Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination
Abstract
1. Introduction
2. Materials and Methods
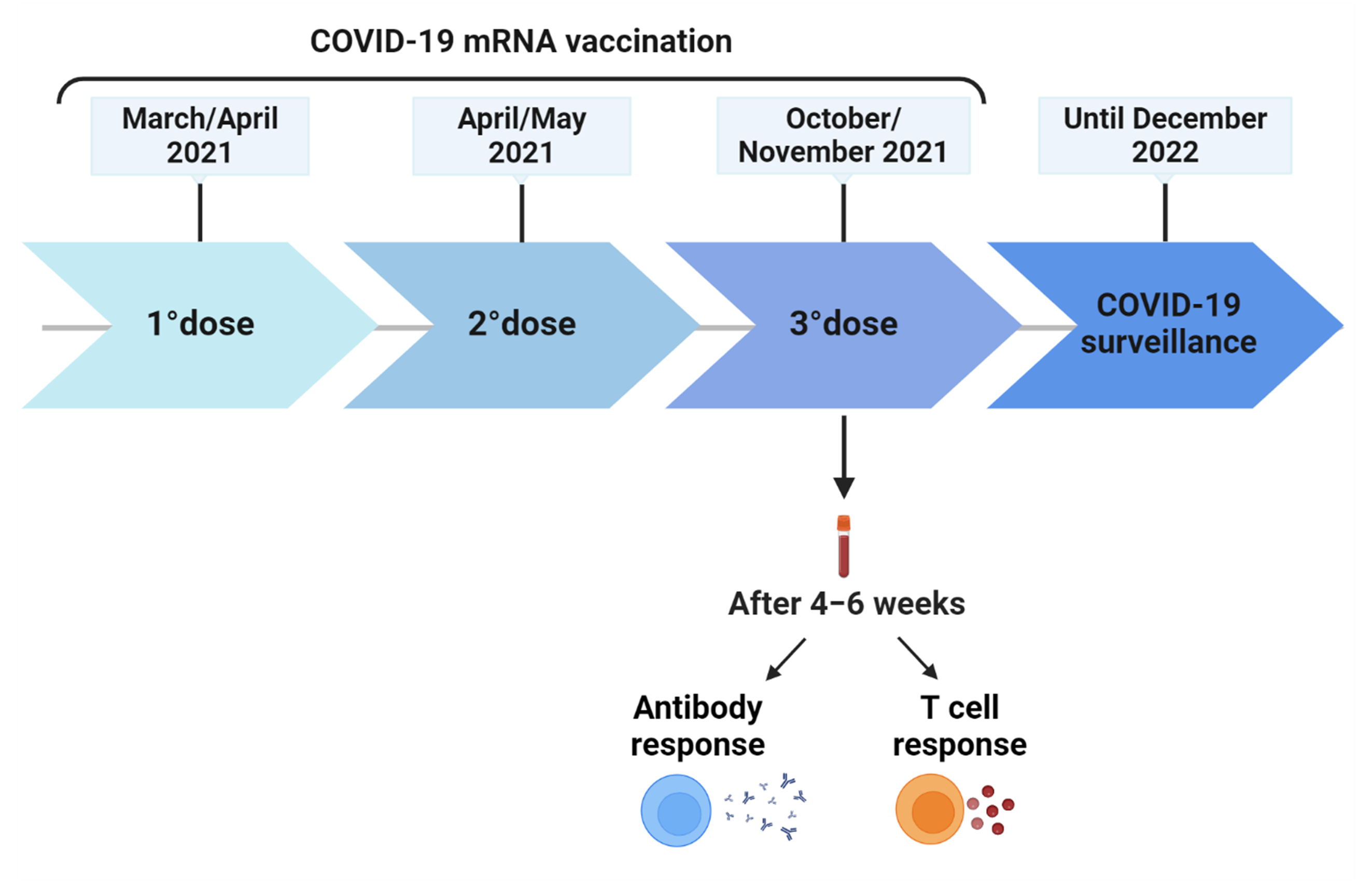
2.1. Study Cohort and Design
2.2. Anti-SARS-CoV-2 Antibodies
2.3. IFN-γ-Spike-Specific T cell Response
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Studied Population
3.2. Incidence of Breakthrough Infections and Clinical Features
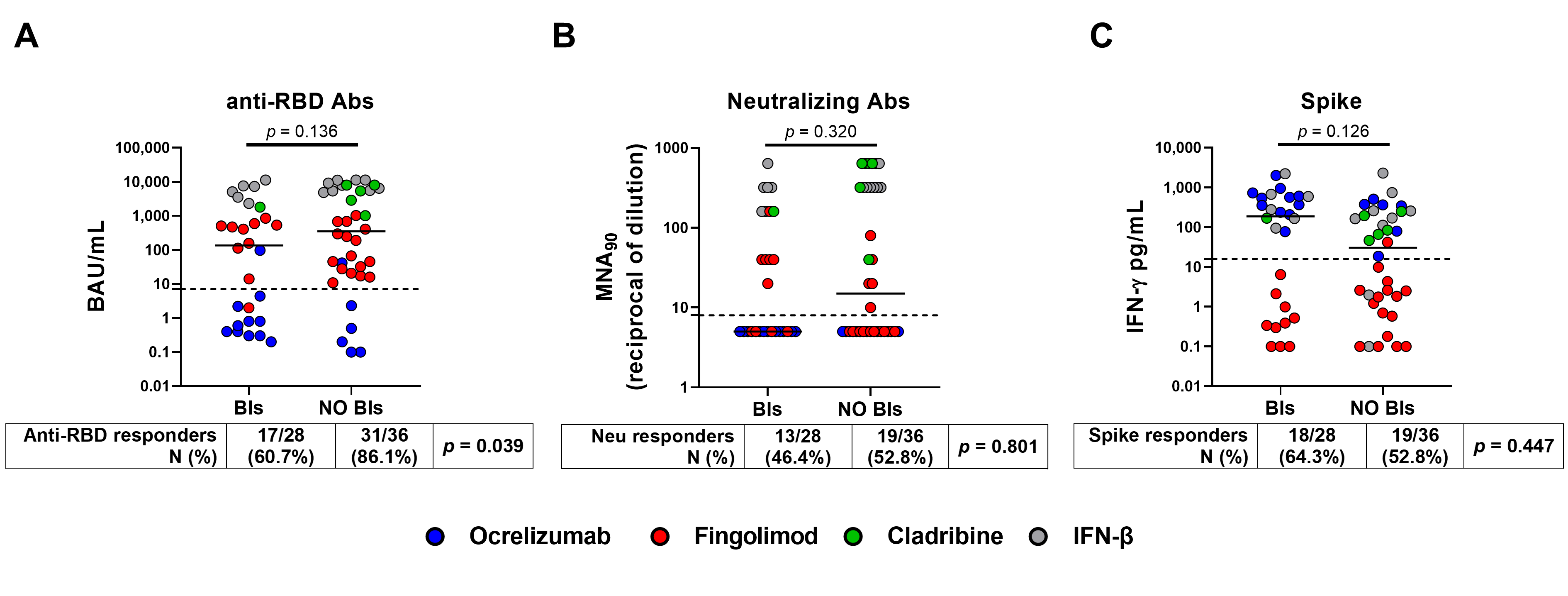
3.3. Incidence of Breakthrough Infections and Immune Response
3.4. COVID-19 Severity
3.5. Time of Swab Negativization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19)—World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 2 May 2023).
- Grifoni, A.; Alonzi, T.; Alter, G.; Noonan, D.M.; Landay, A.L.; Albini, A.; Goletti, D. Impact of Aging on Immunity in the Context of COVID-19, HIV, and Tuberculosis. Front. Immunol. 2023, 14, 1146704. [Google Scholar] [CrossRef]
- Li, Y.; Choudhary, M.C.; Regan, J.; Boucau, J.; Nathan, A.; Speidel, T.; Liew, M.Y.; Edelstein, G.E.; Kawano, Y.; Uddin, R.; et al. SARS-CoV-2 Viral Clearance and Evolution Varies by Type and Severity of Immunodeficiency. Sci. Transl. Med. 2024, 16, eadk1599. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Tortorella, C.; Aiello, A.; Farroni, C.; Ruggieri, S.; Castilletti, C.; Meschi, S.; Cuzzi, G.; Vanini, V.; Palmieri, F.; et al. Humoral and Cellular Response to Spike of Delta SARS-CoV-2 Variant in Vaccinated Patients with Multiple Sclerosis. Front. Neurol. 2022, 13, 881988. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, S.; Aiello, A.; Tortorella, C.; Navarra, A.; Vanini, V.; Meschi, S.; Lapa, D.; Haggiag, S.; Prosperini, L.; Cuzzi, G.; et al. Dynamic Evolution of Humoral and T-Cell Specific Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Followed until the Booster Dose. Int. J. Mol. Sci. 2023, 24, 8525. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, C.; Aiello, A.; Gasperini, C.; Agrati, C.; Castilletti, C.; Ruggieri, S.; Meschi, S.; Matusali, G.; Colavita, F.; Farroni, C.; et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with MS Using Different Disease-Modifying Therapies. Neurology 2022, 98, e541–e554. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Coppola, A.; Ruggieri, S.; Farroni, C.; Altera, A.M.G.; Salmi, A.; Vanini, V.; Cuzzi, G.; Petrone, L.; Meschi, S.; et al. Longitudinal Characterisation of B and T-Cell Immune Responses after the Booster Dose of COVID-19 mRNA-Vaccine in People with Multiple Sclerosis Using Different Disease-Modifying Therapies. J. Neurol. Neurosurg. Psychiatry 2023, 94, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, A.; Chiesa, F.; Conte, S.; Bengtsson, C.; Lee, S.; Minton, N.; Niemcryk, S.; Lindholm, A.; Rosenlund, M.; Piehl, F.; et al. Infections in Patients with Multiple Sclerosis: A National Cohort Study in Sweden. Mult. Scler. Relat. Disord. 2020, 45, 102420. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.B.; Raghavendra, S.; Nooraine, J.; Jaychandran, R. COVID-19 Outcomes in Persons with Multiple Sclerosis Treated with Rituximab. Mult. Scler. Relat. Disord. 2022, 57, 103371. [Google Scholar] [CrossRef]
- Foerch, C.; Friedauer, L.; Bauer, B.; Wolf, T.; Adam, E.H. Severe COVID-19 Infection in a Patient with Multiple Sclerosis Treated with Fingolimod. Mult. Scler. Relat. Disord. 2020, 42, 102180. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef] [PubMed]
- Zaloum, S.A.; Wood, C.H.; Tank, P.; Upcott, M.; Vickaryous, N.; Anderson, V.; Baker, D.; Chance, R.; Evangelou, N.; George, K.; et al. Risk of COVID-19 in People with Multiple Sclerosis Who Are Seronegative Following Vaccination. Mult. Scler. J. 2023, 29, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Tortorella, C.; Haggiag, S.; Ruggieri, S.; Galgani, S.; Gasperini, C. Increased Risk of Death from COVID-19 in Multiple Sclerosis: A Pooled Analysis of Observational Studies. J. Neurol. 2022, 269, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral Immune Response to COVID-19 mRNA Vaccine in Patients with Multiple Sclerosis Treated with High-Efficacy Disease-Modifying Therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012835. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.J.; Mittl, K.; Rowles, W.M.; McPolin, K.; Rajan, J.V.; Laurie, M.T.; Zamecnik, C.R.; Dandekar, R.; Alvarenga, B.D.; Loudermilk, R.P.; et al. Multiple Sclerosis Therapies Differentially Affect SARS-CoV-2 Vaccine–Induced Antibody and T Cell Immunity and Function. JCI Insight 2022, 7, e156978. [Google Scholar] [CrossRef] [PubMed]
- Sainz de la Maza, S.; Walo-Delgado, P.E.; Rodríguez-Domínguez, M.; Monreal, E.; Rodero-Romero, A.; Chico-García, J.L.; Pariente, R.; Rodríguez-Jorge, F.; Ballester-González, R.; Villarrubia, N.; et al. Short- and Long-Term Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines 2023, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Habek, M.; Željko, C.; Savić Mlakar, A.; Bendelja, K.; Rogić, D.; Adamec, I.; Barun, B.; Gabelić, T.; Krbot Skorić, M. Humoral and Cellular Immunity in Convalescent and Vaccinated COVID-19 People with Multiple Sclerosis: Effects of Disease Modifying Therapies. Mult. Scler. Relat. Disord. 2022, 59, 103682. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Arndt, L.; Braun, J.; Fauchere, F.; Vanshylla, K.; Loyal, L.; Henze, L.; Kruse, B.; Dingeldey, M.; Jürchott, K.; Mangold, M.; et al. SARS-CoV-2 mRNA Vaccinations Fail to Elicit Humoral and Cellular Immune Responses in Patients with Multiple Sclerosis Receiving Fingolimod. J. Neurol. Neurosurg. Psychiatry 2022, 93, 960–971. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Bajwa, H.M.; Novak, F.; Nilsson, A.C.; Nielsen, C.; Holm, D.K.; Østergaard, K.; Witt, A.H.; Byg, K.-E.; Johansen, I.S.; Mittl, K.; et al. Persistently Reduced Humoral and Sustained Cellular Immune Response from First to Third SARS-CoV-2 mRNA Vaccination in Anti-CD20-Treated Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2022, 60, 103729. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Gillot, C.; Bayart, J.-L.; Closset, M.; Cabo, J.; Maloteau, V.; Dogné, J.-M.; Douxfils, J.; Favresse, J. Peri-Infection Titers of Neutralizing and Binding Antibodies as a Predictor of COVID-19 Breakthrough Infections in Vaccinated Healthcare Professionals: Importance of the Timing. Clin. Chem. Lab. Med. 2023, 61, 1670–1675. [Google Scholar] [CrossRef]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; d’Andrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2310650. [Google Scholar] [CrossRef] [PubMed]
- Machkovech, H.M.; Hahn, A.M.; Garonzik Wang, J.; Grubaugh, N.D.; Halfmann, P.J.; Johnson, M.C.; Lemieux, J.E.; O’Connor, D.H.; Piantadosi, A.; Wei, W.; et al. Persistent SARS-CoV-2 Infection: Significance and Implications. Lancet Infect. Dis. 2024, 24, e453–e462. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Bohnert, A.S.B.; O’Hare, A.M.; Boyko, E.J.; Maciejewski, M.L.; Smith, V.A.; Bowling, C.B.; Viglianti, E.; Iwashyna, T.J.; Hynes, D.M.; et al. Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann. Intern. Med. 2022, 175, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Tsang, N.N.Y.; So, H.C.; Cowling, B.J.; Leung, G.M.; Ip, D.K.M. Effectiveness of BNT162b2 and CoronaVac COVID-19 Vaccination against Asymptomatic and Symptomatic Infection of SARS-CoV-2 Omicron BA.2 in Hong Kong: A Prospective Cohort Study. Lancet Infect. Dis. 2023, 23, 421–434. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Kirchner, E.; Husni, E.M.; Moss, B.P.; Fernandez, A.P.; Jin, Y.; Calabrese, L.H. Breakthrough SARS-CoV-2 Infections in Patients with Immune-Mediated Disease Undergoing B Cell-Depleting Therapy: A Retrospective Cohort Analysis. Arthritis Rheumatol. 2022, 74, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Novak, F.; Bajwa, H.M.; Coia, J.E.; Nilsson, A.C.; Nielsen, C.; Holm, D.K.; Østergaard, K.; Hvidt, M.V.M.; Byg, K.-E.; Johansen, I.S.; et al. Low Protection from Breakthrough SARS-CoV-2 Infection and Mild Disease Course in Ocrelizumab-Treated Patients with Multiple Sclerosis after Three mRNA Vaccine Doses. J. Neurol. Neurosurg. Psychiatry 2023, 94, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Delgado, S.; Habek, M.; Davydovskaya, M.; Ward, B.J.; Cree, B.A.C.; Totolyan, N.; Pingili, R.; Mancione, L.; Hu, X.; et al. Correction to: COVID-19 Outcomes and Vaccination in People with Relapsing Multiple Sclerosis Treated with Ofatumumab. Neurol. Ther. 2022, 11, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; Schiavetti, I.; Inglese, M.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Visconti, V.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Breakthrough SARS-CoV-2 Infections after COVID-19 mRNA Vaccination in MS Patients on Disease Modifying Therapies during the Delta and the Omicron Waves in Italy. EBioMedicine 2022, 80, 104042. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Galante, A.; Cantu’, M.; Sacco, R.; Mele, F.; Eisler, J.J.; Keller, F.; Bernasconi, E.; Sallusto, F.; Zecca, C.; et al. Longitudinal Postvaccine SARS-CoV-2 Immunoglobulin G Titers, Memory B-Cell Responses, and Risk of COVID-19 in Multiple Sclerosis Over 1 Year. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200043. [Google Scholar] [CrossRef]
- Holroyd, K.B.; Healy, B.C.; Conway, S.; Houtchens, M.; Bakshi, R.; Bhattacharyya, S.; Bose, G.; Galetta, K.; Kaplan, T.; Severson, C.; et al. Humoral Response to COVID-19 Vaccination in MS Patients on Disease Modifying Therapy: Immune Profiles and Clinical Outcomes. Mult. Scler. Relat. Disord. 2022, 67, 104079. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Farber, R.S.; DeAngelis, T.; Leung, T.; Smith, T.; Blanck, R.; Zhovtis-Ryerson, L.; Harel, A. Vaccine-Breakthrough SARS-CoV-2 Infections in People with Multiple Sclerosis and Related Conditions: An Observational Study by the New York COVID-19 Neuro-Immunology Consortium (NYCNIC-2). Mult. Scler. J. 2023, 29, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Zakalik, K.; Awan, S.; Kavak, K.S.; Pennington, P.; Hojnacki, D.; Kolb, C.; Lizarraga, A.A.; Eckert, S.P.; Sarrosa, R.; et al. COVID-19 Vaccination in Multiple Sclerosis and Inflammatory Diseases: Effects from Disease-Modifying Therapy, Long-Term Seroprevalence and Breakthrough Infections. Vaccines 2022, 10, 695. [Google Scholar] [CrossRef]
- van Kempen, Z.L.E.; Stalman, E.W.; Steenhuis, M.; Kummer, L.Y.L.; van Dam, K.P.J.; Wilbrink, M.F.; Ten Brinke, A.; van Ham, S.M.; Kuijpers, T.; Rispens, T.; et al. SARS-CoV-2 Omicron Breakthrough Infections in Patients with Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2023, 94, 280–283. [Google Scholar] [CrossRef]
- Stefanelli, P.; Trentini, F.; Petrone, D.; Mammone, A.; Ambrosio, L.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Zardini, A.; et al. Tracking the Progressive Spread of the SARS-CoV-2 Omicron Variant in Italy, December 2021 to January 2022. Eurosurveillance 2022, 27, 2200125. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Clinical Spectrum. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 3 March 2024).
- Aiello, A.; Najafi Fard, S.; Petruccioli, E.; Petrone, L.; Vanini, V.; Farroni, C.; Cuzzi, G.; Navarra, A.; Gualano, G.; Mosti, S.; et al. Spike Is the Most Recognized Antigen in the Whole-Blood Platform in Both Acute and Convalescent COVID-19 Patients. Int. J. Infect. Dis. 2021, 106, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Matusali, G.; Colavita, F.; Lapa, D.; Meschi, S.; Bordi, L.; Piselli, P.; Gagliardini, R.; Corpolongo, A.; Nicastri, E.; Antinori, A.; et al. SARS-CoV-2 Serum Neutralization Assay: A Traditional Tool for a Brand-New Virus. Viruses 2021, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated Fusogenicity and Pathogenicity of SARS-CoV-2 Omicron Variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Immovilli, P.; Schiavetti, I.; Franceschini, A.; De Mitri, P.; Gelati, L.; Rota, E.; Guidetti, D. Breakthrough COVID-19 in People with Multiple Sclerosis on Disease Modifying Treatments: Is It Still a Severe Disease? Mult. Scler. Relat. Disord. 2024, 85, 105547. [Google Scholar] [CrossRef] [PubMed]
- Spierer, R.; Lavi, I.; Bloch, S.; Mazar, M.; Golan, D. Risk of Breakthrough COVID-19 after Vaccination among People with Multiple Sclerosis on Disease-Modifying Therapies. J. Neurol. 2023, 270, 4632–4639. [Google Scholar] [CrossRef]
- Plebani, M.; Lippi, G. Sex and gender differences in COVID-19: A narrative review. J. Sex Gend. Specif. Med. 2022, 8, 105–111. [Google Scholar]
- Chaudhry, F.; Bulka, H.; Rathnam, A.S.; Said, O.M.; Lin, J.; Lorigan, H.; Bernitsas, E.; Rube, J.; Korzeniewski, S.J.; Memon, A.B.; et al. COVID-19 in Multiple Sclerosis Patients and Risk Factors for Severe Infection. J. Neurol. Sci. 2020, 418, 117147. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple Sclerosis—A Review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Louapre, C.; Collongues, N.; Stankoff, B.; Giannesini, C.; Papeix, C.; Bensa, C.; Deschamps, R.; Créange, A.; Wahab, A.; Pelletier, J.; et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Immovilli, P.; Schiavetti, I.; Cordioli, C.; De Mitri, P.; Grazioli, S.; Guidetti, D.; Sormani, M.P. Lung Involvement Correlates with Disability in MS Patients with COVID-19 Pneumonia. Neurol. Sci. 2022, 43, 6657–6659. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Liu, H.; Wu, N.C.; Lee, C.-C.D.; Zhu, X.; Zhao, F.; Huang, D.; Yu, W.; Hua, Y.; Tien, H.; et al. Structural Basis of a Shared Antibody Response to SARS-CoV-2. Science 2020, 369, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Chandler, T.L.; Yang, A.; Otero, C.E.; Permar, S.R.; Caddy, S.L. Protective Mechanisms of Nonneutralizing Antiviral Antibodies. PLoS Pathog. 2023, 19, e1011670. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.D.; Bouley, A.J.; Jungquist, R.M.; Douglas, E.A.; O’Shea, I.L.; Lathi, E.S. Humoral and T-Cell Responses to SARS-CoV-2 Vaccination in Multiple Sclerosis Patients Treated with Ocrelizumab. Mult. Scler. Relat. Disord. 2022, 57, 103382. [Google Scholar] [CrossRef] [PubMed]
- Räuber, S.; Korsen, M.; Huntemann, N.; Rolfes, L.; Müntefering, T.; Dobelmann, V.; Hermann, A.M.; Kölsche, T.; Lipinski, K.; von Wnuck Lipinski, K.; et al. Immune Response to SARS-CoV-2 Vaccination in Relation to Peripheral Immune Cell Profiles among Patients with Multiple Sclerosis Receiving Ocrelizumab. J. Neurol. Neurosurg. Psychiatry 2022, 93, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Januel, E.; Hajage, D.; Labauge, P.; Maillart, E.; De Sèze, J.; Zephir, H.; Pelletier, J.; Guilloton, L.; Bensa, C.; Heinzlef, O.; et al. Association Between Anti-CD20 Therapies and COVID-19 Severity Among Patients with Relapsing-Remitting and Progressive Multiple Sclerosis. JAMA Netw. Open 2023, 6, e2319766. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.-P. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Palomares Cabeza, V.; Kummer, L.Y.L.; Wieske, L.; Hagen, R.R.; Duurland, M.; Konijn, V.A.L.; van Dam, K.P.J.; Stalman, E.W.; van de Sandt, C.E.; Boekel, L.; et al. Longitudinal T-Cell Responses after a Third SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis on Ocrelizumab or Fingolimod. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1178. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Sette, A.; de Vries, R.D.; Goletti, D. The Importance of Measuring SARS-CoV-2-Specific T-Cell Responses in an Ongoing Pandemic. Pathogens 2023, 12, 862. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics | |
|---|---|
| Total | 64 (100) |
| Age, median (IQR) years | 49 (41.5–55.5) |
| Age class, n (%) | |
| 23–39 | 16 (25.0) |
| 40–49 | 19 (29.7) |
| 50–70 | 29 (45.3) |
| Gender: Female, n (%) | 43 (67.2) |
| Origin: Italian, n (%) | 62 (96.9) |
| Presence of comorbidities, n (%) | 14 (21.9) |
| BMI (kg/m2), median (IQR) | 24 (21–26) |
| Vaccination-First dose with Comirnaty, n (%) | 59 (92.2) |
| MS duration, median (IQR) years | 15 (7–25) |
| MS treatment, n (%) | |
| Cladribine | 6 (9.4) |
| Fingolimod | 26 (40.6) |
| Interferon beta | 15 (23.4) |
| Ocrelizumab | 17 (26.6) |
| MS treatment duration, median months (IQR) | 5 (2–9) |
| EDSS score, median (IQR) | 2 (0.5–4.3) |
| <3 | 37 (58.8) |
| ≥3 | 27 (42.2) |
| MS phenotype, n (%) | |
| Primary-progressive (PP) | 5 (7.8) |
| Relapsing-Remitting (RR) | 59 (92.2) |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.7) |
| Days from booster dose to sample, median (IQR) | 48 (43–51) |
| Among PwMS with SARS-CoV-2 breakthrough infection | 28 (43.7) |
| Days from 3rd dose to infection, median (IQR) | 155 (108–205) |
| Days to negative swab, median (IQR) | 11 (9–15) |
| COVID-19 therapy, n (%) | |
| Anti-viral therapy | 5 (17.9) |
| Monoclonal therapy | 11 (39.3) |
| Non-steroidal anti-inflammatory drugs or paracetamol or no therapy * | 12 (42.9) |
| Severity of COVID-19, n (%) | |
| Mild disease | 21 (75.0) |
| Moderate disease | 5 (17.9) |
| Severe disease | 2 (7.1) |
| Patients’ Characteristics | SARS-CoV-2 BIs | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | IRR | 95%CI | p | IRR | 95%CI | p | |
| Total, n (%) | 36 (56.3) | 28 (43.8) | 64 (100) | ||||||
| Age, median (IQR) years | 50 (45.5–58) | 47.5 (38–53.5) | 49 (41.5–55.5) | ||||||
| 23–39 | 8 (22.2) | 8 (28.6) | 16 (25.0) | Ref. | |||||
| 40–49 | 10 (27.8) | 9 (32.1) | 19 (29.7) | 1.01 | 0.39–2.63 | 0.979 | |||
| 50–70 | 18 (50.0) | 11 (39.3) | 29 (45.3) | 0.66 | 0.27–1.64 | 0.371 | |||
| Gender, n (%) | |||||||||
| Female | 28 (77.8) | 15 (53.6) | 43 (67.2) | Ref. | |||||
| Male | 8 (22.2) | 13 (46.4) | 21 (32.8) | 2.19 | 1.04–4.6 | 0.038 | |||
| Country of birth, n (%) | |||||||||
| Italy | 34 (94.4) | 28 (100) | 62 (96.9) | - | - | - | |||
| Abroad | 2 (5.6) | 0 (0) | 2 (3.1) | - | - | - | |||
| Presence of comorbidities, n (%) | |||||||||
| No | 27 (75.0) | 23 (82.1) | 50 (78.1) | Ref. | |||||
| Yes | 9 (25.0) | 5 (17.9) | 14 (21.9) | 0.69 | 0.26–1.83 | 0.460 | |||
| BMI (kg/m2), median (IQR) | 24 (20.9–25.9) | 24 (22–26.6) | 24 (21.3–26.2) | ||||||
| ≤24 | 18 (50.0) | 14 (50.0) | 32 (50.0) | Ref. | |||||
| >24 | 18 (50.0) | 14 (50.0) | 32 (50.0) | 1.03 | 0.49–2.16 | 0.938 | |||
| Vaccination-First dose, n (%) | |||||||||
| Comirnaty | 33 (91.7) | 26 (92.9) | 59 (92.2) | Ref. | |||||
| Other | 3 (8.3) | 2 (7.1) | 5 (7.8) | 1.06 | 0.25–4.45 | 0.940 | |||
| MS duration (years), median (IQR) | 16.2 (8.5–23.1) | 12.9 (5.8–25) | 15 (7.5–24.5) | ||||||
| ≤15 | 17 (47.2) | 16 (57.1) | 33 (51.6) | Ref. | |||||
| >15 | 19 (52.8) | 12 (42.9) | 31 (48.4) | 0.79 | 0.37–1.67 | 0.539 | |||
| MS treatment, n (%) | |||||||||
| Cladribine | 5 (13.9) | 1 (3.6) | 6 (9.4) | 0.32 | 0.04–2.69 | 0.297 | |||
| Fingolimod | 16 (44.4) | 10 (35.7) | 26 (40.6) | 0.97 | 0.35–2.66 | 0.948 | |||
| Interferon beta | 9 (25.0) | 6 (21.4) | 15 (23.4) | Ref. | |||||
| Ocrelizumab | 6 (16.7) | 11 (39.3) | 17 (26.6) | 2.21 | 0.82–5.97 | 0.118 | |||
| MS treatment duration (years), median (IQR) | 6.5 (2–9.1) | 3.7 (1.8–7.9) | 5.1 (1.9–8.6) | ||||||
| ≤5 | 14 (38.9) | 17 (60.7) | 31 (48.4) | Ref. | |||||
| >5 | 22 (61.1) | 11 (39.3) | 33 (51.6) | 0.49 | 0.23–1.04 | 0.062 | |||
| EDSS score, median (IQR) | 3 (0.5–5) | 1.8 (0.5–3) | 2 (0.5–4.3) | ||||||
| <3 | 17 (47.2) | 20 (71.4) | 37 (58.8) | Ref. | Ref. | ||||
| ≥3 | 19 (52.8) | 8 (28.6) | 27 (42.2) | 0.40 | 0.18–0.9 | 0.029 | 0.22 | 0.08–0.66 | 0.006 |
| MS phenotype, n (%) | |||||||||
| Primary-progressive (PP) | 1 (2.8) | 4 (14.3) | 5 (7.8) | Ref. | Ref. | ||||
| Relapsing-Remitting (RR) | 35 (97.2) | 24 (85.7) | 59 (92.2) | 0.33 | 0.11–0.94 | 0.039 | 0.12 | 0.03–0.46 | 0.002 |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.6) | 1.3 (0.7–1.8) | 1.28 (0.65–1.67) | ||||||
| ≤1.28 | 18 (54.6) | 11 (45.8) | 29 (50.9) | Ref. | |||||
| >1.28 | 15 (45.4) | 13 (54.2) | 28 (49.1) | 1.35 | 0.61–3.02 | 0.461 | |||
| Immune Response | No BI | BI | Total | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|
| 36 (56.3) | 28 (43.8) | 64 (100) | IRR | 95%CI | p | aIRR | 95%CI | p | |
| anti-RBD IgG (BAU/mL) | |||||||||
| continuous, median (IQR) | 352 (24–5513) | 135 (1–1337) | 272 (8–4222) | ||||||
| score: Negative (<7.1) | 5 (13.9) | 11 (39.3) | 16 (25.0) | Ref. | Ref. | ||||
| score: Positive (≥7.1) | 31 (86.1) | 17 (60.7) | 48 (75.0) | 0.33 | 0.15–0.7 | 0.004 | 0.40 | 0.17–0.97 | 0.042 |
| IFN-γ T cell-specific response (pg/mL) | |||||||||
| continuous, median (IQR) | 30 (1–222) | 188 (1–578) | 79 (1–349) | ||||||
| <16 | 17 (47.2) | 10 (35.7) | 27 (42.2) | Ref. | Ref. | ||||
| ≥16 | 19 (52.8) | 18 (64.3) | 37 (57.8) | 1.61 | 0.74–3.49 | 0.228 | 0.55 | 0.22–1.34 | 0.187 |
| Neutralizing antibodies | |||||||||
| continuous, median (IQR) | 15 (5–320) | 5 (5–160) | 8 (5–320) | ||||||
| <10 | 17 (47.2) | 15 (53.6) | 32 (50.0) | Ref. | Ref. | ||||
| ≥10 | 19 (52.8) | 13 (46.4) | 32 (50.0) | 0.83 | 0.39–1.74 | 0.618 | 0.68 | 0.30–1.55 | 0.362 |
| Patients’ Characteristics | COVID-19 Severity | ||||
|---|---|---|---|---|---|
| Mild Disease | Moderate Disease | Severe Disease | Total | p | |
| Total | 21 (75.0) | 5 (17.9) | 2 (7.1) | 28 (100) | |
| Age, median (IQR) years | 47 (39–53) | 49 (30–55) | 47.5 (46–49) | 47.5 (38–53.5) | 0.969 |
| Age class, n (%) | 0.511 | ||||
| 23–39 | 6 (28.6) | 2 (40) | 0 (0) | 8 (28.6) | |
| 40–49 | 6 (28.6) | 1 (20) | 2 (100) | 9 (32.1) | |
| 50–70 | 9 (42.9) | 2 (40) | 0 (0) | 11 (39.3) | |
| Gender, n (%) | 0.293 | ||||
| Female | 13 (61.9) | 2 (40) | 0 (0) | 15 (53.6) | |
| Male | 8 (38.1) | 3 (60) | 2 (100) | 13 (46.4) | |
| Presence of comorbidities, n (%) | 0.696 | ||||
| No | 16 (76.2) | 5 (100) | 2 (100) | 23 (82.1) | |
| Yes | 5 (23.8) | 0 (0) | 0 (0) | 5 (17.9) | |
| BMI (kg/m2), median (IQR) | 24.2 (22.8–26.8) | 22.1 (21.8–23.6) | 24.5 (23.2–25.8) | 24 (22–26.6) | 0.571 |
| Vaccination-First dose type, n (%) | 1.000 | ||||
| Comirnaty | 19 (90.5) | 5 (100) | 2 (100) | 26 (92.9) | |
| Other | 2 (9.5) | 0 (0) | 0 (0) | 2 (7.1) | |
| MS duration, median (IQR) months(?) | 15 (5.7–24.8) | 10.7 (9.1–14) | 19.1 (7.9–30.3) | 12.9 (5.8–25) | 0.763 |
| MS treatment, n (%) | 0.463 | ||||
| Cladribine | 1 (4.8) | 0 (0) | 0 (0) | 1 (3.6) | |
| Fingolimod | 8 (38.1) | 1 (20) | 1 (50) | 10 (35.7) | |
| Interferon beta | 6 (28.6) | 0 (0) | 0 (0) | 6 (21.4) | |
| Ocrelizumab | 6 (28.6) | 4 (80) | 1 (50) | 11 (39.3) | |
| MS treatment duration, median (IQR) months | 4.7 (1.9–8) | 1.9 (1.8–4.1) | 6.7 (3.4–9.9) | 3.7 (1.8–7.9) | 0.310 |
| EDSS score, median (IQR) | 2 (0–2.5) | 1.5 (1–1.5) | 6.5 (6.5–6.5) | 1.8 (0.5–3) | 0.064 |
| <3 | 16 (76.2) | 4 (80.0) | 0 (0) | 20 (71.4) | 0.089 |
| ≥3 | 5 (23.8) | 1 (20.0) | 2 (100) | 8 (28.6) | |
| MS phenotype, n (%) | 0.253 | ||||
| Primary-progressive (PP) | 2 (9.5) | 1 (20) | 1 (50) | 4 (14.3) | |
| Relapsing-Remitting (RR) | 19 (90.5) | 4 (80) | 1 (50) | 24 (85.7) | |
| Lymphocytes count × 103/µL, median (IQR) | 1.3 (0.7–1.7) | 1.5 (1.2–1.7) | 1.5 (0.2–2.7) | 1.3 (0.7–1.8) | 0.912 |
| COVID-19 therapy, n(%) | 0.098 | ||||
| Anti-viral therapy | 4 (19.1) | 1 (20.0) | 0 (0) | 5 (17.9) | |
| Monoclonal therapy | 7 (33.3) | 4 (80.0) | 0 (0) | 11 (39.3) | |
| NSAIDs, paracetamol or no therapy | 10 (47.6) | 0 (0) | 2 (100) | 12 (42.9) | |
| Days from booster dose to infection, median (IQR) | 153 (105–169) | 204 (154–204) | 263 (258–268) | 154.5 (108–204.5) | 0.129 |
| anti-RBD IgG (BAU/mL), median (IQR) | |||||
| continuous | 407 (2–2319) | 1 (0–4) | 268 (0–537) | 135 (1–1337) | 0.160 |
| <7.1, n (%) | 6 (28.6) | 4 (80) | 1 (50) | 11 (39.3) | 0.090 |
| ≥7.1, n (%) | 15 (71.4) | 1 (20) | 1 (50) | 17 (60.7) | |
| IFN-γ T cell-specific response (pg/mL), median (IQR) | |||||
| continuous | 166 (1–564) | 238 (207–353) | 365 (0–729) | 188 (1–578) | 0.957 |
| <16, n (%) | 8 (38.1) | 1 (20) | 1 (50) | 10 (35.7) | 0.823 |
| ≥16, n (%) | 13 (61.9) | 4 (80) | 1 (50) | 18 (64.3) | |
| Neutralizing antibodies, median (IQR) | |||||
| continuous | 20 (5–160) | 5 (5–5) | 23 (5–40) | 5 (5–160) | 0.323 |
| <10, n (%) | 10 (47.6) | 4 (80) | 1 (50) | 15 (53.6) | 0.655 |
| ≥10, n (%) | 11 (52.4) | 1 (20) | 1 (50) | 13 (46.4) | |
| Quantile Regression Model | ||||||
|---|---|---|---|---|---|---|
| Patient’s Characteristic | Univariable | Multivariable | ||||
| Coefficient * | 95%CI | p | Coefficient ** | 95%CI | p | |
| Age class, years n (%) | ||||||
| 23–39 | Ref. | |||||
| 40–49 | 3.00 | −3.07; 9.07 | 0.317 | |||
| 50–70 | 0.00 | −5.78; 5.78 | 1.000 | |||
| Gender, n (%) | ||||||
| Female | Ref. | |||||
| Male | 0.00 | −5.03; 5.03 | 1.000 | |||
| Presence of comorbidities, n (%) | ||||||
| No | Ref. | |||||
| Yes | −5.00 | −10.6; 0.6 | 0.078 | |||
| BMI (kg/m2), median (IQR) | −0.21 | −0.79; 0.37 | 0.459 | |||
| Vaccination-First dose, n (%) | ||||||
| Comirnaty | Ref. | |||||
| Other | −3.00 | −11.63; 5.63 | 0.479 | |||
| MS duration, median (IQR) years | 0.00 | −0.29; 0.29 | 1.000 | |||
| MS treatment, n (%) | ||||||
| Cladribine | 0.00 | −8.52; 8.52 | 1.000 | |||
| Fingolimod | 7.00 | 2.84; 11.16 | 0.002 | |||
| Interferon beta | Ref. | |||||
| Ocrelizumab | 3.00 | −1.16; 7.16 | 0.148 | |||
| MS treatment duration, median (IQR) years | −0.41 | −0.76; −0.05 | 0.028 | |||
| EDSS score, median (IQR) | 0.00 | −1.32; 1.32 | 1.000 | |||
| <3 | Ref. | |||||
| ≥3 | 0.00 | −5.13; 5.13 | 1.000 | |||
| MS phenotype, n (%) | ||||||
| Primary-progressive (PP) | Ref. | |||||
| Relapsing-Remitting (RR) | 0.00 | −7.2; 7.2 | 1.000 | |||
| Lymphocytes count × 103/µL, median (IQR) | −2.36 | −6.46; 1.75 | 0.244 | |||
| COVID-19 therapy, n (%) | ||||||
| Anti-viral therapy | Ref. | |||||
| Monoclonal therapy | 4.00 | −2.3; 10.34 | 0.204 | |||
| NSAIDs, paracetamol or no therapy | −1.00 | −7.13; 6.13 | 0.738 | |||
| Severity of COVID-19, n (%) | ||||||
| Mild illness | Ref. | |||||
| Moderate illness | 4 | −1.90; 9.90 | 0.174 | |||
| Severe illness | 1 | −11.0; 13.0 | 0.865 | |||
| Days from booster dose to sample, median (IQR) | 0.04 | −0.3; 0.39 | 0.796 | |||
| Anti-RBD IgG (BAU/mL), median (IQR) | ||||||
| continuous | −0.0004 | −0.001; 0.0003 | 0.245 | −0.001 | −0.001; 0.0002 | 0.163 |
| score: Negative (<7.1), n (%) | Ref. | Ref. | ||||
| score: Positive (≥7.1), n (%) | −4.00 | −8.31; 0.31 | 0.067 | −2.73 | −7.27; 1.81 | 0.225 |
| <809 | Ref. | Ref. | ||||
| ≥809 | −6.00 | −10.7; −1.3 | 0.015 | −4.39 | −8.8; 0.01 | 0.051 |
| IFN-γ T cell-specific response (pg/mL), median (IQR) | ||||||
| continuous | −0.0018 | −0.006; 0.003 | 0.422 | −0.002 | −0.006; 0.003 | 0.446 |
| <16, n (%) | Ref. | Ref. | ||||
| ≥16, n (%) | −5.00 | −9.77; −0.23 | 0.041 | −5.88 | −10.65; −1.10 | 0.018 |
| Neutralizing antibodies, median (IQR) | ||||||
| continuous | −0.01 | −0.03; 0.01 | 0.178 | −0.01 | −0.03; 0 | 0.135 |
| <10, n (%) | Ref. | Ref. | ||||
| ≥10, n (%) | 0.00 | −3.71; 3.71 | 1.000 | −2.5 | −7.5; 2.5 | 0.310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, A.; Ruggieri, S.; Navarra, A.; Tortorella, C.; Vanini, V.; Haggiag, S.; Prosperini, L.; Cuzzi, G.; Salmi, A.; Quartuccio, M.E.; et al. Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination. Vaccines 2024, 12, 926. https://doi.org/10.3390/vaccines12080926
Aiello A, Ruggieri S, Navarra A, Tortorella C, Vanini V, Haggiag S, Prosperini L, Cuzzi G, Salmi A, Quartuccio ME, et al. Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination. Vaccines. 2024; 12(8):926. https://doi.org/10.3390/vaccines12080926
Chicago/Turabian StyleAiello, Alessandra, Serena Ruggieri, Assunta Navarra, Carla Tortorella, Valentina Vanini, Shalom Haggiag, Luca Prosperini, Gilda Cuzzi, Andrea Salmi, Maria Esmeralda Quartuccio, and et al. 2024. "Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination" Vaccines 12, no. 8: 926. https://doi.org/10.3390/vaccines12080926
APA StyleAiello, A., Ruggieri, S., Navarra, A., Tortorella, C., Vanini, V., Haggiag, S., Prosperini, L., Cuzzi, G., Salmi, A., Quartuccio, M. E., Altera, A. M. G., Meschi, S., Matusali, G., Vita, S., Galgani, S., Maggi, F., Nicastri, E., Gasperini, C., & Goletti, D. (2024). Anti-RBD Antibody Levels and IFN-γ-Specific T Cell Response Are Associated with a More Rapid Swab Reversion in Patients with Multiple Sclerosis after the Booster Dose of COVID-19 Vaccination. Vaccines, 12(8), 926. https://doi.org/10.3390/vaccines12080926








