Comparative Immunogenicity of a High-Dose Hepatitis B Virus (HBV) Vaccine with Rapid Immunization vs. Standard Schedule in HBV Vaccine—Naïve Adults Aged 25–55 in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Vaccines
2.4. Serological Tests
2.5. Statistical Analyses
3. Results
3.1. Demographics Analysis
3.2. Immunogenicity
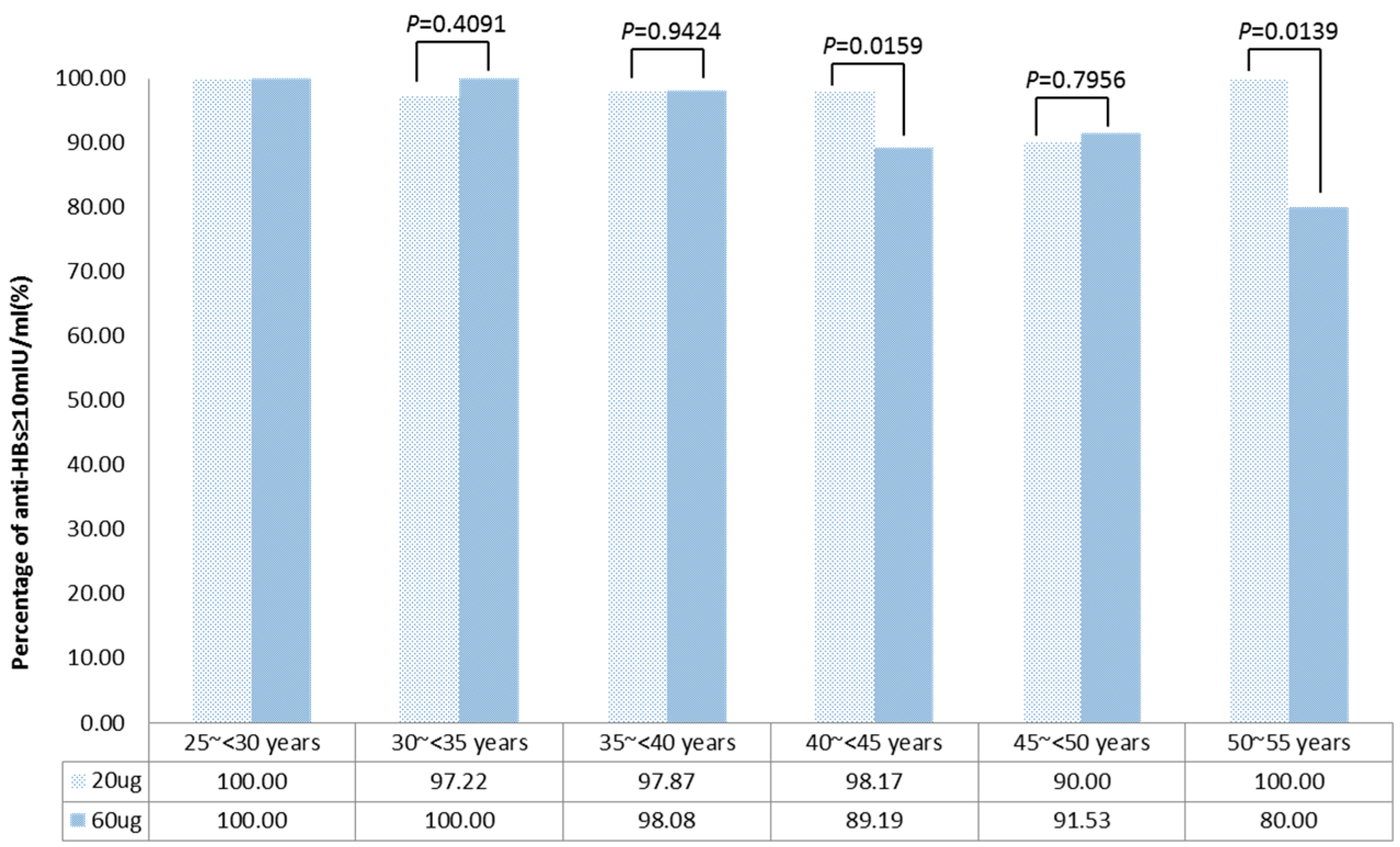
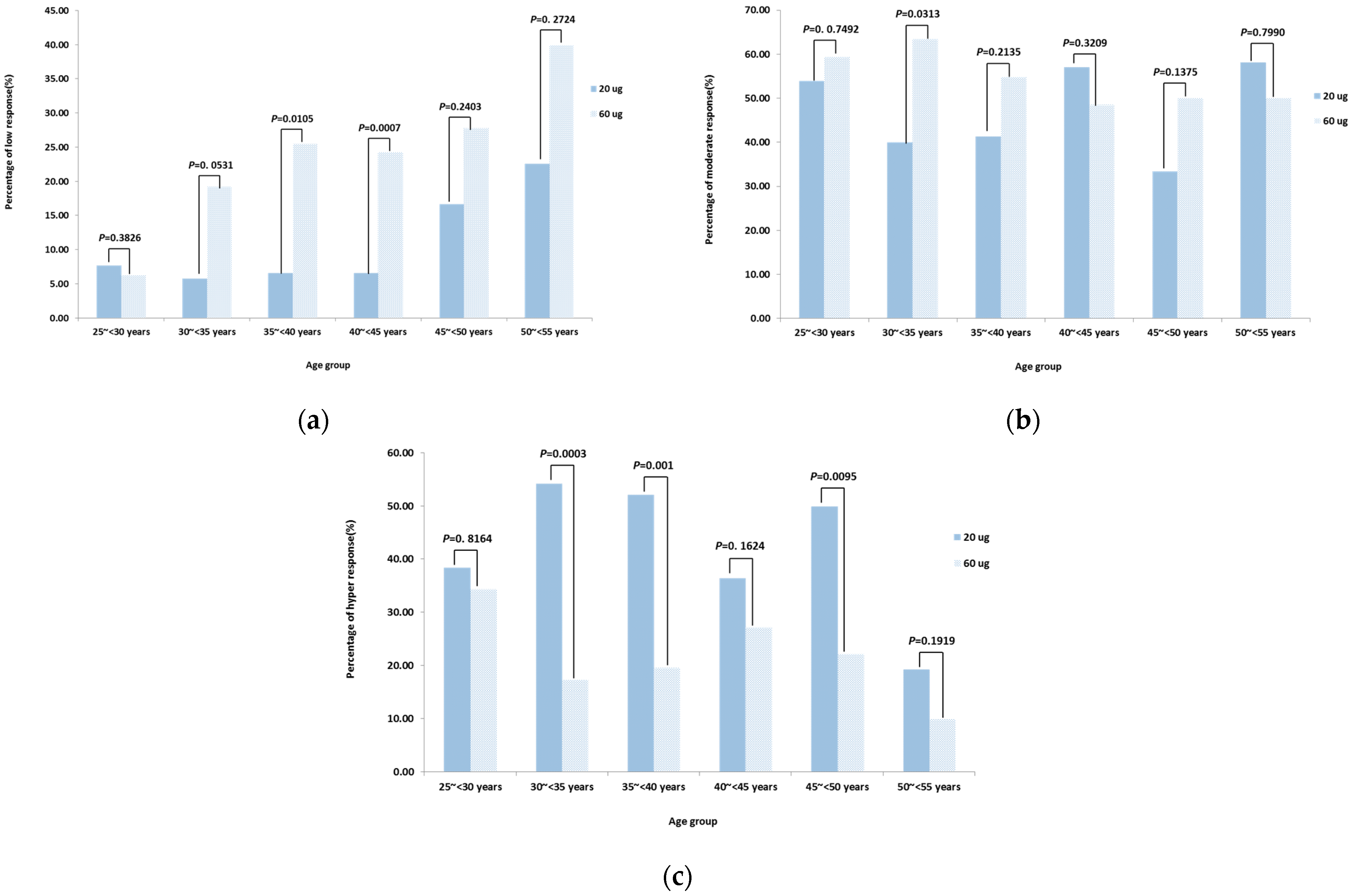
3.3. Immune Response of Adults in Different Age Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Hepatitis Report. 2017. Available online: https://www.afro.who.int/publications/global-hepatitis-report-2017 (accessed on 27 November 2023).
- Waheed, Y.; Siddiq, M.; Jamil, Z.; Najmi, M.H. Hepatitis elimination by 2030: Progress and challenges. World J. Gastroenterol. 2018, 24, 4959–4961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Wu, C.; Zhuang, H. Vaccination against hepatitis B: The Chinese experience. Chin. Med. J. 2009, 122, 98–102. [Google Scholar] [PubMed]
- Cui, F.; Shen, L.; Li, L.; Wang, H.; Wang, F.; Bi, S.; Liu, J.; Zhang, G.; Zheng, H.; Sun, X.; et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg. Infect. Dis. 2017, 23, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.Q. Technical guide for adult hepatitis B immunization in China. Zhonghua Liu Xing Bing Xue Za Zhi 2011, 32, 1199–1203. (In Chinese) [Google Scholar] [PubMed]
- Zhuang, H. The guideline of prevention and treatment for chronic hepatitis B: A 2022 update. J. Pract. Hepatol. 2023, 26, 457–478. [Google Scholar]
- Lu, P.-J.; Hung, M.-C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.B.; Hurley, L.P.; Williams, W.W.; Ramakrishnan, A.; Dean, A.K.; Groom, A.V. Meeting the Challenges of Immunizing Adults. Am. J. Prev. Med. 2015, 49 (Suppl. 4), S455–S464. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Nichol, K.L.; Lipczynski, K. Barriers to adult immunization. Am. J. Med. 2008, 121 (Suppl. 2), S28–S35. [Google Scholar] [CrossRef]
- Trantham, L.; Kurosky, S.K.; Zhang, D.; Johnson, K.D. Adherence with and completion of recommended hepatitis vaccination schedules among adults in the United States. Vaccine 2018, 36, 5333–5339. [Google Scholar] [CrossRef]
- Pan, H.X.; Zeng, Y.; Song, X.F.; Zhang, Y.J.; Xu, K.; Liang, Z.L.; Zhu, F.C. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine 2014, 32, 3706–3712. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Li, M.Q.; Wang, P.; Yang, Z.X.; Wei, L.; Zeng, Y.; Li, Y.P.; Yan, L.; Liu, X.E.; Zhuang, H. Comparative immunogenicity of hepatitis B vaccine with different dosages and schedules in healthy young adults in China. Vaccine 2016, 34, 1034–1039. [Google Scholar] [CrossRef]
- Petra, Z.; Nigel, C. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar]
- Das, K.; Gupta, R.K.; Kumar, V.; Kar, P. Immunogenicity and reactogenicity of a recombinant hepatitis B vaccine in subjects over age of forty years and response of a booster dose among nonresponders. World J. Gastroenterol. 2003, 9, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Gao, Y.H.; Wang, P.; Wei, L.; Xie, C.P.; Yang, Z.X.; Lan, J.; Fang, Z.L.; Zeng, Y.; Yan, L.; et al. Comparison of immunogenicity between hepatitis B vaccines with different dosages and schedules among healthy young adults in China: A 2-year follow-up study. Hum. Vaccines Immunother. 2018, 14, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.D.; Hall, A.J.; Maine, N.; Mendy, M.; Whittle, H.C. What level of hepatitis B antibody is protective? J. Infect. Dis. 1999, 179, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, Y.; Jin, D.; Liu, J.; Zheng, J.; Yuan, N.; Bai, Y.; Yan, T.; Yang, Y.; Liu, Y.; et al. No response to hepatitis B vaccine in infants born to HBsAg(+) mothers is associated to the transplacental transfer of HBsAg. Infect. Dis. 2017, 49, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Poorolajal, J.; Mahmoodi, M.; Majdzadeh, R.; Nasseri-Moghaddam, S.; Haghdoost, A.; Fotouhi, A. Long-term protection provided by hepatitis B vaccine and need for booster dose: A meta-analysis. Vaccine 2010, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanaka, Y. Cross-Protection of Hepatitis B Vaccination among Different Genotypes. Vaccines 2020, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.L.; Lv, J.J.; Liu, J.Y.; Yan, B.Y.; Feng, Y.; Xu, A.Q.; Zhang, L. Persistence of immune memory among adults with normal and high antibody response to primary hepatitis B vaccination: Results from a five-year follow-up study in China. Hum. Vaccines Immunother. 2018, 14, 2485–2490. [Google Scholar] [CrossRef]
- Ma, F.B.; Tang, W.J.; Lu, P.S.; Hu, Y.; Kang, G.D.; Yang, M.W. Study on the Immunogenicity of one dose of 60 mg/1.0ml Recombinant Yeast Derived Hepatitis B Vaccine (Saccharomyces Cerevisiae) Among Health Population Older than 16 Years. Shiyong Yufang Yixue 2013, 20, 653–655. (In Chinese) [Google Scholar]
- Yang, S.; Tian, G.; Cui, Y.; Ding, C.; Deng, M.; Yu, C.; Xu, K.; Ren, J.; Yao, J.; Li, Y.; et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 2016, 6, 27251. [Google Scholar] [CrossRef]
- Hepatitis B Foundation: Vaccine Non-Responders. Available online: https://www.hepb.org/prevention-and-diagnosis/vaccination/vaccine-non-responders/ (accessed on 20 January 2024).
- Corrao, G.; Calleri, M.; Zotti, M.; Barral, C.; Russo, R.; Garella, D.; Moiraghi Ruggenini, A. Immune response to anti-HBV vaccination: Study of conditioning factors. Eur. J. Epidemiol. 1988, 4, 492–496. [Google Scholar] [CrossRef]
- Fisman, D.N.; Agrawal, D.; Leder, K. The effect of age on immunologic response to recombinant hepatitis B vaccine: A meta-analysis. Clin. Infect. Dis. 2002, 35, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bao, H.; Yao, J.; Chen, Y.; Lu, S.; Li, J.; Jiang, Z.G.; Ren, J.J.; Xu, K.J.; Ruan, B.; et al. Suitable hepatitis B vaccine for adult immunization in China: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2019, 15, 220–227. [Google Scholar] [CrossRef]
- Hepatitis B Vaccines: WHO Position Paper. July 2017. Available online: https://www.who.int/publications/i/item/WER9227 (accessed on 19 January 2024).
- West, D.J.; Calandra, G.B. Vaccine induced immunologic memory for hepatitis B surface antigen: Implications for policy on booster vaccination. Vaccine 1996, 14, 1019–1027. [Google Scholar] [CrossRef]
- Pileggi, C.; Papadopoli, R.; Bianco, A.; Pavia, M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine 2017, 35, 6302–6307. [Google Scholar] [CrossRef]
- Banatvala, J.E.; Damme, P. Hepatitis B vaccine—Do we need boosters? J. Viral Hepat. 2003, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J. Boosters for Hepatitis B Vaccination? Need for an Evidence-Based Policy. Hepatology 2010, 51, 1485–1486. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Wu, Z.W.; Zhou, H.S.; Gao, Z.; Hao, Z.Y.; Jin, F.; Zhang, Y.H.; Li, M.J.; Wang, F.; Li, Q.; et al. Long-term protection at 20–31 years after primary vaccination with plasma-derived hepatitis B vaccine in a Chinese rural community. Hum. Vaccines Immunother. 2020, 16, 16–20. [Google Scholar] [CrossRef]
- Jan, C.F.; Huang, K.C.; Chien, Y.C.; Greydanus, D.E.; Davies, H.D.; Chiu, T.Y.; Huang, L.M.; Chen, C.J.; Chen, D.S. Determination of Immune Memory to Hepatitis B Vaccination Through Early Booster Response in College Students. Hepatology 2010, 51, 1547–1554. [Google Scholar] [CrossRef]


| 20 µg | 60 µg | p Value | |
|---|---|---|---|
| Subjects, no | 289 | 294 | |
| Age, Mean (SD) | 40.3 (7.1) | 39.5 (7.7) | 0.1934 |
| 25~<30 years, N (%) | 26 (9.0) | 32 (10.9) | 0.0116 |
| 30~<35 years, N (%) | 36 (12.5) | 52 (17.7) | |
| 35~<40 years, N (%) | 47 (16.3) | 52 (17.7) | |
| 40~<45 years, N (%) | 109 (37.7) | 74 (25.2) | |
| 45~<50 years, N (%) | 40 (13.8) | 59 (20.1) | |
| 50~55 years, N (%) | 31 (10.7) | 25 (8.5) | |
| Sex, N (%) | |||
| Male | 126 (43.6) | 145 (49.3) | 0.1661 |
| Female | 163 (56.4) | 149 (50.7) | |
| BMI, Mean (SD) | 23.8 (3.1) | 23.9 (3.0) | 0.4939 |
| History of smoke, N (%) | 76 (26.3) | 97 (33.0) | 0.0769 |
| History of drink, N (%) | 80 (27.7) | 81 (27.6) | 0.9719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Wang, H.; Liu, X.; Pang, X.; Zhang, W. Comparative Immunogenicity of a High-Dose Hepatitis B Virus (HBV) Vaccine with Rapid Immunization vs. Standard Schedule in HBV Vaccine—Naïve Adults Aged 25–55 in China. Vaccines 2024, 12, 923. https://doi.org/10.3390/vaccines12080923
Qiu Q, Wang H, Liu X, Pang X, Zhang W. Comparative Immunogenicity of a High-Dose Hepatitis B Virus (HBV) Vaccine with Rapid Immunization vs. Standard Schedule in HBV Vaccine—Naïve Adults Aged 25–55 in China. Vaccines. 2024; 12(8):923. https://doi.org/10.3390/vaccines12080923
Chicago/Turabian StyleQiu, Qian, Huai Wang, Xiuying Liu, Xinghuo Pang, and Wei Zhang. 2024. "Comparative Immunogenicity of a High-Dose Hepatitis B Virus (HBV) Vaccine with Rapid Immunization vs. Standard Schedule in HBV Vaccine—Naïve Adults Aged 25–55 in China" Vaccines 12, no. 8: 923. https://doi.org/10.3390/vaccines12080923
APA StyleQiu, Q., Wang, H., Liu, X., Pang, X., & Zhang, W. (2024). Comparative Immunogenicity of a High-Dose Hepatitis B Virus (HBV) Vaccine with Rapid Immunization vs. Standard Schedule in HBV Vaccine—Naïve Adults Aged 25–55 in China. Vaccines, 12(8), 923. https://doi.org/10.3390/vaccines12080923





