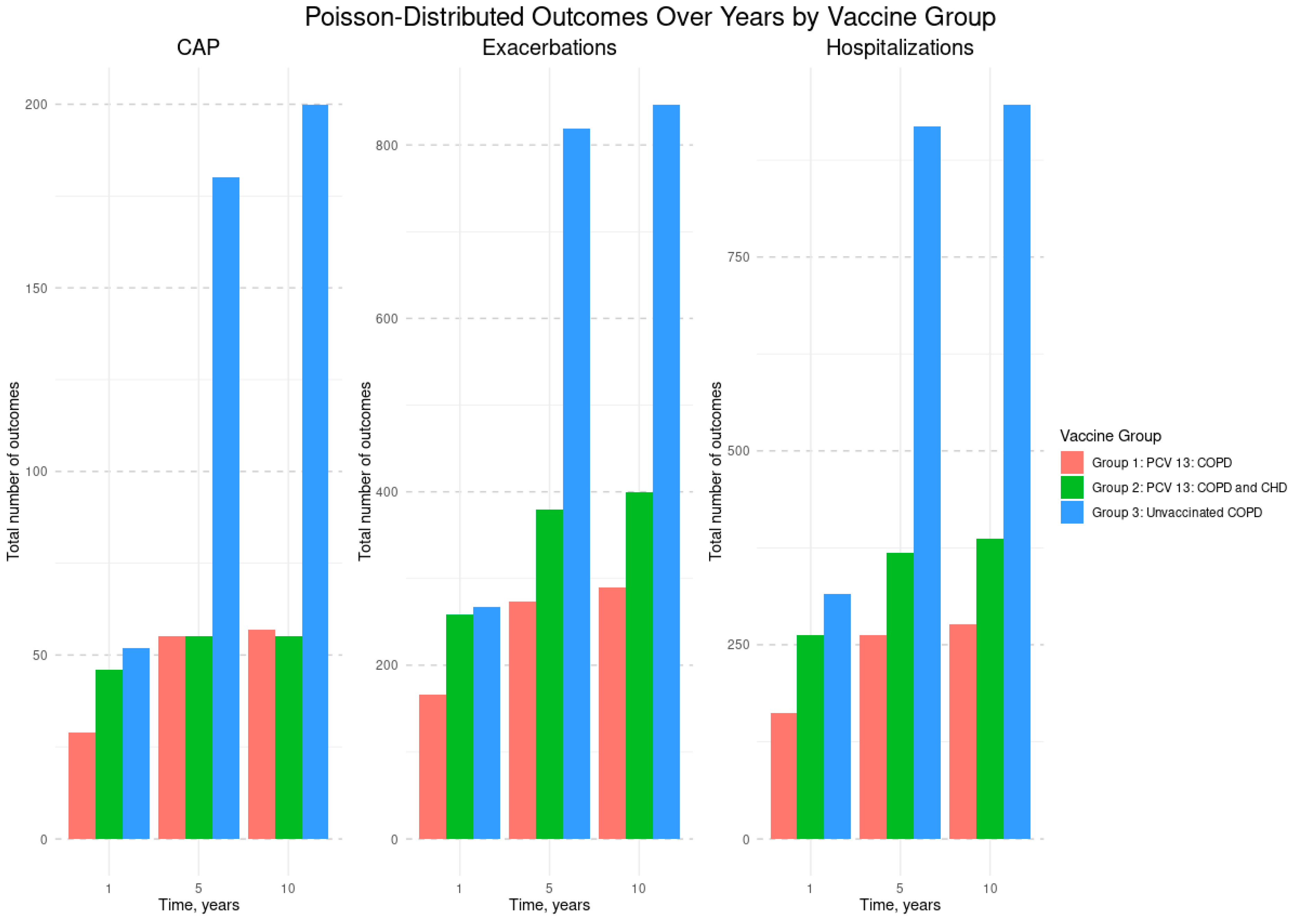
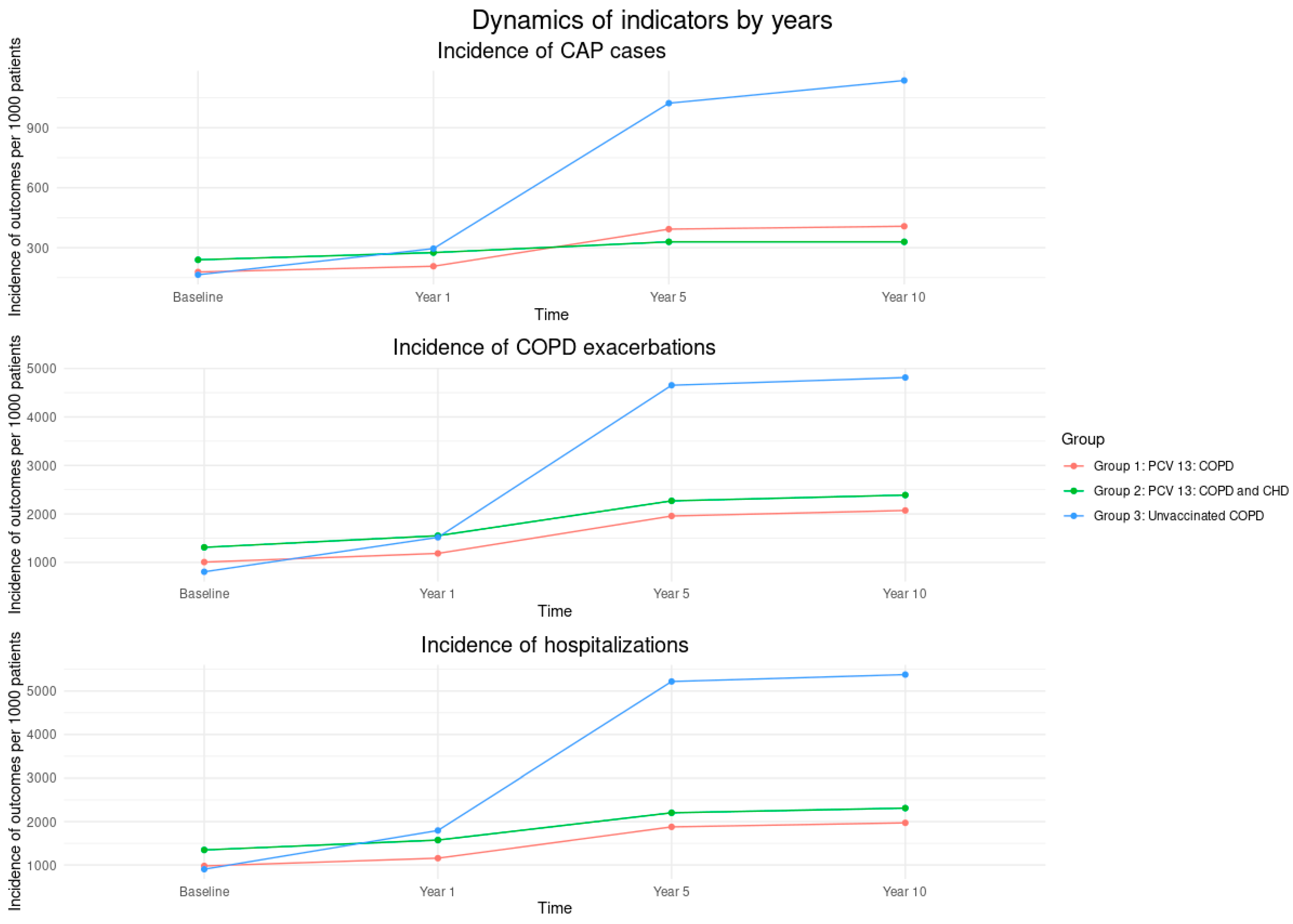
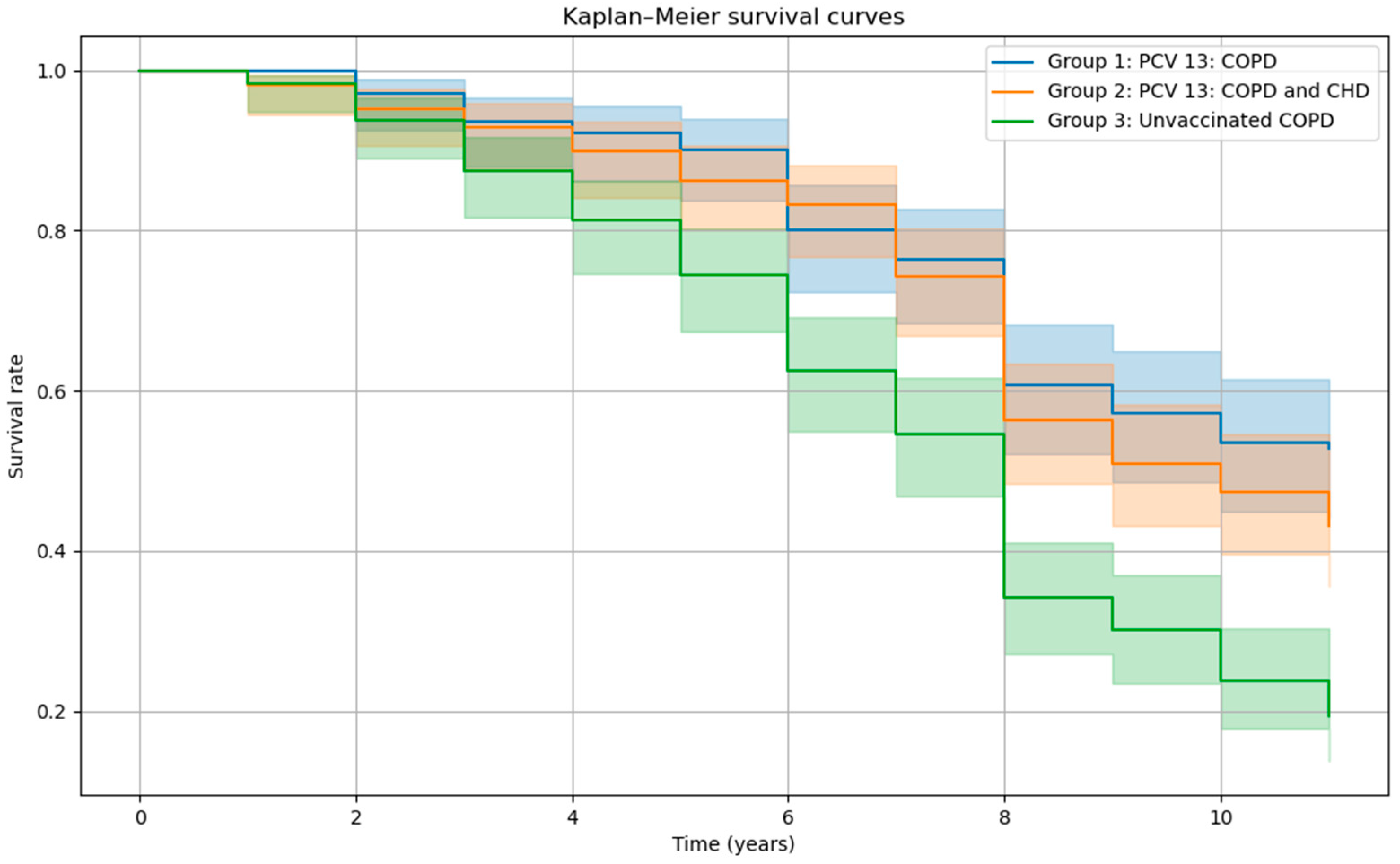
| Variables | Vaccinated PCV13, n = 307 | Unvaccinated, n = 176 | p-Values | |
|---|---|---|---|---|
| Group | COPD | COPD + Stable Angina | COPD | |
| Sample size, n | 140 | 167 | 176 | 0.123 |
| Age (years), median (Q25–Q75) | 61 58–64 | 62 59–65 | 65 61–68 | 0.327 |
| Smoking history (years), median (Q25–Q75) | 16.00 12.00–21.00 | 13.00 11.00–15.00 | 15.00 12.00–20.00 | 0.156 |
| COPD history (years), median (Q25–Q75) | 13 11–15 | 9 7–10.50 | 13 11–15 | 0.012 |
| COPD stages (GOLD), n (%) | 0.483 | |||
| II | 22 (15.7) | 33 (19.8) | 26 (14.7) | |
| III | 106 (75.7) | 116 (69.5) | 138 (78.4) | |
| IV | 12 (8.6) | 18 (10.7) | 12 (6.9) | |
| Functional class of angina pectoris, n (%) | - | - | - | 0.176 |
| II | - | 31 (18.5) | - | |
| III | - | 97 (58.1) | - | |
| IV | - | 39 (23.4) | - | |
| # CAP per year | 25 | 44 | 24 | 0.176 |
| # COPD exacerbations per year | 148 | 229 | 142 | 0.235 |
| # Hospitalizations per year | 141 | 235 | 159 | 0.312 |
| MRC dyspnea scale (points), median (Q25–Q75) | 3 2–3 | 3 2–3 | 3.20 3–4 | 0.076 |
| SpO2 (%), median (Q25–Q75) | 91 90–92 | 88 86–89 | 94 92–95 | 0.021 |
| FEV1 (%), median (Q25–Q75) | 48 30–51 | 46 35–58 | 51 47.8–56 | 0.032 |
| 6MWD, (meters), median (Q25–Q75) | 230 190–300 | 217 190–245 | 218 190–240 | 0.014 |
| Model #1: CAP | Estimates (95% CI) | p-Value |
|---|---|---|
| Group | ||
| Unvaccinated: COPD | reference | |
| PCV13 vaccinated: COPD | −0.38 (−0.72, −0.03) | 0.032 |
| PCV13 vaccinated: COPD and stable angina | −0.18 (−0.45, 0.09) | 0.192 |
| Time | ||
| Year 1 | reference | |
| Year 5 | 1.24 (0.98, 1.50) | <0.0001 |
| Year 10 | 1.35 (1.09, 1.60) | <0.0001 |
| Interaction: Group × Time | ||
| Unvaccinated: COPD × Year 1 | reference | |
| PCV13 vaccinated: COPD × Year 5 | −0.60 (−0.97, −0.24) | 0.001 |
| PCV13 vaccinated: COPD × Year 10 | −0.67 (−1.04, −0.30) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 5 | −1.06 (−1.36, −0.77) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 10 | −1.17 (−1.46, −0.88) | <0.0001 |
| CAP at baseline | 1.11 (0.86, 1.36) | <0.0001 |
| Model #2: COPD exacerbations | ||
| Group | ||
| Unvaccinated: COPD | reference | |
| PCV13 vaccinated: COPD | −0.37 (−0.45, −0.29) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina | −0.30 (−0.41, −0.20) | <0.0001 |
| Time | ||
| Year 1 | reference | |
| Year 5 | 1.12 (1.08, 1.16) | <0.0001 |
| Year 10 | 1.16 (1.11, 1.20) | <0.0001 |
| Interaction: Group × Time | ||
| Unvaccinated: COPD × Year 1 | reference | |
| PCV13 vaccinated: COPD × Year 5 | −0.62 (−0.73, −0.51) | <0.0001 |
| PCV13 vaccinated: COPD × Year 10 | −0.60 (−0.71, −0.48) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 5 | −0.74 (−0.81, −0.67) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 10 | −0.72 (−0.80, −0.64) | <0.0001 |
| COPD exacerbations at baseline | 0.61 (0.46, 0.76) | <0.0001 |
| Model #3: Hospitalizations | ||
| Group | ||
| Unvaccinated: COPD | reference | |
| PCV13 vaccinated: COPD | −0.69 (−0.77, −0.61) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina | −0.76 (−0.85, −0.67) | <0.0001 |
| Time | ||
| Year 1 | reference | |
| Year 5 | 1.07 (1.038, 1.10) | <0.0001 |
| Year 10 | 1.10 (1.065, 1.13) | <0.0001 |
| Interaction: Group × Time | ||
| Unvaccinated: COPD × Year 1 | reference | |
| PCV13 vaccinated: COPD × Year 5 | −0.58 (−0.68, −0.49) | <0.0001 |
| PCV13 vaccinated: COPD × Year 10 | −0.57 (−0.67, −0.46) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 5 | −0.73 (−0.79, −0.67) | <0.0001 |
| PCV13 vaccinated: COPD and stable angina × Year 10 | −0.71 (−0.78, −0.65) | <0.0001 |
| Hospitalizations at baseline | 1.15 (1.03, 1.28) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, G.L.; Avdeev, S.N.; Antonov, V.N.; Blinova, E.V.; Osikov, M.V. 10-Year Effects of the 13-Valent Pneumococcal Conjugate Vaccine in Patients with Chronic Obstructive Pulmonary Disease and Stable Angina Pectoris. Vaccines 2025, 13, 1000. https://doi.org/10.3390/vaccines13101000
Ignatova GL, Avdeev SN, Antonov VN, Blinova EV, Osikov MV. 10-Year Effects of the 13-Valent Pneumococcal Conjugate Vaccine in Patients with Chronic Obstructive Pulmonary Disease and Stable Angina Pectoris. Vaccines. 2025; 13(10):1000. https://doi.org/10.3390/vaccines13101000
Chicago/Turabian StyleIgnatova, Galina L., Sergey N. Avdeev, Vladimir N. Antonov, Elena V. Blinova, and Mikhail V. Osikov. 2025. "10-Year Effects of the 13-Valent Pneumococcal Conjugate Vaccine in Patients with Chronic Obstructive Pulmonary Disease and Stable Angina Pectoris" Vaccines 13, no. 10: 1000. https://doi.org/10.3390/vaccines13101000
APA StyleIgnatova, G. L., Avdeev, S. N., Antonov, V. N., Blinova, E. V., & Osikov, M. V. (2025). 10-Year Effects of the 13-Valent Pneumococcal Conjugate Vaccine in Patients with Chronic Obstructive Pulmonary Disease and Stable Angina Pectoris. Vaccines, 13(10), 1000. https://doi.org/10.3390/vaccines13101000






