SARS-CoV-2 Evolution: Implications for Diagnosis, Treatment, Vaccine Effectiveness and Development
Abstract
1. Introduction
2. The Close Relationship Between Mutations and Vaccines
2.1. The Pre-Omicron Era
2.2. The Era of Omicron and Subvariants
3. Pan-Coronavirus Vaccine Strategies
Nasal Vaccines
4. Hybrid Immunity and Its Long-Term Effects
5. Implications of Main Variants and Vaccines for Pregnancy: Risks for the Mother, Fetus, and Child After Birth
6. Viral Evolution Impacts on Diagnosis
6.1. Reverse Transcription Polymerase Chain Reaction
6.2. Reverse Transcription Loop-Mediated Isothermal Amplification
6.3. Rapid Antigen Detection Tests
6.4. Genome Sequencing
6.5. Serological Tests
6.6. Future Perspectives on Diagnosis
7. Pharmacological Therapies Against SARS-CoV-2
7.1. Monoclonal Antibody Treatment
| Monoclonal Antibody | Variants Efficacy | Status | FDA Setting | Clinical Trial |
|---|---|---|---|---|
| Bamlanivimab (LY-CoV555) | Not effective against variants. | Authorization revoked | Adults and pediatric patients (12 years of age and older and weighing at least 40 kg) with positive results | Cohort: 1097 people [181] |
| REGN-COV2 (Casirivimab (REGN10933) and Imdevimab (REGN10933)) | Not effective against variants. | FDA partially revoked, EMA-approved | Adults and pediatric individuals (12 years of age and older and weighing at least 40 kg) who were at high risk for progression to severe COVID-19 | Cohort: 799 people [159] |
| Bebtelovimab | Effective against variants, not Omicron BQ.1 or XBB. | Authorization revoked | Adults and pediatric patients (12 years of age and older and weighing at least 40 kg) with positive results | Cohort: 706 people [182] |
| Evusheld (Cilgavimab + Tixagevimab) | Effective against variants, not Omicron. | FDA revoked, EMA-approved | Adults and pediatric patients (12 years of age and older and weighing at least 40 kg) who were immunocompromised | Cohort: 5197 people [183] |
| Sotrovimab (VIR-7831) (Xevudy) | Effective against variants. Efficacy reduced in Omicron. | FDA revoked, EMA-approved | Adults and pediatric patients (12 years of age and older and weighing at least 40 kg) with positive results; not recommended for those requiring oxygen | Cohort: 583 people [184] |
| Regdanvimab (CT-P59) (Regkirona) | Proven efficacy. Efficacy reduced in new variants. | EMA-approved | Cohort: 1315 people [185] | |
| Pemgarda (Pemivibart) | Efficacy against new Variants. | FDA-approved, EMA in review | Adults and pediatric patients (12 years of age and older and weighing at least 40 kg) who were immunocompromised | Cohort: 775 people [186] |
7.2. Antiviral Treatment
7.2.1. RNA-Dependent RNA Polymerase Inhibitors
7.2.2. Protease Inhibitors
| Antiviral | Status | FDA Setting | Clinical Trial |
|---|---|---|---|
| Remdesivir (Veklury) | FDA- and EMA-approved | Adult and pediatric patients 12 years of age and older requiring hospitalization and weighing at least 40 kg. | Cohort: 1062 people [205] |
| Molnupiravir (Lagevrio) | FDA authorization and EMA withdrawal | Adults with positive results for direct SARS-CoV-2 viral testing who are at high risk of progression to severe COVID-19 and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate. | Cohort: 1433 people [222] |
| Nirmatrelvir/ritonavir (Paxlovid) | FDA- and EMA-approved | Mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. | Cohort: 2246 people [233] |
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. 20 January 2020 Novel Coronavirus (2019 n-CoV) Situation Report-1. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf (accessed on 12 November 2024).
- WHO. 22 December 2020. Weekly Epidemiological Update. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020#:~:text=Globally%20in%20the%20past%20week,the%20start%20of%20the%20pandemic. (accessed on 12 November 2024).
- WHO. 28 December 2021. Weekly Epidemiological Update on COVID-19. Edition 72. Available online: https://iris.who.int/handle/10665/350973 (accessed on 12 November 2024).
- Angius, F.; Pala, G.; Manzin, A. SARS-CoV-2 and Its Variants: The Pandemic of Unvaccinated. Front. Microbiol. 2021, 12, 749634. [Google Scholar] [CrossRef] [PubMed]
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; et al. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021, 538, 88–91. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Vaqar, S. Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls; Treasure Island (FL) Ineligible Companies. Disclosure: Abdul Bari Akbar Samad Declares No Relevant Financial Relationships with In-Eligible Companies. Disclosure: Sarosh Vaqar Declares No Relevant Financial Relationships with Ineligible Companies; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- WHO. Coronavirus (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 12 November 2024).
- WHO. 21 December 2022. Weekly Epidemiological Update on COVID-19. Edition 123. Available online: https://iris.who.int/handle/10665/365535 (accessed on 12 November 2024).
- Willyard, C. What the Omicron wave is revealing about human immunity. Nature 2022, 602, 22–25. [Google Scholar] [CrossRef]
- Pather, S.; Madhi, S.A.; Cowling, B.J.; Moss, P.; Kamil, J.P.; Ciesek, S.; Muik, A.; Tureci, O. SARS-CoV-2 Omicron variants: Burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front. Immunol. 2023, 14, 1130539. [Google Scholar] [CrossRef]
- Sreepadmanabh, M.; Sahu, A.K.; Chande, A. COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development. J. Biosci. 2020, 45, 148. [Google Scholar] [CrossRef] [PubMed]
- Dryden-Peterson, S.; Kim, A.; Kim, A.Y.; Caniglia, E.C.; Lennes, I.T.; Patel, R.; Gainer, L.; Dutton, L.; Donahue, E.; Gandhi, R.T.; et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study. Ann. Intern. Med. 2023, 176, 77–84. [Google Scholar] [CrossRef] [PubMed]
- CDC. 22 November 2022. CDC Reports on Bivalent COVID-19 Vaccine, Paxlovid Effectiveness. Available online: https://www.aha.org/news/headline/2022-11-22-cdc-reports-bivalent-covid-19-vaccine-paxlovid-effectiveness (accessed on 12 November 2024).
- Hansen, K.; Makkar, S.R.; Sahner, D.; Fessel, J.; Hotaling, N.; Sidky, H. Paxlovid (nirmatrelvir/ritonavir) effectiveness against hospitalization and death in N3C: A target trial emulation study. medRxiv 2023. [Google Scholar] [CrossRef]
- Paxlovid. Emergency Use Authorization. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=17109 (accessed on 12 November 2024).
- WHO. 22 December 2023. COVID-19 Epidemiological Update. Edition 162. Available online: https://iris.who.int/handle/10665/375379 (accessed on 12 November 2024).
- Alqahtani, M.; Abdulrahman, A.; Mustafa, F.; Alawadhi, A.I.; Alalawi, B.; Mallah, S.I. Evaluation of Rapid Antigen Tests Using Nasal Samples to Diagnose SARS-CoV-2 in Symptomatic Patients. Front. Public Health 2021, 9, 728969. [Google Scholar] [CrossRef]
- Anand, A.; Vialard, F.; Esmail, A.; Ahmad Khan, F.; O’Byrne, P.; Routy, J.P.; Dheda, K.; Pant Pai, N. Self-tests for COVID-19: What is the evidence? A living systematic review and meta-analysis (2020–2023). PLoS Glob. Public Health 2024, 4, e0002336. [Google Scholar] [CrossRef] [PubMed]
- Mukoka, M.; Sibanda, E.; Watadzaushe, C.; Kumwenda, M.; Abok, F.; Corbett, E.L.; Ivanova, E.; Choko, A.T. COVID-19 self-testing using antigen rapid diagnostic tests: Feasibility evaluation among health-care workers and general population in Malawi. PLoS ONE 2023, 18, e0289291. [Google Scholar] [CrossRef] [PubMed]
- News, U.T. COVID Variant XEC Sees Rapid Global Growth: What to Know About the New Strain. Available online: https://www.usatoday.com/story/news/health/2024/09/16/xec-covid-variant/75253344007/ (accessed on 12 November 2024).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob. Chall. 2017, 1, 33–46. [Google Scholar] [CrossRef]
- Mike-Honey. COVID-19-Genomes. Available online: https://github.com/Mike-Honey/covid-19-genomes?tab=readme-ov-file (accessed on 12 November 2024).
- Cook, T.M.; Farrar, J.J. COVID-19 vaccines: One step towards the beginning of the end of the global impact of the pandemic. Anaesthesia 2021, 76, 435–443. [Google Scholar] [CrossRef]
- Khan, K.; Lustig, G.; Romer, C.; Reedoy, K.; Jule, Z.; Karim, F.; Ganga, Y.; Bernstein, M.; Baig, Z.; Jackson, L.; et al. Evolution and neutralization escape of the SARS-CoV-2 BA.2.86 subvariant. Nat. Commun. 2023, 14, 8078. [Google Scholar] [CrossRef]
- Wee, L.E.; Sim, X.Y.J.; Conceicao, E.P.; Aung, M.K.; Goh, J.Q.; Yeo, D.W.T.; Gan, W.H.; Chua, Y.Y.; Wijaya, L.; Tan, T.T.; et al. Containment of COVID-19 cases among healthcare workers: The role of surveillance, early detection, and outbreak management. Infect. Control. Hosp. Epidemiol. 2020, 41, 765–771. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Winger, A.; Caspari, T. The Spike of Concern-The Novel Variants of SARS-CoV-2. Viruses 2021, 13, 1002. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Sakr, M.M.; El-Housseiny, G.S.; Wasfi, R.; Aboshanab, K.M. State of the art in epitope mapping and opportunities in COVID-19. Future Sci. OA 2023, 16, FSO832. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Zhang, B.; Huo, J.; Huang, Y.; Teo, S.C.; Li, Y.F.; Toh, L.K.; Lam, K.P.; Xu, S.Y. Homologous or Heterologous mRNA Booster Vaccination Induces Robust Neutralizing Antibody Responses Against SARS-CoV-2 Omicron Variant in Individuals Receiving mRNA or Inactivated Virus Priming Vaccination. Lancet, 2022; Preprint. [Google Scholar] [CrossRef]
- Mengist, H.M.; Kombe Kombe, A.J.; Mekonnen, D.; Abebaw, A.; Getachew, M.; Jin, T. Mutations of SARS-CoV-2 spike protein: Implications on immune evasion and vaccine-induced immunity. Semin. Immunol. 2021, 55, 101533. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Yang, H.; Sun, R.; Li, C.; Guo, D. The emerging role of SARS-CoV-2 nonstructural protein 1 (nsp1) in epigenetic regulation of host gene expression. FEMS Microbiol. Rev. 2024, 48, fuae023. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.W.; Tang, C.; Wei, H.C.; Du, B.; Chen, C.; Wang, M.; Zhou, Y.; Yu, M.X.; Cheng, L.; Kuivanen, S.; et al. Genomic monitoring of SARS-CoV-2 uncovers an Nsp1 deletion variant that modulates type I interferon response. Cell Host Microbe 2021, 29, 489–502.e488. [Google Scholar] [CrossRef]
- Davis, C.; Logan, N.; Tyson, G.; Orton, R.; Harvey, W.; Haughney, J.; Perkins, J.; The COVID-19 Genomics UK (COG-UK) Consortium; Peacock, T.P.; Barclay, W.S.; et al. Reduced neutralization of the Delta (B.1.617.2) SARS-CoV-2 variant of concern following vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Ozkaya, E.; Yazici, M.; Baran, I.; Cetin, N.S.; Tosun, I.; Buruk, C.K.; Kaklikkaya, N.; Aydin, F.; Doymaz, M.Z. Neutralization of Wild-Type and Alpha SARS-CoV-2 Variant by CoronaVac(R) Vaccine and Natural Infection-Induced Antibodies. Curr. Microbiol. 2023, 80, 162. [Google Scholar] [CrossRef] [PubMed]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Aragones, M.; Tubert, J.E.; et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: Test negative case-control study. BMJ 2021, 375, e068848. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef]
- Gupta, S.L.; Jaiswal, R.K. An Assessment of the Bivalent Vaccine as a Second Booster for COVID-19. Vaccines 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Kimura, I.; Yamasoba, D.; Tamura, T.; Nao, N.; Suzuki, T.; Oda, Y.; Mitoma, S.; Ito, J.; Nasser, H.; Zahradnik, J.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell 2022, 185, 3992–4007.e3916. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022, 185, 2422–2433.e2413. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Kurhade, C.; Xia, H.; Liu, M.; Xie, X.; Ren, P.; Shi, P.Y. Cross-neutralization of Omicron BA.1 against BA.2 and BA.3 SARS-CoV-2. Nat. Commun. 2022, 13, 2956. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; Houhamdi, L.; Delorme, L.; Colson, P.; Gautret, P. Reinfections with Different SARS-CoV-2 Omicron Subvariants, France. Emerg. Infect. Dis. 2022, 28, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Suzuki, R.; Uriu, K.; Itakura, Y.; Zahradnik, J.; Kimura, K.T.; Deguchi, S.; Wang, L.; Lytras, S.; Tamura, T.; et al. Convergent evolution of SARS-CoV-2 Omicron subvariants leading to the emergence of BQ.1.1 variant. Nat. Commun. 2023, 14, 2671. [Google Scholar] [CrossRef]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat. Commun. 2023, 14, 2800. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhattacharya, M.; Dhama, K.; Lee, S.S.; Chakraborty, C. Can the RBD mutation R346X provide an additional fitness to the “variant soup,” including offspring of BQ and XBB of SARS-CoV-2 Omicron for the antibody resistance? Mol. Ther. Nucleic Acids 2023, 32, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Bormann, M.; Brochhagen, L.; Alt, M.; Otte, M.; Thummler, L.; van de Sand, L.; Kraiselburd, I.; Thomas, A.; Gosch, J.; Brass, P.; et al. Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5. Front. Immunol. 2023, 14, 1150667. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Z.; Guo, Y.; Guo, H.; Jian, L.; Xiao, J.; Yao, X.; Yu, H.; Cheng, T.; Zhang, Y.; et al. Evolving spike mutations in SARS-CoV-2 Omicron variants facilitate evasion from breakthrough infection-acquired antibodies. Cell Discov. 2023, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Tamura, T.; Yamasoba, D.; Oda, Y.; Ito, J.; Kamasaki, T.; Nao, N.; Hashimoto, R.; Fujioka, Y.; Suzuki, R.; Wang, L.; et al. Comparative pathogenicity of SARS-CoV-2 Omicron subvariants including BA.1, BA.2, and BA.5. Commun. Biol. 2023, 6, 772. [Google Scholar] [CrossRef]
- Chye, H.; Chew, C.W.Y.; Yeo, H.P.; Tambyah, P.A.; Young, B.E.; Tan, G.G.Y.; Tan, B.H.; Vasoo, S.; Chan, C.E.Z. Neutralization escape of emerging subvariants XBB.1.5/1.9.1 and XBB.2.3 from current therapeutic monoclonal antibodies. J. Med. Virol. 2023, 95, e29074. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Panchal, A.R.; et al. Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell Rep. 2023, 42, 112443. [Google Scholar] [CrossRef]
- Sugano, A.; Kataguchi, H.; Ohta, M.; Someya, Y.; Kimura, S.; Maniwa, Y.; Tabata, T.; Takaoka, Y. SARS-CoV-2 Omicron XBB.1.5 May Be a Variant That Spreads More Widely and Faster Than Other Variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Scarpa, F.; Azzena, I.; Ciccozzi, A.; Giovanetti, M.; Locci, C.; Casu, M.; Fiori, P.L.; Borsetti, A.; Cella, E.; Quaranta, M.; et al. Integrative Genome-Based Survey of the SARS-CoV-2 Omicron XBB.1.16 Variant. Int. J. Mol. Sci. 2023, 24, 13573. [Google Scholar] [CrossRef]
- Kaku, Y.; Kosugi, Y.; Uriu, K.; Ito, J.; Hinay, A.A., Jr.; Kuramochi, J.; Sadamasu, K.; Yoshimura, K.; Asakura, H.; Nagashima, M.; et al. Antiviral efficacy of the SARS-CoV-2 XBB breakthrough infection sera against omicron subvariants including EG.5. Lancet Infect. Dis. 2023, 23, e395–e396. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e278. [Google Scholar] [CrossRef]
- Zhang, L.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Dopfer-Jablonka, A.; Stankov, M.V.; Schulz, S.R.; Jack, H.M.; Behrens, G.M.N.; Pohlmann, S.; et al. Neutralisation sensitivity of SARS-CoV-2 lineages EG.5.1 and XBB.2.3. Lancet Infect. Dis. 2023, 23, e391–e392. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Collier, A.Y.; Hachmann, N.P.; Miller, J.; Rowe, M.; Schonberg, E.D.; Rodrigues, S.L.; LaPiana, A.; Patio, R.C.; Anand, T.; et al. Neutralization escape by SARS-CoV-2 Omicron subvariant BA.2.86. Vaccine 2023, 41, 6904–6909. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Yang, Y.; Westerberg, M.; Oling, S.; Muschiol, S.; Sato, K.; Peacock, T.P.; Karlsson Hedestam, G.B.; Albert, J.; Murrell, B. Sensitivity of the SARS-CoV-2 BA.2.86 variant to prevailing neutralising antibody responses. Lancet Infect. Dis. 2023, 23, e462–e463. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Ito, J.; Kosugi, Y.; Tanaka, Y.L.; Mugita, Y.; Guo, Z.; Hinay, A.A., Jr.; Putri, O.; Kim, Y.; Shimizu, R.; et al. Transmissibility, infectivity, and immune evasion of the SARS-CoV-2 BA.2.86 variant. Lancet Infect. Dis. 2023, 23, e460–e461. [Google Scholar] [CrossRef]
- Chalkias, S.; McGhee, N.; Whatley, J.L.; Essink, B.; Brosz, A.; Tomassini, J.E.; Girard, B.; Wu, K.; Edwards, D.K.; Nasir, A.; et al. Safety and Immunogenicity of XBB.1.5-Containing mRNA Vaccines. medRxiv 2023. [Google Scholar] [CrossRef]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef]
- Cheng, S.M.S.; Mok, C.K.P.; Li, J.K.C.; Chan, K.K.P.; Luk, K.S.; Lee, B.H.W.; Gu, H.; Chan, K.C.K.; Tsang, L.C.H.; Yiu, K.Y.S.; et al. Cross-neutralizing antibody against emerging Omicron subvariants of SARS-CoV-2 in infection-naive individuals with homologous BNT162b2 or BNT162b2(WT + BA.4/5) bivalent booster vaccination. Virol. J. 2024, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.S.; Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Cross-Reactivity Assessment of Vaccine-Derived SARS-CoV-2 T Cell Responses against BA.2.86 and JN.1. Viruses 2024, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Faraone, J.N.; Hsu, C.C.; Chamblee, M.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Horowitz, J.C.; Mallampalli, R.K.; Saif, L.J.; et al. Characteristics of JN.1-derived SARS-CoV-2 subvariants SLip, FLiRT, and KP.2 in neutralization escape, infectivity and membrane fusion. bioRxiv 2024. [Google Scholar] [CrossRef]
- Kaku, Y.; Yo, M.S.; Tolentino, J.E.; Uriu, K.; Okumura, K.; Genotype to Phenotype Japan, C.; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 KP.3, LB.1, and KP.2.3 variants. Lancet Infect. Dis. 2024, 24, e482–e483. [Google Scholar] [CrossRef]
- Happle, C.; Hoffmann, M.; Kempf, A.; Nehlmeier, I.; Stankov, M.V.; Calderon Hampel, N.; Witte, T.; Pohlmann, S.; Behrens, G.M.N.; Dopfer-Jablonka, A. Humoral immunity after mRNA SARS-CoV-2 omicron JN.1 vaccination. Lancet Infect. Dis. 2024, 24, e674–e676. [Google Scholar] [CrossRef] [PubMed]
- CDC; IDSA. COVID 19 Real-Time Learning Network. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/ (accessed on 12 November 2024).
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J.; Sun, P.; Qin, J.; Yang, X.; Chen, D.; Zhang, Y.; Zhong, N.; Wang, Z. ARS-CoV-2 epitope-specific T cells: Immunity response feature, TCR repertoire characteristics and cross-reactivity. Front. Immunol. 2023, 10, 1146196. [Google Scholar] [CrossRef]
- Augusto, D.G.; Hollenbach, J.A. HLA variation and antigen presentation in COVID-19 and SARS-CoV-2 infection. Curr. Opin. Immunol. 2022, 76, 102178. [Google Scholar] [CrossRef]
- Brand, M.; Kesmir, C. Evolution of SARS-CoV-2-specific CD4+ T cell epitopes. Immunogenetics 2023, 75, 283–293. [Google Scholar] [CrossRef]
- Cohen, A.A.; Gnanapragasam, P.N.P.; Lee, Y.E.; Hoffman, P.R.; Ou, S.; Kakutani, L.M.; Keeffe, J.R.; Wu, H.J.; Howarth, M.; West, A.P.; et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Renauer, P.A.; Okten, A.; Fang, Z.; Park, J.J.; Zhou, X.; Lin, Q.; Dong, M.B.; Filler, R.; Xiong, Q.; et al. Variant-specific vaccination induces systems immune responses and potent in vivo protection against SARS-CoV-2. Cell Rep. Med. 2022, 3, 100634. [Google Scholar] [CrossRef]
- Cankat, S.; Demael, M.U.; Swadling, L. In search of a pan-coronavirus vaccine: Next-generation vaccine design and immune mechanisms. Cell. Mol. Immunol. 2024, 21, 103–118. [Google Scholar] [CrossRef]
- Joyce, M.G.; Chen, W.H.; Sankhala, R.S.; Hajduczki, A.; Thomas, P.V.; Choe, M.; Chang, W.; Peterson, C.E.; Martinez, E.; Morrison, E.B.; et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hoffmann, M.A.G.; Yang, Z.; Huey-Tubman, K.E.; Cohen, A.A.; Gnanapragasam, P.N.P.; Nakatomi, L.M.; Storm, K.N.; Moon, W.J.; Lin, P.J.C.; West, A.P., Jr.; et al. ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell 2023, 186, 2380–2391.e2389. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Steinberger, P.; Wilkinson, J.; Klais, G.; Kundi, M.; Wiedermann, U. SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity. Vaccines 2024, 12, 795. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Scent of a vaccine. Science 2021, 373, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.E.; Vidarsson, G. Antibodies and their receptors: Different potential roles in mucosal defense. Front. Immunol. 2013, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Jarlhelt, I.; Nielsen, S.K.; Jahn, C.X.H.; Hansen, C.B.; Perez-Alos, L.; Rosbjerg, A.; Bayarri-Olmos, R.; Skjoedt, M.O.; Garred, P. SARS-CoV-2 Antibodies Mediate Complement and Cellular Driven Inflammation. Front. Immunol. 2021, 12, 767981. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Dotiwala, F.; Upadhyay, A.K. Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Pabst, R. Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)-Structure, function and species differences. Vaccine 2015, 33, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; St John, A.L. Promises and challenges of mucosal COVID-19 vaccines. Vaccine 2023, 41, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wang, R.; Jin, Y.; Song, Y.; Wang, T. From intramuscular to nasal: Unleashing the potential of nasal spray vaccines against coronavirus disease 2019. Clin. Transl. Immunol. 2024, 13, e1514. [Google Scholar] [CrossRef] [PubMed]
- Altay Benetti, A.; Tan, E.Y.Z.; Chang, Z.W.; Bae, K.H.; Thwin, M.T.; Muthuramalingam, R.P.K.; Liao, K.-C.; Wan, Y.; Ng, L.F.P.; Renia, L.; et al. Design and Characterization of a New Formulation for the Delivery of COVID-19-mRNA Vaccine to the Nasal Mucosa. Vaccines 2024, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Jakaew, P.; Jearanaiwitayakul, T.; Midoeng, P.; Masrinoul, P.; Sunintaboon, P.; Ubol, S. Responses of primary human nasal epithelial cells to COVID-19 vaccine candidate. Asian Pac. J. Allergy Immunol. 2024. [Google Scholar] [CrossRef]
- Lobaina, Y.; Chen, R.; Suzarte, E.; Ai, P.; Musacchio, A.; Lan, Y.; Chinea, G.; Tan, C.; Silva, R.; Guillen, G.; et al. A Nasal Vaccine Candidate, Containing Three Antigenic Regions from SARS-CoV-2, to Induce a Broader Response. Vaccines 2024, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- NIH. NIH-Sponsored Trial of Nasal COVID-19 Vaccine Opens. Available online: https://www.nih.gov/news-events/news-releases/nih-sponsored-trial-nasal-covid-19-vaccine-opens (accessed on 12 November 2024).
- Ntziora, F.; Kostaki, E.G.; Karapanou, A.; Mylona, M.; Tseti, I.; Sipsas, N.V.; Paraskevis, D.; Sfikakis, P.P. Protection of vaccination versus hybrid immunity against infection with COVID-19 Omicron variants among Health-Care Workers. Vaccine 2022, 40, 7195–7200. [Google Scholar] [CrossRef]
- Ali, A.; Dwyer, D.; Wu, Q.; Wang, Q.; Dowling, C.A.; Fox, D.A.; Khanna, D.; Poland, G.A.; Mao-Draayer, Y. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine 2021, 39, 6111–6116. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreno, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef]
- Bowman, K.A.; Stein, D.; Shin, S.; Ferbas, K.G.; Tobin, N.H.; Mann, C.; Fischinger, S.; Ollmann Saphire, E.; Lauffenburger, D.; Rimoin, A.W.; et al. Hybrid Immunity Shifts the Fc-Effector Quality of SARS-CoV-2 mRNA Vaccine-Induced Immunity. mBio 2022, 13, e0164722. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.L.; Jaiswal, R.K. Relevant of neutralizing antibody during SARS-CoV-2 infection and their therapeutic usage. Mol. Biol. Rep. 2022, 49, 10137–10140. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Khan, M.S.; Shakya, M.; Verma, C.K. Exploring immunogenic CD8 + T-cell epitopes for peptide-based vaccine development against evolving SARS-CoV-2 variants: An immunoinformatics approach. VirusDisease 2024, 35, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Bretones, M.; Fouchier, R.A.; Koopmans, M.P.; van Nierop, G.P. Impact of antigenic evolution and original antigenic sin on SARS-CoV-2 immunity. J. Clin. Investig. 2023, 133, e162192. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, P.; Tkaczyk, I.; Monticolo, M.; Jędrzejczak, A.M.; Krata, N.; Pączek, L.; Foroncewicz, B.; Mucha, K. Hybrid Immunity Provides the Best COVID-19 Humoral Response in Immunocompromised Patients with or without SARS-CoV-2 Infection History. Vaccines 2023, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, G.; Koosha, K.; Vogt, T.; Stein, A.; Ziegler, L.; Asam, C.; Weps, M.; Schwagerl, V.; Richter, L.; Hepp, N.; et al. Hybrid immunity by two COVID-19 mRNA vaccinations and one breakthrough infection provides a robust and balanced cellular immune response as basic immunity against severe acute respiratory syndrome coronavirus 2. J. Med. Virol. 2024, 96, e29739. [Google Scholar] [CrossRef]
- Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination-Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; Leier, H.C.; McBride, S.K.; Schoen, D.; Lyski, Z.L.; Lee, D.X.; Messer, W.B.; Curlin, M.E.; Tafesse, F.G. An extended interval between vaccination and infection enhances hybrid immunity against SARS-CoV-2 variants. JCI Insight 2023, 8, e165265. [Google Scholar] [CrossRef] [PubMed]
- Reeves, E.L.; Neelam, V.; Carlson, J.M.; Olsen, E.O.; Fox, C.J.; Woodworth, K.R.; Nestoridi, E.; Mobley, E.; Montero Castro, S.; Dzimira, P.; et al. Pregnancy and infant outcomes following SARS-CoV-2 infection in pregnancy during delta variant predominance—Surveillance for Emerging Threats to Pregnant People and Infants. Am. J. Obstet. Gynecol. MFM 2024, 6, 101265. [Google Scholar] [CrossRef]
- Greenberg, G.C.; Vishwakarma, N.; Tirupattur, M.P.; Sprague, H.M.; Katwa, L.C. Implications of COVID-19 Pandemic on Pregnancy: Current Status and Controversies. COVID 2023, 3, 859–873. [Google Scholar] [CrossRef]
- Gunther, J.; Ziert, Y.; Andresen, K.; Pecks, U.; von Versen-Hoynck, F.; Network, C. Variability in COVID-19 symptom presentation during pregnancy and its impact on maternal and infant outcomes across the pandemic. Int. J. Infect. Dis. 2024, 146, 107157. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Rasmussen, S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 177–186. [Google Scholar] [CrossRef]
- Chaubey, I.; Vijay, H.; Govindaraj, S.; Babu, H.; Cheedarla, N.; Shankar, E.M.; Vignesh, R.; Velu, V. Impact of COVID-19 Vaccination on Pregnant Women. Pathogens 2023, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.C.; Gunier, R.B.; Rego, A.; Sentilhes, L.; Rauch, S.; Gandino, S.; Teji, J.S.; Thornton, J.G.; Kachikis, A.B.; Nieto, R.; et al. Maternal vaccination against COVID-19 and neonatal outcomes during Omicron: INTERCOVID-2022 study. Am. J. Obstet. Gynecol. 2024, 231, 460.e1–460.e17. [Google Scholar] [CrossRef] [PubMed]
- CDC. COVID-19 Vaccination for People Who Are Pregnant or Breastfeeding. Available online: https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html#:~:text=Everyone%20ages%206%20months%20and,pregnancy%20is%20safe%20and%20effective. (accessed on 12 November 2024).
- Ciapponi, A.; Berrueta, M.; Argento, F.J.; Ballivian, J.; Bardach, A.; Brizuela, M.E.; Castellana, N.; Comande, D.; Gottlieb, S.; Kampmann, B.; et al. Safety and Effectiveness of COVID-19 Vaccines During Pregnancy: A Living Systematic Review and Meta-analysis. Drug Saf. 2024, 47, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Asirwatham, A.; Hillman, M.; Khan, L.; Sangermano, L.M.; Leung, K.; Leftwich, H.K. SARS-CoV-2 Variants in Pregnancy [ID 2683546]. Obstet. Gynecol. 2024, 143, 5S–6S. [Google Scholar] [CrossRef]
- Lam, J.N.; Nehira, J.; Phung, O.; Deng, B. Systematic Review: Safety and Efficacy of mRNA COVID-19 Vaccines in Pregnant Women. J. Pharm. Pract. 2024, 37, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.W.; Zhang, Y.; Zhang, H.C.; Xu, T.; Zhang, W.H. Era of molecular diagnosis for pathogen identification of unexplained pneumonia, lessons to be learned. Emerg. Microbes Infect. 2020, 9, 597–600. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- WHO. Diagnostic Testing for SARS-CoV-2: Interim Guidance, 11 September 2020. Available online: https://iris.who.int/handle/10665/334254 (accessed on 12 November 2024).
- Salazar-Ardiles, C.; Asserella-Rebollo, L.; Cornejo, C.; Arias, D.; Vasquez-Munoz, M.; Toledo, C.; Andrade, D.C. Molecular diagnostic approaches for SARS-CoV-2 detection and pathophysiological consequences. Mol. Biol. Rep. 2023, 50, 10367–10382. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.M.; Bitew, M.; Fokam, J.; Lelo, E.A.; Ahidjo, A.; Asmamaw, K.; Beloumou, G.A.; Bulimo, W.D.; Buratti, E.; Chenwi, C.; et al. Diagnostic performance of a colorimetric RT -LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa. EClinicalMedicine 2021, 40, 101101. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lyu, J.; Zheng, X.; Liang, Z.; Lei, B.; Chen, H.; Mai, Y.; Huang, H.; Sun, B. Evolution of the newest diagnostic methods for COVID-19: A Chinese perspective. J. Zhejiang Univ. Sci. B 2023, 24, 463–484. [Google Scholar] [CrossRef]
- Gao, Y.P.; Huang, K.J.; Wang, F.T.; Hou, Y.Y.; Xu, J.; Li, G. Recent advances in biological detection with rolling circle amplification: Design strategy, biosensing mechanism, and practical applications. Analyst 2022, 147, 3396–3414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Zhao, W. Emerging Microtechnologies and Automated Systems for Rapid Bacterial Identification and Antibiotic Susceptibility Testing. SLAS Technol. 2017, 22, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Alvarez, C.; Lam, Q.; Baldwin, D.A.; Chernoff, J. Low saliva pH can yield false positives results in simple RT-LAMP-based SARS-CoV-2 diagnostic tests. PLoS ONE 2021, 16, e0250202. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Q.; Li, J.; Chen, J.; Wang, Z.; Zhang, X.; Fang, Z.; Li, H.; Zhao, Y.; Yu, P.; et al. Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection. mSphere 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- American Society for Microbiology. How the SARS-CoV-2 EUA Antigen Tests Work. Available online: https://asm.org/articles/2020/august/how-the-sars-cov-2-eua-antigen-tests-work#:~:text=This%20system%20uses%20chromatogenic%20digital,bind%20to%20the%20N%20antigen. (accessed on 12 November 2024).
- Nicolai, E.; Tomassetti, F.; Pignalosa, S.; Redi, S.; Marino, M.; Basile, U.; Ciotti, M. The Evolution of Serological Assays during Two Years of the COVID-19 Pandemic: From an Easy-to-Use Screening Tool for Identifying Current Infections to Laboratory Algorithms for Discovering Immune Protection and Optimizing Vaccine Administration. COVID 2024, 4, 1272–1290. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. [Google Scholar] [CrossRef]
- Vashist, S.K. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef]
- Kretschmer, A.; Kossow, A.; Grune, B.; Schildgen, O.; Mathes, T.; Schildgen, V. False positive rapid antigen tests for SARS-CoV-2 in the real-world and their economic burden. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Fukumori, T.; Nishihara, Y.; Sekine, T.; Okuda, N.; Nishimura, T.; Fujikura, H.; Hirai, N.; Imakita, N.; Kasahara, K. Another false-positive problem for a SARS-CoV-2 antigen test in Japan. J. Clin. Virol. 2020, 131, 104612. [Google Scholar] [CrossRef] [PubMed]
- Barlev-Gross, M.; Weiss, S.; Ben-Shmuel, A.; Sittner, A.; Eden, K.; Mazuz, N.; Glinert, I.; Bar-David, E.; Puni, R.; Amit, S.; et al. Spike vs nucleocapsid SARS-CoV-2 antigen detection: Application in nasopharyngeal swab specimens. Anal. Bioanal. Chem. 2021, 413, 3501–3510. [Google Scholar] [CrossRef] [PubMed]
- NIH. How Rapid Antigen Tests Perform Against Viral Variants. Available online: https://covid19.nih.gov/news-and-stories/how-rapid-antigen-tests-perform-against-viral-variants#:~:text=In%20a%20study%2C%20rapid%20antigen,99%25%20of%20potential%20future%20variants. (accessed on 12 November 2024).
- Lau, K.A.; Horan, K.; Goncalves da Silva, A.; Kaufer, A.; Theis, T.; Ballard, S.A.; Rawlinson, W.D. Proficiency testing for SARS-CoV-2 whole genome sequencing. Pathology 2022, 54, 615–622. [Google Scholar] [CrossRef]
- Shen, Z.; Xiao, Y.; Kang, L.; Ma, W.; Shi, L.; Zhang, L.; Zhou, Z.; Yang, J.; Zhong, J.; Yang, D.; et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 713–720. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Sidiq, Z.; Hanif, M.; Dwivedi, K.K.; Chopra, K.K. Benefits and limitations of serological assays in COVID-19 infection. Indian J. Tuberc. 2020, 67, S163–S166. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed]
- FDA. Sherlock CRISPR SARS-CoV-2 Kit. Available online: https://www.fda.gov/media/137747/download (accessed on 12 November 2024).
- Li, J.; Zhang, K.; Lin, G.; Li, J. CRISPR-Cas system: A promising tool for rapid detection of SARS-CoV-2 variants. J. Med. Virol. 2024, 96, e29356. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Guo, L.; Zhou, X.; Zhu, Y.; He, Q.; Han, H.; Feng, Q. Machine learning techniques for CT imaging diagnosis of novel coronavirus pneumonia: A review. Neural Comput. Appl. 2022, 36, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Sadeghi, M.; Sharifpour, A.; Fakhar, M.; Zakariaei, Z.; Sadeghi, M.; Rokni, M.; Zakariaei, A.; Banimostafavi, E.S.; Hajati, F. Potential diagnostic application of a novel deep learning- based approach for COVID-19. Sci. Rep. 2024, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Valu, D.D.; Parikh, P.K.; Tiwari, N.; Chhipa, A.S.; Shukla, S.; Patel, S.S.; Balar, P.C.; Paiva-Santos, A.C.; Patravale, V. Conventional and Novel Diagnostic Tools for the Diagnosis of Emerging SARS-CoV-2 Variants. Vaccines 2023, 11, 374. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lam, C.Y.; Tan, P.H.; Tsang, H.F.; Wong, S.C. Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 8155. [Google Scholar] [CrossRef]
- Mohring, J.; Leithauser, N.; Wlazlo, J.; Schulte, M.; Pilz, M.; Munch, J.; Kufer, K.H. Estimating the COVID-19 prevalence from wastewater. Sci. Rep. 2024, 14, 14384. [Google Scholar] [CrossRef] [PubMed]
- Drozdzal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybycinski, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Los, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updat. 2021, 59, 100794. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schafer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- Focosi, D.; Franchini, M.; Maggi, F.; Shoham, S. COVID-19 therapeutics. Clin. Microbiol. Rev. 2024, 37, e0011923. [Google Scholar] [CrossRef]
- Knowlson, C.; Byrne, A.; Wilkinson, J.; Whitmore, C.; Torgerson, D. The evidence base for emergency use authorizations for COVID-19 treatments: A rapid review. Health Sci. Rep. 2023, 6, e1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- FDA. Authorization (EUA) for Emergency Use of Casirivimab and Imdevimab. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 12 November 2024).
- Westendorf, K.; Zentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef] [PubMed]
- Liew, M.N.Y.; Kua, K.P.; Lee, S.W.H.; Wong, K.K. SARS-CoV-2 neutralizing antibody bebtelovimab—A systematic scoping review and meta-analysis. Front. Immunol. 2023, 14, 1100263. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Rigopoulos, E.A.; Kaiafa, G.; Daios, S.; Karlafti, E.; Ztriva, E.; Polychronopoulos, G.; Gogos, C.; Savopoulos, C. Tixagevimab/Cilgavimab in SARS-CoV-2 Prophylaxis and Therapy: A Comprehensive Review of Clinical Experience. Viruses 2022, 15, 118. [Google Scholar] [CrossRef]
- Keam, S.J. Tixagevimab + Cilgavimab: First Approval. Drugs 2022, 82, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, H.; Yang, Z.; Hu, X.; Mahmmod, Y.S.; Zhu, X.; Zhao, C.; Zhai, J.; Zhang, X.X.; Luo, S.; et al. SARS-CoV-2: An Updated Review Highlighting Its Evolution and Treatments. Vaccines 2022, 10, 2145. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Casadevall, A. A Critical Analysis of the Use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld) for COVID-19 Prophylaxis and Treatment. Viruses 2022, 14, 1999. [Google Scholar] [CrossRef] [PubMed]
- Vellas, C.; Kamar, N.; Izopet, J. Resistance mutations in SARS-CoV-2 omicron variant after tixagevimab-cilgavimab treatment. J. Infect. 2022, 85, e162–e163. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, D.K.; Lee, J.; Kim, Y.I.; Seo, J.M.; Kim, Y.G.; Jeong, J.H.; Kim, M.; Kim, J.I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef]
- Ison, M.G.; Kim, J.Y.; Sandulescu, O.; Preotescu, L.-L.; Martinez, N.E.R.; Dobryanska, M.; Birlutiu, V.; Miftode, E.G.; Gaibu, N.; Caliman-Sturdza, O.A.; et al. 546. Therapeutic Effect of Regdanvimab in Patients with Mild to Moderate COVID-19: Day 28 Results from a Multicentre, Randomised, Controlled Pivotal Trial. Open Forum Infect. Dis. 2021, 8, S375. [Google Scholar] [CrossRef]
- Ryu, D.K.; Kang, B.; Noh, H.; Woo, S.J.; Lee, M.H.; Nuijten, P.M.; Kim, J.I.; Seo, J.M.; Kim, C.; Kim, M.; et al. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem. Biophys. Res. Commun. 2021, 578, 91–96. [Google Scholar] [CrossRef]
- Syed, Y.Y. Regdanvimab: First Approval. Drugs 2021, 81, 2133–2137. [Google Scholar] [CrossRef]
- Bock, A. New Guidance Helps Clinicians Use Pemivibart to Protect Immunocompromised Patients From COVID-19. JAMA 2024, 332, 1127–1129. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Zhang, R.M.; Ho, J.; Mohri, H.; Valdez, R.; Manthei, D.M.; Gordon, A.; Liu, L.; Ho, D.D. Antibody neutralisation of emerging SARS-CoV-2 subvariants: EG.5.1 and XBC.1.6. Lancet Infect. Dis. 2023, 23, e397–e398. [Google Scholar] [CrossRef] [PubMed]
- Roe, T.L.; Brady, T.; Schuko, N.; Nguyen, A.; Beloor, J.; Guest, J.D.; Aksyuk, A.A.; Tuffy, K.M.; Zhang, T.; Streicher, K.; et al. Molecular Characterization of AZD7442 (Tixagevimab-Cilgavimab) Neutralization of SARS-CoV-2 Omicron Subvariants. Microbiol. Spectr. 2023, 11, e0033323. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Zhang, Z.; Liu, X.; Wang, P.; Cao, J.; Liang, Q.; Qu, J.; Zhou, M. Serum neutralization of SARS-CoV-2 Omicron BA.2, BA.2.75, BA.2.76, BA.5, BF.7, BQ.1.1 and XBB.1.5 in individuals receiving Evusheld. J. Med. Virol. 2023, 95, e28932. [Google Scholar] [CrossRef] [PubMed]
- Pochtovyi, A.A.; Kustova, D.D.; Siniavin, A.E.; Dolzhikova, I.V.; Shidlovskaya, E.V.; Shpakova, O.G.; Vasilchenko, L.A.; Glavatskaya, A.A.; Kuznetsova, N.A.; Iliukhina, A.A.; et al. In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1. Vaccines 2023, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Consortium, C.-G.U.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 2023, 21, 112–124. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Rodrigues Falci, D.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 1236–1246. [Google Scholar] [CrossRef]
- McCreary, E.K.; Kip, K.E.; Collins, K.; Minnier, T.E.; Snyder, G.M.; Steiner, A.; Meyers, R.; Borneman, T.; Adam, M.; Thurau, L.; et al. Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era. Open Forum Infect Dis 2022, 9, ofac517. [Google Scholar] [CrossRef]
- Shertel, T.; Lange, N.W.; Salerno, D.M.; Hedvat, J.; Jennings, D.L.; Choe, J.Y.; Brown, R.S., Jr.; Pereira, M.R. Bebtelovimab for Treatment of COVID-19 in Ambulatory Solid Organ Transplant Recipients. Transplantation 2022, 106, e463–e464. [Google Scholar] [CrossRef]
- Lilly, E. Lilly’s Neutralizing Antibody Bamlanivimab (LY-CoV555) Prevented COVID-19 at Nursing Homes in the BLAZE-2 Trial, Reducing Risk by Up to 80 Percent for Residents. Available online: https://investor.lilly.com/news-releases/news-release-details/lillys-neutralizing-antibody-bamlanivimab-ly-cov555-prevented (accessed on 12 November 2024).
- Lilly, E. Bebtelovimab. Available online: https://covid19.lilly.com/ (accessed on 12 November 2024).
- Arena, C.T. Evusheld (Tixagevimab and Cilgavimab) for the Prevention of COVID-19. Available online: https://www.clinicaltrialsarena.com/projects/evusheld-tixagevimab-cilgavimab/ (accessed on 12 November 2024).
- GSK. GSK and Vir Biotechnology Announce Sotrovimab (VIR-7831) Receives Emergency Use Authorization from the US FDA for Treatment of Mild-to-Moderate COVID-19 in High-Risk Adults and Paediatric Patients. Available online: https://www.gsk.com/en-gb/media/press-releases/gsk-and-vir-biotechnology-announce-sotrovimab-vir-7831-receives-emergency-use-authorization-from-the-us-fda/ (accessed on 12 November 2024).
- Celltrion. Available online: https://www.celltrion.com/en-us (accessed on 12 November 2024).
- Invivyd. NVIVYD ANNOUNCES PEMGARDA™ (PEMIVIBART) DEMONSTRATED 84% RELATIVE RISK REDUCTION IN SYMPTOMATIC COVID-19 COMPARED TO PLACEBO IN AN EXPLORATORY ANALYSIS FROM ONGOING CANOPY PHASE 3 CLINICAL TRIAL. August 2024. Available online: https://investors.invivyd.com/news-releases/news-release-details/invivyd-announces-pemgardatm-pemivibart-demonstrated-84-relative/#:~:text=Release%20Details-,Invivyd%20Announces%20PEMGARDA™%20(pemivibart)%20Demonstrated%2084%25%20Relative%20Risk,CANOPY%20Phase%203%20Clinical%20Trial&text=WALTHAM%2C%20Mass.%2C%20Aug.,NEWSWIRE)%20%2D%2D%20Invivyd%2C%20Inc. (accessed on 12 November 2024).
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Liu, H.; Wei, P.; Zhang, Q.; Chen, Z.; Aviszus, K.; Downing, W.; Peterson, S.; Reynoso, L.; Downey, G.P.; Frankel, S.K.; et al. 501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to bamlanivimab in vitro. MAbs 2021, 13, 1919285. [Google Scholar] [CrossRef]
- Suryadevara, N.; Shrihari, S.; Gilchuk, P.; VanBlargan, L.A.; Binshtein, E.; Zost, S.J.; Nargi, R.S.; Sutton, R.E.; Winkler, E.S.; Chen, E.C.; et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell 2021, 184, 2316–2331.e2315. [Google Scholar] [CrossRef]
- Yamasoba, D.; Kosugi, Y.; Kimura, I.; Fujita, S.; Uriu, K.; Ito, J.; Sato, K.; Genotype to Phenotype Japan, C. Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect. Dis. 2022, 22, 942–943. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F.; McConnell, S.; Casadevall, A. Very low levels of remdesivir resistance in SARS-COV-2 genomes after 18 months of massive usage during the COVID19 pandemic: A GISAID exploratory analysis. Antivir. Res. 2022, 198, 105247. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schafer, A.; Dinnon, K.H., 3rd; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; Andre, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef]
- Kalil, A.C. Treating COVID-19-Off-Label Drug Use, Compassionate Use, and Randomized Clinical Trials During Pandemics. JAMA 2020, 323, 1897–1898. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Yuan, Z.; Pavel, M.A.; Wang, H.; Kwachukwu, J.C.; Mediouni, S.; Jablonski, J.A.; Nettles, K.W.; Reddy, C.B.; Valente, S.T.; Hansen, S.B. Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture. Commun. Biol. 2022, 5, 958. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, G.; Manaresi, E.; Cross, E.M.; Hoad, M.; Akbari, N.; Pavan, S.; Ariawan, D.; Bua, G.; Petersen, G.F.; Forwood, J.; et al. Importin alpha/beta-dependent nuclear transport of human parvovirus B19 nonstructural protein 1 is essential for viral replication. Antivir. Res. 2023, 213, 105588. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Mims, C.A. Ia antigens and Fc receptors of mouse peritoneal macrophages as determinants of susceptibility to lactic dehydrogenase virus. J. Gen. Virol. 1985, 66 Pt 7, 1469–1477. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Rawlinson, S.M.; Hearps, A.C.; Jans, D.A. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. Screen 2011, 16, 192–200. [Google Scholar] [CrossRef]
- Zapata-Cardona, M.I.; Florez-Alvarez, L.; Guerra-Sandoval, A.L.; Chvatal-Medina, M.; Guerra-Almonacid, C.M.; Hincapie-Garcia, J.; Hernandez, J.C.; Rugeles, M.T.; Zapata-Builes, W. In vitro and in silico evaluation of antiretrovirals against SARS-CoV-2: A drug repurposing approach. AIMS Microbiol. 2023, 9, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Way, G.; Madahar, V. Target Virus or Target Ourselves for COVID-19 Drugs Discovery?-Lessons learned from anti-influenza virus therapies. Med. Drug Discov. 2020, 5, 100037. [Google Scholar] [CrossRef] [PubMed]
- Rismanbaf, A. Potential Treatments for COVID-19; a Narrative Literature Review. Arch. Acad. Emerg. Med. 2020, 8, e29. [Google Scholar] [PubMed]
- Iketani, S.; Mohri, H.; Culbertson, B.; Hong, S.J.; Duan, Y.; Luck, M.I.; Annavajhala, M.K.; Guo, Y.; Sheng, Z.; Uhlemann, A.C.; et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 2023, 613, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Li, Y. Review: The Landscape of Antiviral Therapy for COVID-19 in the Era of Widespread Population Immunity and Omicron-Lineage Viruses. Clin. Infect. Dis. 2024, 78, 908–917. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Gotte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Hobartner, C.; Cramer, P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.; Le, N.T.; Selisko, B.; Eydoux, C.; Alvarez, K.; Guillemot, J.C.; Decroly, E.; Peersen, O.; Ferron, F.; Canard, B. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites. Antivir. Res. 2020, 178, 104793. [Google Scholar] [CrossRef] [PubMed]
- Malin, J.J.; Suarez, I.; Priesner, V.; Fatkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin Microbiol. Rev. 2020, 34, 10-1128. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, E.H.; Casel, M.A.B.; Kim, Y.I.; Sun, R.; Kwak, M.J.; Yoo, J.S.; Yu, M.; Yu, K.M.; Jang, S.G.; et al. SARS-CoV-2 variants with NSP12 P323L/G671S mutations display enhanced virus replication in ferret upper airways and higher transmissibility. Cell Rep. 2023, 42, 113077. [Google Scholar] [CrossRef]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.; Li, J.; et al. Mutations in the SARS-CoV-2 RNA-dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Nooruzzaman, M.; Johnson, K.E.E.; Rani, R.; Finkelsztein, E.J.; Caserta, L.C.; Kodiyanplakkal, R.P.; Wang, W.; Hsu, J.; Salpietro, M.T.; Banakis, S.; et al. Emergence of transmissible SARS-CoV-2 variants with decreased sensitivity to antivirals in immunocompromised patients with persistent infections. Nat. Commun. 2024, 15, 7999. [Google Scholar] [CrossRef]
- Barnard, D.L.; Hubbard, V.D.; Burton, J.; Smee, D.F.; Morrey, J.D.; Otto, M.J.; Sidwell, R.W. Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and beta-D-N4-hydroxycytidine. Antivir. Chem. Chemother. 2004, 15, 15–22. [Google Scholar] [CrossRef]
- Painter, G.R.; Bowen, R.A.; Bluemling, G.R.; DeBergh, J.; Edpuganti, V.; Gruddanti, P.R.; Guthrie, D.B.; Hager, M.; Kuiper, D.L.; Lockwood, M.A.; et al. The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus infection. Antivir. Res. 2019, 171, 104597. [Google Scholar] [CrossRef]
- Reynard, O.; Nguyen, X.N.; Alazard-Dany, N.; Barateau, V.; Cimarelli, A.; Volchkov, V.E. Identification of a New Ribonucleoside Inhibitor of Ebola Virus Replication. Viruses 2015, 7, 6233–6240. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Gotte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef] [PubMed]
- Swanstrom, R.; Schinazi, R.F. Lethal mutagenesis as an antiviral strategy. Science 2022, 375, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, S.; Yi, D.; Li, Q.; Ma, L.; Zhang, Y.; Wang, J.; Li, X.; Guo, F.; Lin, R.; et al. A cell-based assay to discover inhibitors of SARS-CoV-2 RNA dependent RNA polymerase. Antivir. Res. 2021, 190, 105078. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Strizki, J.M.; Gaspar, J.M.; Howe, J.A.; Hutchins, B.; Mohri, H.; Nair, M.S.; Kinek, K.C.; McKenna, P.; Goh, S.L.; Murgolo, N. Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance. Antimicrob. Agents Chemother. 2024, 68, e0095323. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.; Hisner, R.; Donovan-Banfield, I.; Hartman, H.; Lochen, A.; Peacock, T.P.; Ruis, C. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature 2023, 623, 594–600. [Google Scholar] [CrossRef]
- FDA. FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. 23 December 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain (accessed on 12 November 2024).
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. beta-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2023, 401, 281–293. [Google Scholar] [CrossRef]
- EMA. Lagevrio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lagevrio (accessed on 12 November 2024).
- Focosi, D.; McNally, D.; Maggi, F. The fitness of molnupiravir-signed SARS-CoV-2 variants: Imputation analysis based on prescription counts and GISAID analyses by country. Intervirology 2024, 67, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Zhang, Y.W.; Huang, P.; Ge, G.B. The SARS-CoV-2 main protease (M(pro)): Structure, function, and emerging therapies for COVID-19. MedComm 2022, 3, e151. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Ekanayake, K.B.; Otting, G.; Nitsche, C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg. Med. Chem. Lett. 2022, 62, 128629. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Charness, M.E.; Gupta, K.; Stack, G.; Strymish, J.; Adams, E.; Lindy, D.C.; Mohri, H.; Ho, D.D. Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment. N. Engl. J. Med. 2022, 387, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
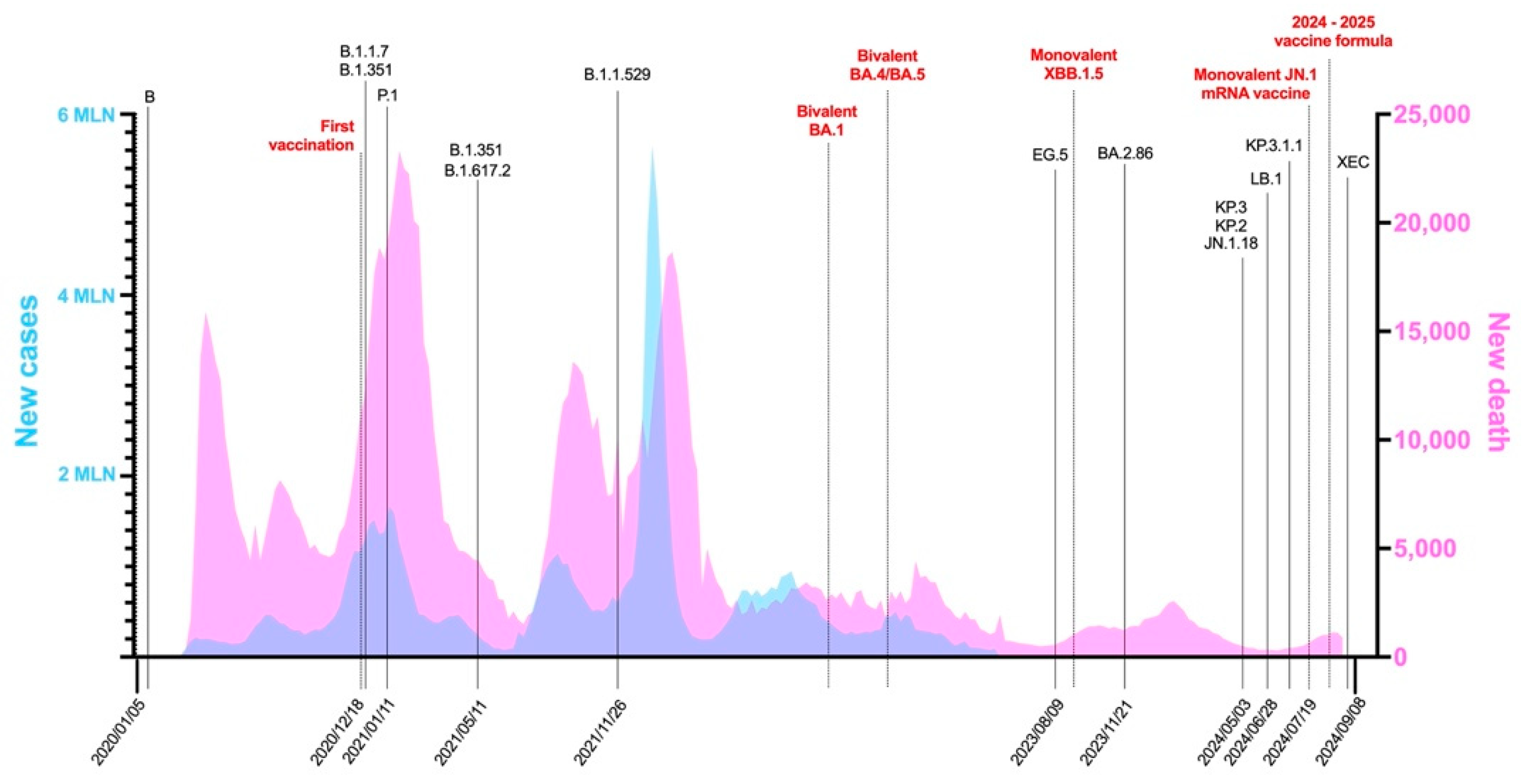
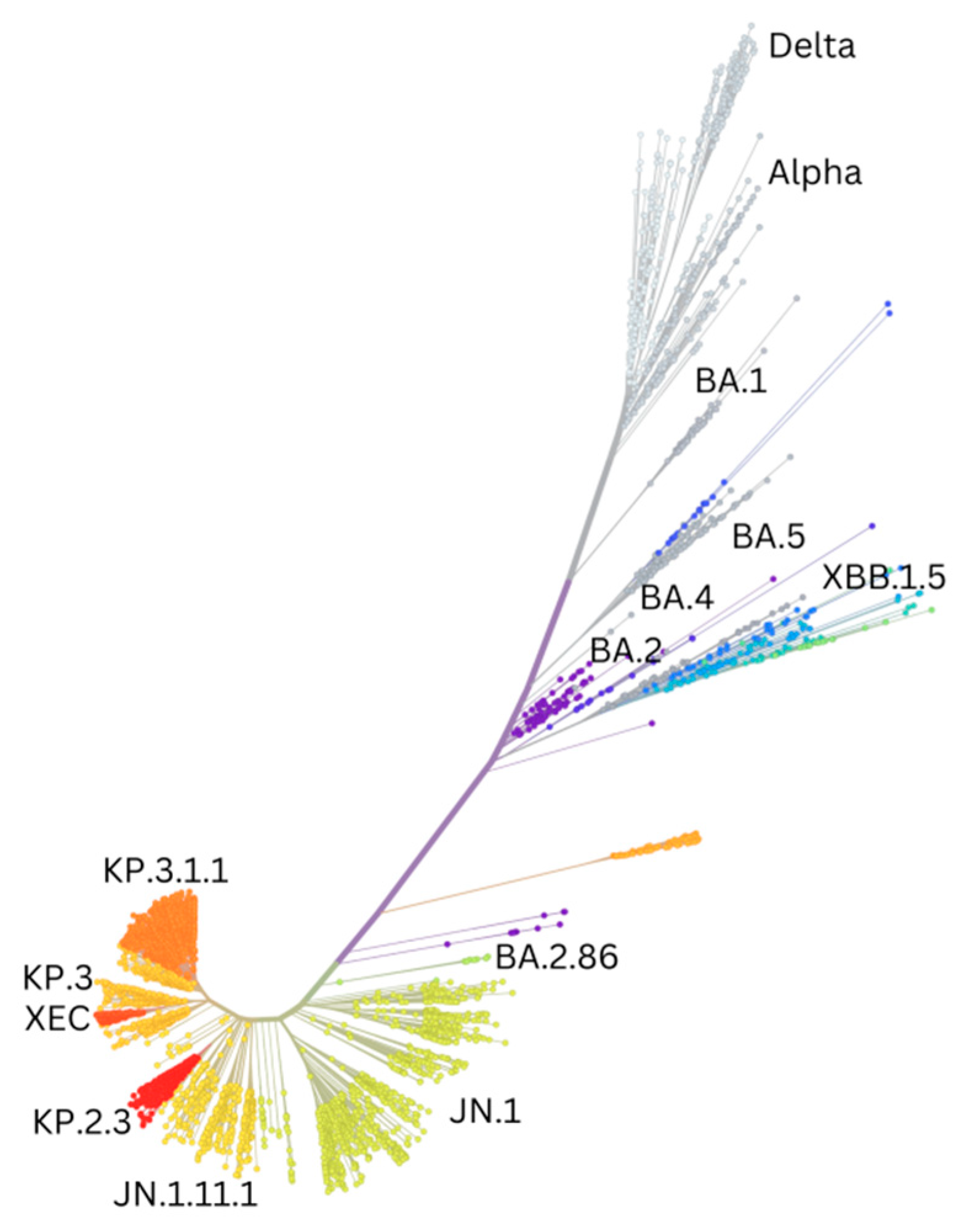



| Variant | PANGO Lineage | Date of Designation | Risk Assessment Update | Designation | Next Strain Clade | Relevant Genetic Features | Earliest Documented Samples | Prototype GenBank Accession Number |
|---|---|---|---|---|---|---|---|---|
| Alpha | B.1.1.7 | 18 Dec 2020 | 20 Sep 2021 | Previous VOC | 20I (V1) | S:N501Y S:Δ69/70 S:P681H S:T716I S:S982A | United Kingdom, Sep 2020 | MZ344997.1 |
| Beta | B.1.351 | 18 Dec 2020 | 20 Sep 2021 | Previous VOC | 20H (V2) | S:E484K | South Africa, May 2020 | MW598419.1 |
| Gamma | P.1 | 11 Jan 2021 | 20 Sep 2021 | Previous VOC | 20J (V3) | S:E484K | Brazil, Nov 2020 | MW642250.1 |
| Epsilon | B.1.427/B.1.429 | 5 Mar 2021 | 6 Jul 2021 | VOI | 21C | S:L452R | USA, Mar 2020 | MW453103.1 |
| Zeta | P.2 | 17 Mar 2021 | 6 Jul 2021 | VOI | 20B/S.484K | S:E484K | Brazil, Apr 2020 | MW523796.1 |
| Eta | B.1.525 | 17 Mar 2021 | 20 Sep 2021 | VOI | 21D | S:E484K S:F888 | Multiple countries, Dec 2020 | MW560924.1 |
| Theta | P.3 | 24 Mar 2021 | 6 Jul 2021 | VOI | 21E | S:E484K S:N501Y | Philippines, Jan 2021 | NA |
| Iota | B.1.526 | 24 Mar 2021 | 20 Sep 2021 | VOI | 21F | S:E484K S:D614G | USA, Nov 2020 | MW643362.1 |
| Kappa | B.1.617.1 | 4 Apr 2021 | 20 Sep 2021 | VOI | 21B | S:L452R S:E484Q | India, Oct 2020 | MW966601.1 |
| Delta | B.1.617.2 | 11 May 2021 | Previous VOC: 7 Jun 2022 | Previous VOC | 21A, 21I, 21J | S:L452R S:T478K S:P681R S:D614G | India, Oct 2020 | MZ009823.1 |
| Lambda | C.37 | 14 Jun 2021 | 9 Mar 2022 | VOI | 21G | S:L452Q S:F490S | Peru, Dec 2020 | MW850639.1 |
| Mu | B.1.621 | 30 Aug 2021 | 9 Mar 2022 | VOI | 21H | S:T95I S:Y144S S:R346K S:E484K S:N501Y | Colombia, Jan 2021 | OQ248293.1 |
| Omicron | B.1.1.529 (includes BA.1, BA.2, BA.3, BA.4, BA.5) | 26 Nov 2021 | - | VOC | 21K, 21L, 21M, 22A, 22B, 22C, 22D | S:R346K S:L452X S:F486V | Multiple countries, Nov 2021 | OL672836.1 |
| BA.2.75 | BA.2.75 | 06 Jul 2022 | 10 Apr 2024 | VUM | 22D | BA.2 + S:K147E S:W152R S:F157L S:I210V S:G257S S:D339H S:G446S S:N460K S:Q493R reversion | 31 Dec 2021 | ON990685.1 |
| BQ.1 | BQ.1 | 21 Sep 2022 | VUM | 22E | BA.5 + S:R346T S:K444T S:N460K | 07 Feb 2022 | OP412163.1 | |
| XBB | XBB | 12 Oct 2022 | 10 Apr 2024 | VUM | 22F | BA.2+ S:V83A S:Y144- S:H146Q S:Q183E S:V213E S:G252V S:G339H S:R346T S:L368I S:V445P S:G446S S:N460K S:F486S S:F490S | 19 Aug 2022 | OR098785.1 |
| BA.5 | BA.5 | 20 Nov 2022 | VUM | 22B, 22E | BA.5 + one or more of these mutations: S:R346X S:K444X S:V445X S:N450D or S:N460X | 07 Feb 2022 | ON249995.1 | |
| BA.4.6 | BA.4.6 | 20 Nov 2022 | VUM | 22A | BA.4 + S:R346T S:N658S | 20 Jul 2020 | OR325409.1 | |
| BA.2.3.20 | BA.2.3.20 | 20 Nov 2022 | VUM | 21L | BA.2 + S:M153T S:N164K S:H245N S:G257D S:K444R S:N450D S:L452M S:N460K S:E484R | 15 Aug 2022 | PP847689.1 | |
| XBB.1.5 | XBB.1.5 | 11 Jan 2023 | 7 Jun 2024 | VOI | 23A | Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e., BJ.1 and BM.1.1.1, with a breakpoint in S1. XBB.1 + S:F486P (similar Spike genetic profile as XBB.1.9.1) Includes XBB.1.5.70 (23G): XBB.1.5 + S:L455F and S:F456L | 21 Oct 2022 | OP790748.1 |
| CH.1.1 | CH.1.1 | 8 Feb 2023 | VUM | 22D | BA.2.75 + S:L452R S:F486S | 27 Jul 2022 | PP848047.1 | |
| XBF | XBF | 8 Feb 2023 | VUM | Recombinant of BA.5.2.3 and CJ.1 (BA.2.75.3 sublineage) BA.5 + S:K147E S:W152R S:F157L S:I210V S:G257S S:G339H S:R346T S:G446S S:N460K S:F486P S:F490S | 27 Jul 2022 | PP848029.1 | ||
| BF.7 | BF.7 | 9 Feb 2023 | VUM | 22B | BA.5 + S:R346T | 24 Jan 2022 | PP848045.1 | |
| XBB.1.9.1 | XBB.1.9.1 | 30 Mar 2023 | 10 Apr 2024 | VUM | 23D | Recombinant of BA.2.10.1 and BA.2.75 sublineages (i.e., BJ.1 and BM.1.1.1 XBB.1 + S:F486P S:Q613H) | 05 Dec 2022 | PP846633.1 |
| XBB.1.16 | XBB.1.16 | 17 Apr 2023 | 7 Jun 2024 | VOI | 23B | Recombinant of BA.2.10.1 and BA.2.75 sublineages (i.e., BJ.1 and BM.1.1.1 XBB.1 + S:E180V S:K478R S:F486P) | 09 Jan 2023 | PP846659.1 |
| XBB.1.9.2 | XBB.1.9.2 | 26 Apr 2023 | 10 Apr 2024 | VUM | 23D | XBB.1 + S:F486P | 05 Dec 2022 | PP846644.1 |
| XBB.2.3 | XBB.2.3 | 17 May 2023 | 10 Apr 2024 | VUM | 23E | Recombinant of BA.2.10.1 and BA.2.75 sublineages (i.e., BJ.1 and BM.1.1.1 XBB + S:D253G S:F486P S:P521S) | 09 Dec 2022 | PP846522.1 |
| EG.5 | EG.5 | 09 Aug 2023 | 28 Jun 2024 | VOI | Not Assigned | XBB.1.9.2 + S:F456L, which includes EG.5.1 (23F): EG.5 + S:Q52H HK.3 (23H): EG.5 + S:Q52H S:L455F HV.1: EG.5 + S:Q52H S:F157L S:L452R | 17 Feb 2023 | OQ873579.1 |
| DV.7 | DV.7 | 23 Oct 2023 | 10 Apr 2024 | VUM | 23C | CH.1.1 + S:N185D S:L858I | 19 Jan 2023 | PP846399.1 |
| BA.2.86 | BA.2.86 | 21 Nov 2023 | - | VOI | 23I | Mutations relative to BA.2 | 24 Jul 2023 | PP092736.1 |
| JN.1 | JN.1 | 18 Dec 2023 | Updated on 15 Apr 2024 | VOI | 24A | BA.2.86 + S:L455S | 25 Aug 2023 | PP846619.1 |
| JN.1.7 | JN.1.7 | 03 May 2024 | - | VUM | 24A | JN.1 + S:T572I S:E1150D | 25 Sep 2023 | NA |
| JN.1.18 | JN.1.18 | 03 May 2024 | - | VUM | 24A | JN.1 + S:R346T | 11 Nov 2023 | NA |
| KP.2 | KP.2 | 03 May 2024 | - | VUM | 24B | JN.1 + S:R346T S:F456L S:V1104L | 02 Jan 2024 | NA |
| KP.3 | KP.3 | 03 May 2024 | - | VUM | 24C | JN.1 + S:F456L S:Q493E S:V1104L | 11 Feb 2024 | NA |
| LB.1 | LB.1 | 28 Jun 2024 | - | VUM | 24A | JN.1 + S:S31- S:Q183H S:R346T S:F456L | 26 Feb 2024 | NA |
| KP.3.1.1 | KP.3.1.1 | 19 Jul 2024 | - | VUM | 24E | KP.3 + S:S31- | 27 Mar 2024 | NA |
| XEC | XEC | 24 Sep 2024 | - | VUM | 24F | JN.1 + S:T22N S:F59S S:F456L S:Q493E S:V1104L | 16 May 2024 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angius, F.; Puxeddu, S.; Zaimi, S.; Canton, S.; Nematollahzadeh, S.; Pibiri, A.; Delogu, I.; Alvisi, G.; Moi, M.L.; Manzin, A. SARS-CoV-2 Evolution: Implications for Diagnosis, Treatment, Vaccine Effectiveness and Development. Vaccines 2025, 13, 17. https://doi.org/10.3390/vaccines13010017
Angius F, Puxeddu S, Zaimi S, Canton S, Nematollahzadeh S, Pibiri A, Delogu I, Alvisi G, Moi ML, Manzin A. SARS-CoV-2 Evolution: Implications for Diagnosis, Treatment, Vaccine Effectiveness and Development. Vaccines. 2025; 13(1):17. https://doi.org/10.3390/vaccines13010017
Chicago/Turabian StyleAngius, Fabrizio, Silvia Puxeddu, Silvio Zaimi, Serena Canton, Sepehr Nematollahzadeh, Andrea Pibiri, Ilenia Delogu, Gualtiero Alvisi, Meng Ling Moi, and Aldo Manzin. 2025. "SARS-CoV-2 Evolution: Implications for Diagnosis, Treatment, Vaccine Effectiveness and Development" Vaccines 13, no. 1: 17. https://doi.org/10.3390/vaccines13010017
APA StyleAngius, F., Puxeddu, S., Zaimi, S., Canton, S., Nematollahzadeh, S., Pibiri, A., Delogu, I., Alvisi, G., Moi, M. L., & Manzin, A. (2025). SARS-CoV-2 Evolution: Implications for Diagnosis, Treatment, Vaccine Effectiveness and Development. Vaccines, 13(1), 17. https://doi.org/10.3390/vaccines13010017









