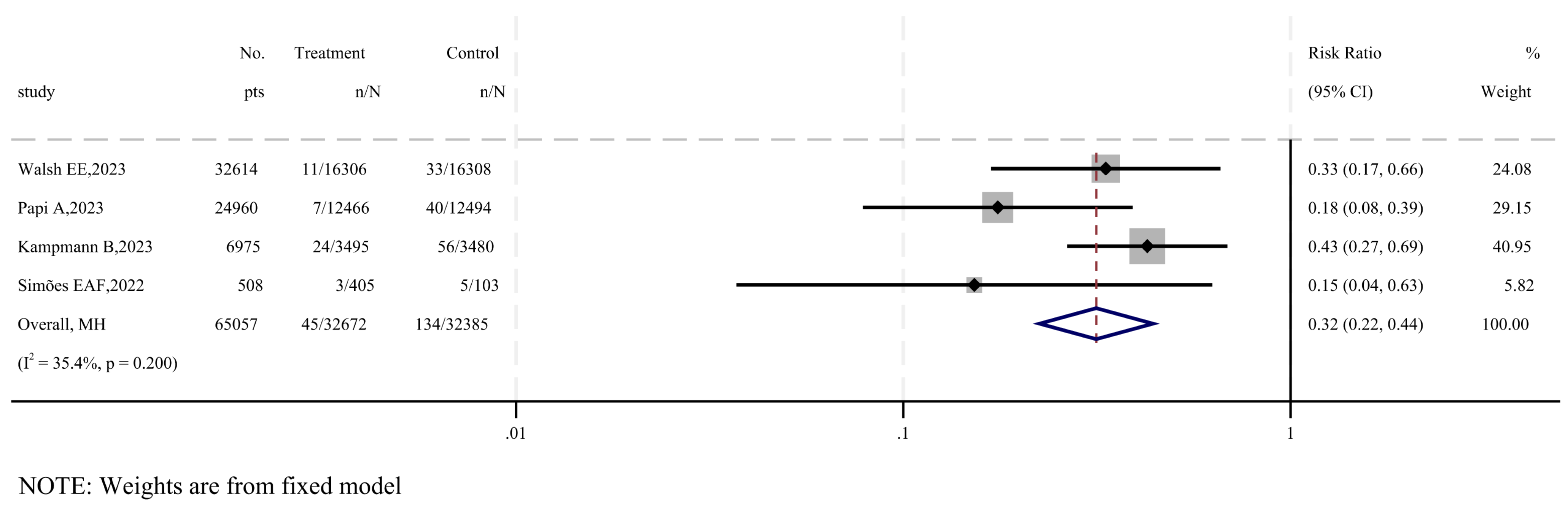Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Outcome Measures
2.4. Quality Assessment
2.5. Data Analysis
3. Results
3.1. Search Results and Study Characteristics
3.2. Quality Assessment
3.3. The Efficacy of RSV Subunit Vaccines
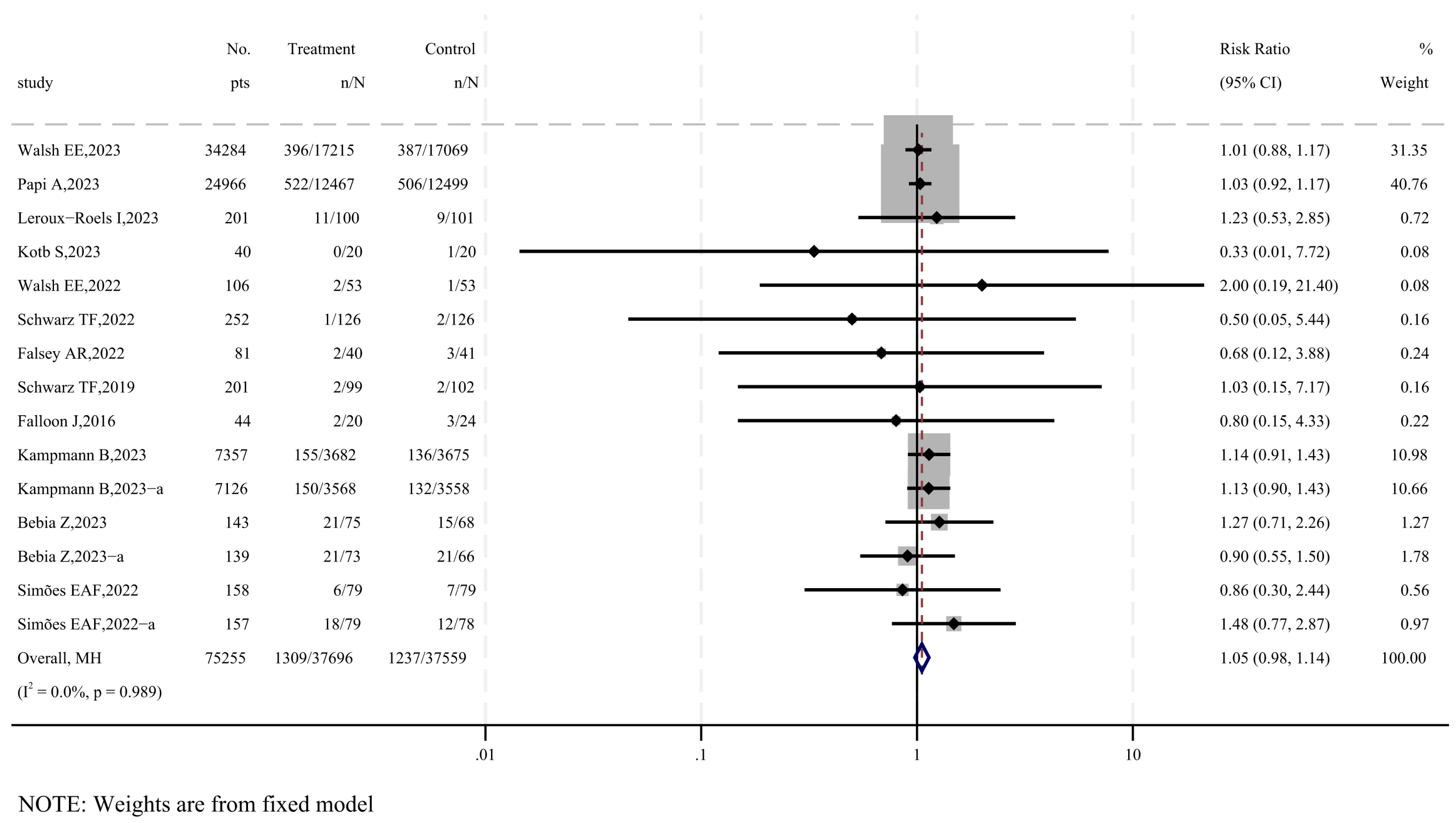
3.4. The Safety of RSV Subunit Vaccines
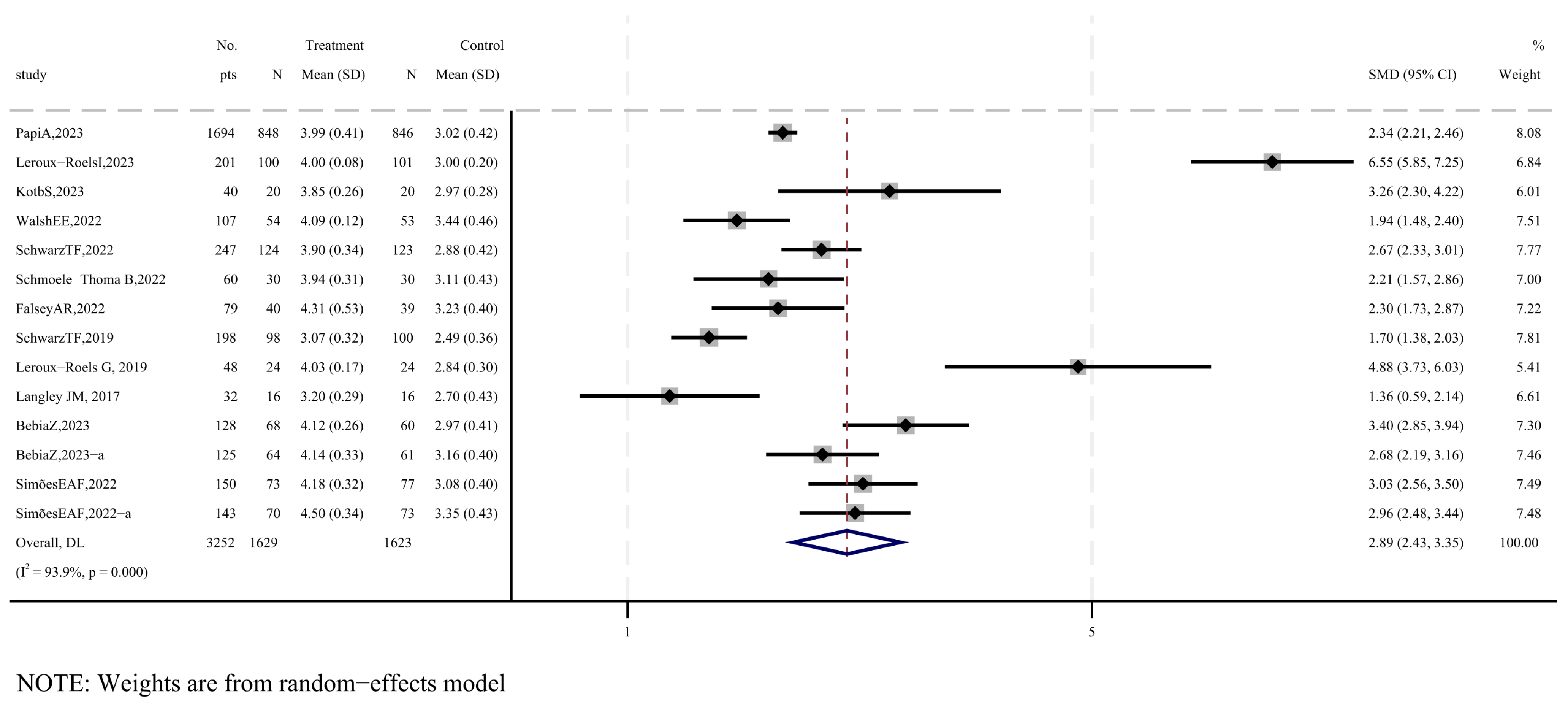
3.5. Immunogenicity of RSV Subunit Vaccines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, B.S.; Anderson, L.J. Challenges and opportunities for respiratory syncytial virus vaccines. Curr. Top. Microbiol. Immunol. 2013, 372, 391–404. [Google Scholar] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.J.; Mwananyanda, L.; MacLeod, W.B.; Kwenda, G.; Pieciak, R.; Mupila, Z.; Murphy, C.; Chikoti, C.; Forman, L.; Berklein, F. Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: A 3-year, systematic, post-mortem surveillance project. Lancet Glob. Health 2022, 10, e269–e277. [Google Scholar] [CrossRef]
- Cohen, C.; Zar, H.J. Deaths from RSV in young infants-the hidden community burden. Lancet Glob. Health 2022, 10, e169–e170. [Google Scholar] [CrossRef]
- Gatt, D.; Martin, I.; AlFouzan, R.; Moraes, T.J. Prevention and Treatment Strategies for Respiratory Syncytial Virus (RSV). Pathogens 2023, 12, 154. [Google Scholar] [CrossRef]
- Tam, C.C.; Yeo, K.T.; Tee, N.; Lin, R.; Mak, T.M.; Thoon, K.C.; Jit, M.; Yung, C.F. Burden and Cost of Hospitalization for Respiratory Syncytial Virus in Young Children, Singapore. Emerg. Infect. Dis. 2020, 26, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2023, 23, 1631–1640. [Google Scholar] [CrossRef]
- Simoes, E.A.; Tan, D.H.; Ohlsson, A.; Sales, V.; Wang, E.E. Respiratory syncytial virus vaccine: A systematic overview with emphasis on respiratory syncytial virus subunit vaccines. Vaccine 2001, 20, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Britton, P.N.; King, C.L.; Booy, R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: A systematic review. Influenza Other Respir. Viruses 2021, 15, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Reprint--preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023); Cochrane: Hoboken, NJ, USA, 2023; Available online: www.training.cochrane.org/handbook (accessed on 30 May 2024).
- Bebia, Z.; Reyes, O.; Jeanfreau, R.; Kantele, A.; De Leon, R.G.; Sánchez, M.G.; Banooni, P.; Gardener, G.J.; Rasero, J.L.B.; Pardilla, M.B.E. Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus Vaccine (RSVPreF3) in Mothers and Their Infants: A Phase 2 Randomized Trial. J. Infect. Dis. 2023, 228, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhao, G.; Dong, A.; He, Z.; Wang, J.; Jiang, B.; Wang, B.; Wang, M.; Huai, X.; Zhang, S.; et al. A First-in-Human Trial to Evaluate the Safety and Immunogenicity of a G Protein-Based Recombinant Respiratory Syncytial Virus Vaccine in Healthy Adults 18-45 Years of Age. Vaccines 2023, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Falloon, J.; Ji, F.; Curtis, C.; Bart, S.; Sheldon, E.; Krieger, D.; Dubovsky, F.; Lambert, S.; Takas, T.; Villafana, T.; et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine 2016, 34, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults with Concomitant Inactivated Influenza Vaccine. J. Infect. Dis. 2022, 225, 2056–2066. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simoes, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Perez Marc, G.; Radley, D.; Shittu, E. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef]
- Kotb, S.; Haranaka, M.; Folschweiller, N.; Nakanwagi, P.; Verheust, C.; De Schrevel, N.; David, M.P.; Mesaros, N.; Hulstrøm, V. Safety and immunogenicity of a respiratory syncytial virus prefusion F protein (RSVPreF3) candidate vaccine in older Japanese adults: A phase I, randomized, observer-blind clinical trial. Respir. Investig. 2023, 61, 261–269. [Google Scholar] [CrossRef]
- Langley, J.M.; Aggarwal, N.; Toma, A.; Halperin, S.A.; McNeil, S.A.; Fissette, L.; Dewé, W.; Leyssen, M.; Toussaint, J.F.; Dieussaert, I. A Randomized, Controlled, Observer-Blinded Phase 1 Study of the Safety and Immunogenicity of a Respiratory Syncytial Virus Vaccine with or without Alum Adjuvant. J. Infect. Dis. 2017, 215, 24–33. [Google Scholar] [CrossRef]
- Langley, J.M.; MacDonald, L.D.; Weir, G.M.; MacKinnon-Cameron, D.; Ye, L.; McNeil, S.; Schepens, B.; Saelens, X.; Stanford, M.M.; Halperin, S.A. A Respiratory Syncytial Virus Vaccine Based on the Small Hydrophobic Protein Ectodomain Presented with a Novel Lipid-Based Formulation Is Highly Immunogenic and Safe in Adults: A First-in-Humans Study. J. Infect. Dis. 2018, 218, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G.; De Boever, F.; Maes, C.; Nguyen, T.L.; Baker, S.; Gonzalez Lopez, A. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 2019, 37, 2694–2703. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Davis, M.G.; Steenackers, K.; Essink, B.; Vandermeulen, C.; Fogarty, C.; Andrews, C.P.; Kerwin, E.; David, M.P.; Fissette, L. Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial. J. Infect. Dis. 2023, 227, 761–772. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Schmoele-Thoma, B.; Zareba, A.M.; Jiang, Q.; Maddur, M.S.; Danaf, R.; Mann, A.; Eze, K.; Fok-Seang, J.; Kabir, G.; Catchpole, A. Vaccine Efficacy in Adults in a Respiratory Syncytial Virus Challenge Study. N. Engl. J. Med. 2022, 386, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.F.; Johnson, C.; Grigat, C.; Apter, D.; Csonka, P.; Lindblad, N.; Nguyen, T.L.; Gao, F.F.; Qian, H.; Tullio, A.N. Three Dose Levels of a Maternal Respiratory Syncytial Virus Vaccine Candidate Are Well Tolerated and Immunogenic in a Randomized Trial in Nonpregnant Women. J. Infect. Dis. 2022, 225, 2067–2076. [Google Scholar] [CrossRef]
- Schwarz, T.F.; McPhee, R.A.; Launay, O.; Leroux-Roels, G.; Talli, J.; Picciolato, M.; Gao, F.; Cai, R.; Nguyen, T.L.; Dieussaert, I. Immunogenicity and Safety of 3 Formulations of a Respiratory Syncytial Virus Candidate Vaccine in Nonpregnant Women: A Phase 2, Randomized Trial. J. Infect. Dis. 2019, 220, 1816–1825. [Google Scholar] [CrossRef]
- Simões, E.A.F.; Center, K.J.; Tita, A.T.N.; Swanson, K.A.; Radley, D.; Houghton, J.; McGrory, S.B.; Gomme, E.; Anderson, M.; Roberts, J.P. Prefusion F Protein-Based Respiratory Syncytial Virus Immunization in Pregnancy. N. Engl. J. Med. 2022, 386, 1615–1626. [Google Scholar] [CrossRef]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Perez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.N.; Graham, B.S.; Karron, R.A.; Munoz, F.M.; Falsey, A.R.; Anderson, L.J.; Marshall, V.; Kim, S.; Beeler, J.A. Challenges and opportunities in RSV vaccine development: Meeting report from FDA/NIH workshop. Vaccine 2016, 34, 4843–4849. [Google Scholar] [CrossRef]
- Yu, J.; Powers, J.H., 3rd; Vallo, D.; Falloon, J. Evaluation of Efficacy Endpoints for a Phase IIb Study of a Respiratory Syncytial Virus Vaccine in Older Adults Using Patient-Reported Outcomes with Laboratory Confirmation. Value Health J. Int. Soc. Pharmacoecon. Outcomes Res. 2020, 23, 227–235. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines. Available online: https://www.who.int/publications/i/item/WHO-IVB-17.11 (accessed on 30 May 2024).
- Ruckwardt, T.J.; Morabito, K.M.; Phung, E.; Crank, M.C.; Costner, P.J.; Holman, L.A.; Chang, L.A.; Hickman, S.P.; Berkowitz, N.M.; Gordon, I.J. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: A phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir. Med. 2021, 9, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]

| No | First Author, Year | Country | Phase | Target Population; Age | Sample Size | Sex (% Female) | Vaccine Name | Adjuvant | Efficacy | Safety | Immunogenicity | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RSV-ARI | RSV-LRTI | RSV-SRLI | Local or System AEs | Serious AEs | GMT | |||||||||
| 1 | Bebia Z, 2023 [17] | multicenter | phase 2 | Pregnant Women; 18–40 y | 143 | 100 | RSVPreF3 | None | NR | NR | NR | YES | YES | YES |
| 2 | Cheng X, 2023 [18] | Australia | phase 1 | Adults; 18–45 y | 60 | 76.7 | BARS13 | cyclosporine A | NR | NR | NR | YES | YES | NR |
| 3 | Falloon J, 2016 [19] | United States | phase 1a | Old Adults; ≥60 y | 44 | 51.2 | RSV sF | glucopyranosyl lipid A | NR | NR | NR | YES | YES | NR |
| 4 | Falsey AR, 2022 [20] | United States | phase 1/2 | Old Adults; 65–85 y | 81 | 63 | RSVpreF | None | NR | NR | NR | YES | YES | YES |
| 5 | Kampmann B, 2023 [21] | multicenter | phase 3 | Pregnant Women; 18–49 y | 7392 | 100 | RSVpreF | None | NR | YES | YES | YES | YES | NR |
| 6 | Kotb S, 2023 [22] | Japan | phase 1 | Old Adults; 60–80 y | 40 | 50 | RSVPreF3 | AS01 E | NR | NR | NR | YES | YES | YES |
| 7 | Langley JM, 2017 [23] | Canada | phase 1 | Adults; 18–44 y | 32 | 0 | RSV-PreF | None | NR | NR | NR | YES | YES | YES |
| 8 | Langley JM, 2018 [24] | Canada | phase 1 | Old Adults; 50–64 y | 16 | 72.5 | DPX-RSV | None | NR | NR | NR | YES | YES | NR |
| 9 | Leroux-Roels G, 2019 [25] | Belgium | phase 1 | Adults; 18–45 y | 48 | 66.7 | RSV F | Al(OH)3 | NR | NR | NR | YES | YES | YES |
| 10 | Leroux-Roels I, 2023 [26] | multicenter | phase 1/2 | Old Adults; 60–80 y | 201 | 57.2 | RSVPreF3 | AS01 E | NR | NR | NR | YES | YES | YES |
| 11 | Papi A, 2023 [27] | multicenter | phase 3 | Old Adults; ≥60 y | 24,966 | 51.7 | RSVpreF3 | AS01 E | YES | YES | YES | YES | YES | YES |
| 12 | Schmoele-Thoma B, 2022 [28] | United Kingdom | phase 2a | Adults; 18–50 y | 70 | 29 | RSVpreF | None | YES | NR | NR | YES | YES | YES |
| 13 | Schwarz TF, 2022 [29] | multicenter | phase 1/2 | Adults; 18–45 y | 252 | 100 | RSVPreF3 | None | NR | NR | NR | YES | YES | YES |
| 14 | Schwarz TF, 2019 [30] | multicenter | phase 2 | Adults; 18–45 y | 201 | 100 | RSVpreF | None | NR | NR | NR | YES | YES | YES |
| 15 | Simões EAF, 2022 [31] | multicenter | phase 2b | Pregnant Women; 18–49 y | 158 | 100 | RSVpreF | None | NR | YES | YES | YES | YES | YES |
| 16 | Walsh EE, 2022 [32] | United States | phase 1/2 | Adults; 18–49 y | 107 | 63.6 | RSVpreF | None | NR | NR | NR | YES | YES | YES |
| 17 | Walsh EE, 2023 [33] | multicenter | phase 3 | Old Adults; ≥60 y | 34,284 | 49.2 | RSVpreF | None | YES | YES | NR | YES | YES | NR |
| Adverse Events | No. of Studies | Reactions/Total | RR (95% CI) | Heterogeneity I2 (%) | p-Heterogeneity | |
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| Local adverse events (any) | 7 | 1932/5625 | 974/5543 | 3.58 (2.00, 6.41) | 97.5 | 0.000 |
| Systemic adverse events(any) | 7 | 1665/5625 | 1400/5534 | 1.40 (1.05, 1.85) | 91.0 | 0.000 |
| Pain | 16 | 2320/8895 | 664/8772 | 4.00 (2.92, 5.49) | 82.9 | 0.000 |
| Redness | 13 | 465/8851 | 83/8724 | 5.49 (4.36, 6.91) | 42.1 | 0.055 |
| Swelling | 12 | 334/7957 | 76/7837 | 3.75 (1.90, 7.38) | 60.9 | 0.003 |
| Fatigue | 15 | 2780/8887 | 2317/8764 | 1.23 (1.05, 1.44) | 67.3 | 0.000 |
| Headaches | 15 | 2097/8887 | 1685/8764 | 1.37 (1.08, 1.74) | 86.6 | 0.000 |
| Myalgia | 11 | 1447/7706 | 943/7585 | 1.52 (1.41, 1.63) | 33.6 | 0.130 |
| Nausea | 9 | 923/8510 | 882/8774 | 1.05 (0.97, 1.15) | 48.9 | 0.048 |
| Pyrexia | 10 | 333/8598 | 207/8479 | 1.91 (1.10, 3.32) | 75.5 | 0.000 |
| Arthralgia | 7 | 488/4022 | 428/3981 | 1.14 (1.01, 1.29) | 12.6 | 0.334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lu, Y.; Bai, Y.; Zhu, B.; Chang, F.; Lu, Y. Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2024, 12, 879. https://doi.org/10.3390/vaccines12080879
Wu Y, Lu Y, Bai Y, Zhu B, Chang F, Lu Y. Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines. 2024; 12(8):879. https://doi.org/10.3390/vaccines12080879
Chicago/Turabian StyleWu, Yuhang, Yuqiong Lu, Yuwei Bai, Bingde Zhu, Feng Chang, and Yun Lu. 2024. "Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Vaccines 12, no. 8: 879. https://doi.org/10.3390/vaccines12080879
APA StyleWu, Y., Lu, Y., Bai, Y., Zhu, B., Chang, F., & Lu, Y. (2024). Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines, 12(8), 879. https://doi.org/10.3390/vaccines12080879