Persistence of Antibodies against Measles, Mumps, and Rubella after the Two-Dose MMR Vaccination: A 7-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
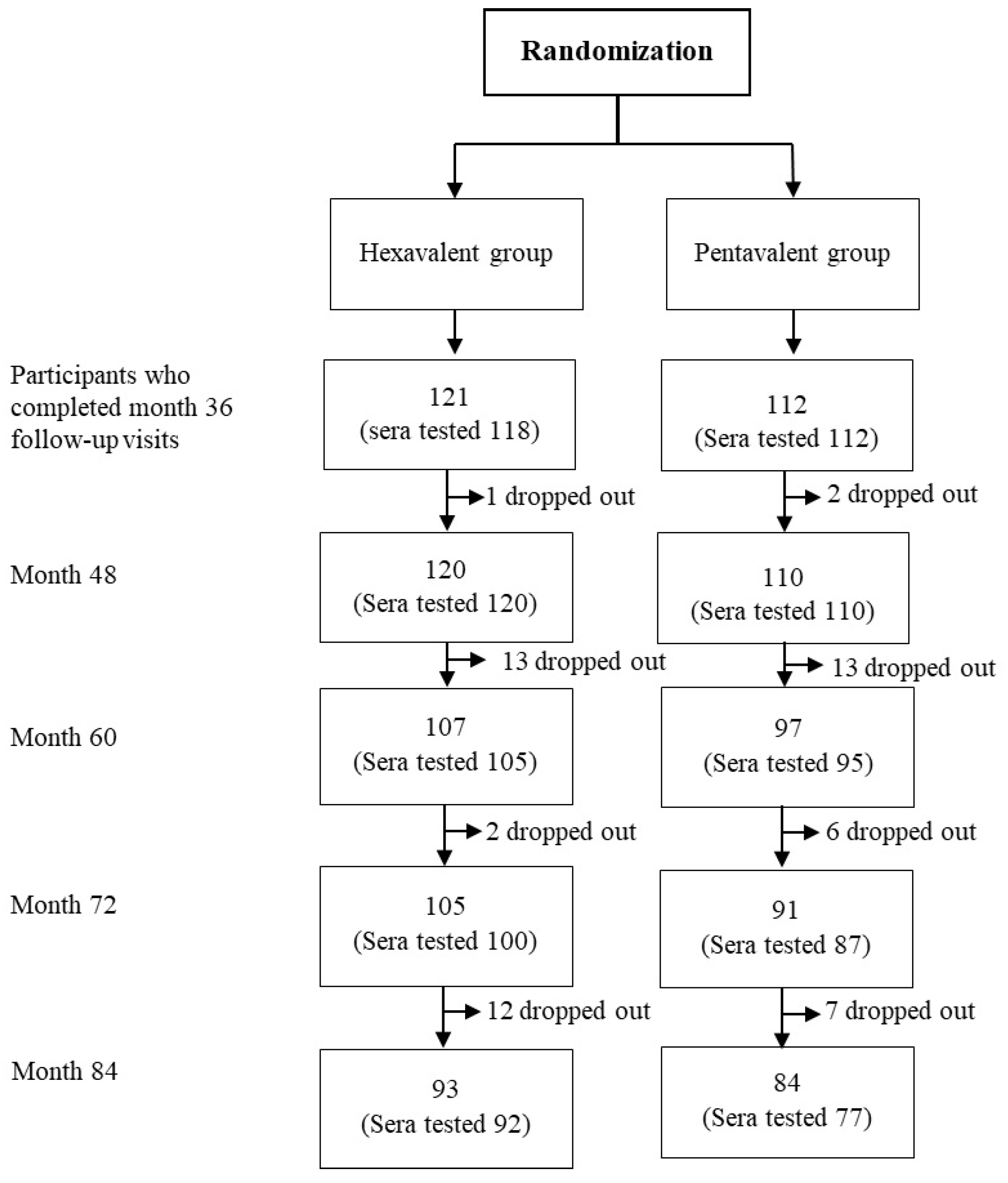
2.1. Study Design
2.2. Study Vaccines and Blood Sampling
2.3. Laboratory Testing
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Study Participants
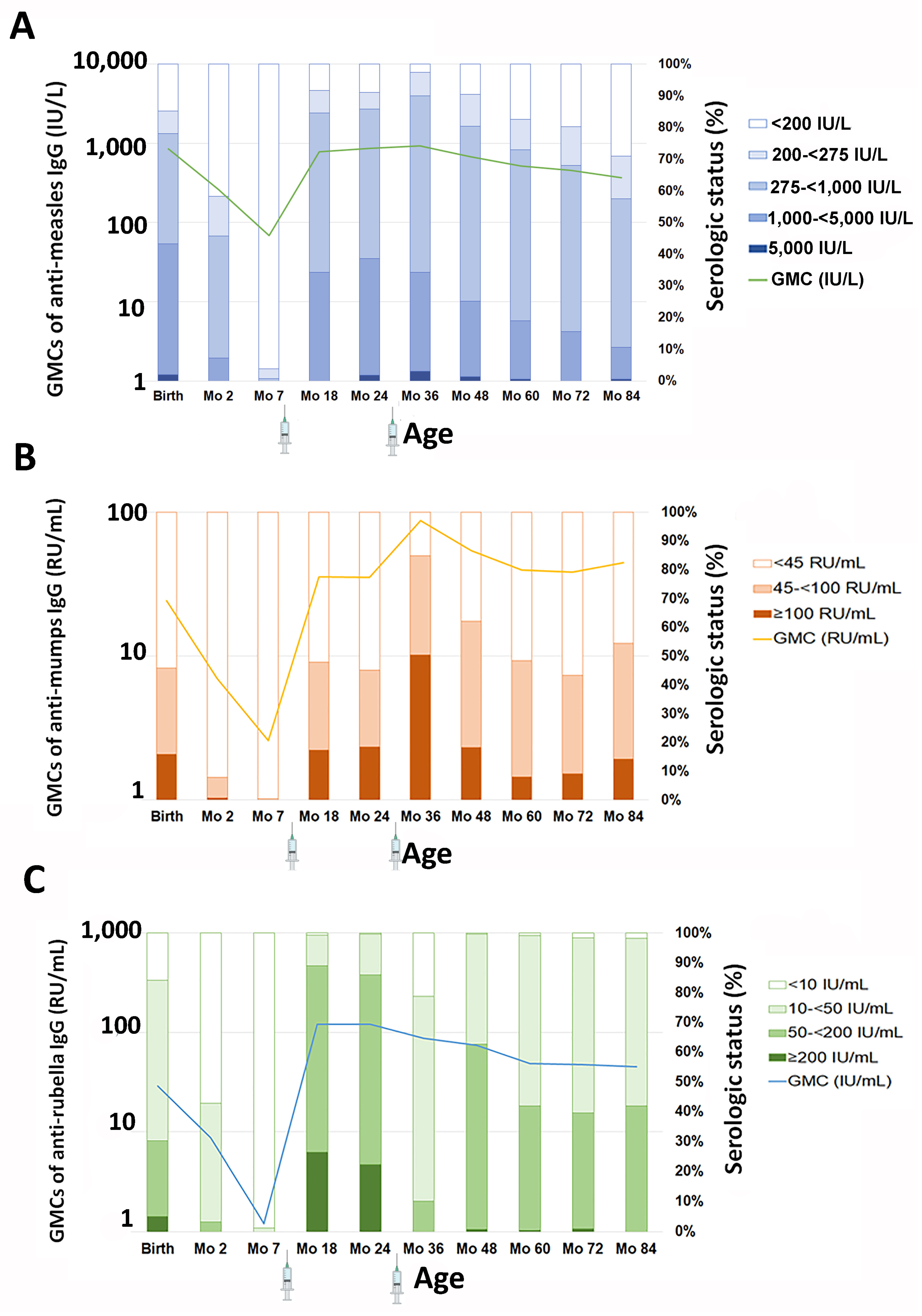
3.2. GMC and Serologic Status of Anti-Measles IgG
3.3. GMC and Serologic Status of Anti-Mumps IgG
3.4. GMC and Serologic Status of Anti-Rubella IgG
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramson, O.; Dagan, R.; Tal, A.; Sofer, S. Severe complications of measles requiring intensive care in infants and young children. Arch. Pediatr. Adolesc. Med. 1995, 149, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Berche, P. History of measles. Presse Med. 2022, 51, 104149. [Google Scholar] [CrossRef]
- World Health Organization. Strategic Plan for Measles and Rubella Elimination in WHO South-East Asia Region: 2020–2024. Available online: https://www.who.int/southeastasia/publications-detail/9789290227427 (accessed on 23 May 2024).
- Immunization Agenda 2030. A Global Strategy to Leave no One Behind. Available online: https://www.immunizationagenda2030.org/ (accessed on 20 June 2024).
- World Health Organization. Measles and Rubella Strategic Framework 2021–2030. Available online: https://www.immunizationagenda2030.org/images/documents/measles_rubella_initiative_Digital3.pdf (accessed on 20 June 2024).
- Wodi, A.P.; Murthy, N.; McNally, V.V.; Daley, M.F.; Cineas, S. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 6–10. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Vaccine Schedules in all Countries in the EU/EEA. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=8&SelectedCountryIdByDisease=-1 (accessed on 23 May 2024).
- Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec. 2017, 92, 205–227.
- Lalwani, S.; Chatterjee, S.; Balasubramanian, S.; Bavdekar, A.; Mehta, S.; Datta, S.; Povey, M.; Henry, O. Immunogenicity and safety of early vaccination with two doses of a combined measles-mumps-rubella-varicella vaccine in healthy Indian children from 9 months of age: A phase III, randomised, non-inferiority trial. BMJ Open 2015, 5, e007202. [Google Scholar] [CrossRef]
- World Health Organization. Table 1: Summary of WHO Position Papers—Recommendations for Routine Immunization. Available online: https://www.who.int/publications/m/item/table1-summary-of-who-position-papers-recommendations-for-routine-immunization (accessed on 23 May 2024).
- Wanlapakorn, N.; Puenpa, J.; Thongmee, T.; Srimuan, D.; Thatsanathorn, T.; Vongpunsawad, S.; Poovorawan, Y. Antibodies to measles, mumps, and rubella virus in Thai children after two-dose vaccination at 9 months and 2.5 years: A longitudinal study. Vaccine 2020, 38, 4016–4023. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Immunization Thailand 2023 Country Profile. Available online: https://www.who.int/publications/m/item/immunization-thailand-2023-country-profile (accessed on 23 May 2024).
- Wanawanakorn, K. Factors Affecting Coverage of measles vaccine of the target group in Su-ghai Kolok District. JPMAT 2019, 9, 190–196. [Google Scholar]
- Wongsanuphat, S.; Thitichai, P.; Jaiyong, R.; Plernprom, P.; Thintip, K.; Jitpeera, C.; Suphanchaimat, R. Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019. Int. J. Environ. Res. Public Health 2020, 17, 4627. [Google Scholar] [CrossRef]
- Jinarong, T.; Chootong, R.; Vichitkunakorn, P.; Songwathana, P. Muslim parents’ beliefs and factors influencing complete immunization of children aged 0–5 years in a Thai rural community: A qualitative study. BMC Public Health 2023, 23, 1348. [Google Scholar] [CrossRef]
- Daya, S.; Lillahkul, N.; Noin, J. Experience of Parents of Thai Muslim Childhood Aged—5 Years in Yala Province Who Rejected the Service of Expanded Program Immunization with Vaccine. J. Dep. Med. Serv. 2018, 43, 137–141. [Google Scholar]
- Ngaovithunvong, V.; Wanlapakorn, N.; Tesapirat, L.; Suratannon, N.; Poovorawan, Y. Mumps antibody in the Thai population 17 years after the universal measles mumps rubella vaccination program. J. Infect. Dev. Ctries. 2016, 10, 735–740. [Google Scholar] [CrossRef]
- Wang, D.; Nie, T.; Pan, F.; Wang, Y.; Wang, J.; Qin, W. Loss of protective immunity of two-dose mumps-containing vaccine over time: Concerns with the new strategy of the mumps immunization program in China. Hum. Vaccines Immunother. 2021, 17, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Angsuwatcharakon, P.; Puthanakit, T.; Bunjoungmanee, P.; Anugulruengkitt, S.; Srimuan, P.; Kowitdamrong, E.; Savangsindh, P.; Sophonphan, J.; Tantawichien, T.; Tangsathapornpong, A. High seroprevalence of rubella in Thai children with a 2-dose MMR national immunization policy. Vaccine 2021, 39, 6206–6209. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Maertens, K.; Vongpunsawad, S.; Puenpa, J.; Tran, T.M.P.; Hens, N.; Van Damme, P.; Thiriard, A.; Raze, D.; Locht, C.; et al. Quantity and Quality of Antibodies After Acellular Versus Whole-cell Pertussis Vaccines in Infants Born to Mothers Who Received Tetanus, Diphtheria, and Acellular Pertussis Vaccine During Pregnancy: A Randomized Trial. Clin. Infect. Dis. 2020, 71, 72–80. [Google Scholar] [CrossRef]
- Meng, Q.H.; Liu, Y.; Yu, J.Q.; Li, L.J.; Shi, W.; Shen, Y.J.; Li, L.; Zhan, S.N.; Yang, F.; Wang, Y.J.; et al. Seroprevalence of Maternal and Cord Antibodies Specific for Diphtheria, Tetanus, Pertussis, Measles, Mumps and Rubella in Shunyi, Beijing. Sci. Rep. 2018, 8, 13021. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Sarawanangkoor, N.; Srimuan, D.; Thatsanathorn, T.; Thongmee, T.; Poovorawan, Y. Antibody persistence to diphtheria toxoid, tetanus toxoid, Bordetella pertussis antigens, and Haemophilus influenzae type b following primary and first booster with pentavalent versus hexavalent vaccines. Hum. Vaccin. Immunother. 2024, 20, 2352909. [Google Scholar] [CrossRef] [PubMed]
- Puthanakit, T.; Anugulruengkitt, S.; Angsuwatcharakon, P.; Bunjoungmanee, P.; Kowitdamrong, E.; Primsirikunawut, A.; Intarakhao, S.; Chetsonwisorn, P.; Sophonphan, J.; Tangsathapornpong, A. Low Measles Seropositivity Rate among Thai Adolescents in the Thai National Immunization Program. Vaccines 2022, 10, 1269. [Google Scholar] [CrossRef]
- Seagle, E.E.; Bednarczyk, R.A.; Hill, T.; Fiebelkorn, A.P.; Hickman, C.J.; Icenogle, J.P.; Belongia, E.A.; McLean, H.Q. Measles, mumps, and rubella antibody patterns of persistence and rate of decline following the second dose of the MMR vaccine. Vaccine 2018, 36, 818–826. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Stefanizzi, P.; De Nitto, S.; Larocca, A.M.V.; Germinario, C.; Tafuri, S. Long-term Immunogenicity of Measles Vaccine: An Italian Retrospective Cohort Study. J. Infect. Dis. 2020, 221, 721–728. [Google Scholar] [CrossRef]
- Franconeri, L.; Antona, D.; Cauchemez, S.; Lévy-Bruhl, D.; Paireau, J. Two-dose measles vaccine effectiveness remains high over time: A French observational study, 2017–2019. Vaccine 2023, 41, 5797–5804. [Google Scholar] [CrossRef]
- Anugulruengkitt, S.; Angsuwatcharakon, P.; Puthanakit, T.; Bunjoungmanee, P.; Srimuan, P.; Kowitdamrong, E.; Sawangsinth, P.; Sophonphan, J.; Tantawichien, T.; Tangsathapornpong, A. Seroprevalence of mumps among children and adolescents in Thailand, 2020. Vaccine 2022, 40, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Wijmenga-Monsuur, A.J.; van Houten, M.A.; Veldhuijzen, I.K.; Ten Hulscher, H.I.; Kerkhof, J.; van der Klis, F.R.; van Binnendijk, R.S. A Third Dose of Measles-Mumps-Rubella Vaccine to Improve Immunity Against Mumps in Young Adults. J. Infect. Dis. 2020, 221, 902–909. [Google Scholar] [CrossRef]
- Marin, M.; Marlow, M.; Moore, K.L.; Patel, M. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus-Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. Mmwr. Morb. Mortal. Wkly. Rep. 2018, 67, 33–38. [Google Scholar] [CrossRef]
- Cutts, F.T.; Hanson, M. Seroepidemiology: An underused tool for designing and monitoring vaccination programmes in low- and middle-income countries. Trop. Med. Int. Health 2016, 21, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles Reported Cases and Incidence, Thailand. Available online: https://immunizationdata.who.int/global/wiise-detail-page/measles-reported-cases-and-incidence?CODE=THA&YEAR= (accessed on 20 June 2024).
- World Health Organization. Mumps—Number of Reported Cases. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/mumps---number-of-reported-cases (accessed on 20 June 2024).
| Time Points | Antibody | Overall GMCs (95% CI) | GMCs of Children Receiving Hexavalent Vaccine (95% CI) | GMCs of Children Receiving Pentavalent Vaccine (95% CI) |
|---|---|---|---|---|
| Month 48 | Anti-measles IgG (IU/L) | 678.3 (598.8–768.3) | 716.1 (600.1–854.5) | 639.3 (536.3–762.1) |
| Anti-mumps IgG (RU/mL) | 54.2 (49.5–59.4) | 58.8 (52.4–65.9) | 49.6 (43.0–57.2) | |
| Anti-rubella IgG (IU/mL) | 73.8 (67.9–80.2) | 77.6 (69.6–86.5) | 69.8 (61.5–79.3) | |
| n | 230 | 120 | 110 | |
| Month 60 | Anti-measles IgG (IU/L) | 514.5 (445.8–593.9) | 532.1 (437.9–646.4) | 495.8 (400.8–613.3) |
| Anti-mumps IgG (RU/mL) | 39.7 (35.8–44.0) | 44.9 (39.3–51.3) | 34.6 (29.5–40.5) | |
| Anti-rubella IgG (IU/mL) | 48.7 (44.2–53.6) | 52.3 (45.9–59.6) | 45.0 (39.1–51.8) | |
| n | 200 | 105 | 95 | |
| Month 72 | Anti-measles IgG (IU/L) | 456.4 (397.6–523.9) | 450.7 (374.3–542.7) | 463.2 (376.7–569.5) |
| Anti-mumps IgG (RU/mL) | 38.1 (34.0–42.8) | 43.4 (37.2–50.6) | 32.9 (27.8–38.8) | |
| Anti-rubella IgG (IU/mL) | 47.4 (42.8–52.6) | 49.7 (43.5–56.7) | 44.9 (38.2–52.9) | |
| n | 187 | 100 | 87 | |
| Month 84 | Anti-measles IgG (IU/L) | 367.4 (313.9–430.1) | 360.5 (291.8–445.3) | 375.9 (296.4–476.6) |
| Anti-mumps IgG (RU/mL) | 44.5 (39.7–49.9) | 48.2 (41.7–55.8) | 40.5 (33.9–48.4) | |
| Anti-rubella IgG (IU/mL) | 44.9 (40.6–49.7) | 46.8 (41.0–53.3) | 42.8 (36.6–50.2) | |
| n | 169 | 92 | 77 |
| 48 Months | 60 Months | 72 Months | 84 Months | |
|---|---|---|---|---|
| Anti-measles IgG | ||||
| <200 IU/L | 9.6% (5.8–13.4) | 17.5% (12.2–22.8) | 19.8% (14.1–25.5) | 29.0% (22.2–35.8) |
| 200–<275 IU/L | 10.0% (6.1–13.9) | 9.5% (5.4–13.6) | 12.3% (7.6–17.0) | 13.6% (8.4–18.8) |
| 275–<1000 IU/L | 55.2% (48.8–61.6) | 54.0% (47.1–60.9) | 52.4% (45.2–59.6) | 46.7% (39.2–54.3) |
| 1000–<5000 IU/L | 23.9% (18.4–29.4) | 18.5% (13.1–23.9) | 15.5% (10.3–20.7) | 10.1% (5.5–14.6) |
| ≥5000 IU/L | 1.3% (−0.2–2.8) | 0.5% (−0.5–1.5) | 0.0% (0.0–0.0) | 0.6% (−0.6–1.7) |
| Anti-mumps IgG | ||||
| <45 RU/mL | 37.8% (31.6–44.1) | 51.5% (44.6–58.4) | 56.7% (49.6–63.8) | 45.6% (38.1–53.1) |
| 45–<100 RU/mL | 43.9% (37.5–50.3) | 40.5% (33.7–47.3) | 34.2% (27.4–41.0) | 40.2% (32.8–47.6) |
| ≥100 RU/mL | 18.3% (13.3–23.3) | 8.0% (4.2–11.8) | 9.1% (5.0–13.2) | 14.2% (8.9–19.5) |
| Anti-rubella IgG | ||||
| <10 IU/mL | 0.4%(−0.4–1.3) | 1.0% (−0.4–2.4) | 1.6% (−0.2–3.4) | 1.8% (−0.2–3.8) |
| 10–<50 IU/mL | 37.0% (30.7–43.2) | 57.0% (50.1–63.9) | 58.8% (51.8–65.9) | 56.2% (48.7–63.7) |
| 50–<200 IU/mL | 61.7% (55.5–68.0) | 41.5% (34.7–48.3) | 38.5% (31.5–45.5) | 42.0%(34.6–49.5) |
| ≥200 IU/mL | 0.9% (−0.3–2.1) | 0.5% (−0.5–1.5) | 1.1% (−0.4–2.5) | 0.0% (0.0–0.0) |
| GMC Ratios | 95% CI | p-Value | |
|---|---|---|---|
| Anti-measles IgG | |||
| Study month | |||
| 36 | 1 (ref) | ||
| 84 | 0.40 | (0.37–0.43) | <0.001 |
| Vaccine type | |||
| MM | 1.33 | (0.57–3.12) | 0.51 |
| MP | 0.98 | (0.72–1.35) | 0.92 |
| PM | 0.97 | (0.69–1.35) | 0.85 |
| PP | 1 (ref) | ||
| Weight at month 24 (kg) | 0.99 | (0.91–1.09) | 0.93 |
| Length at month 24 (cm) | 0.98 | (0.93–1.02) | 0.32 |
| Sex | |||
| Male | 1 (ref) | ||
| Female | 0.99 | (0.78–1.28) | 0.99 |
| Anti-mumps IgG | |||
| Study month | |||
| 36 | 1 (ref) | ||
| 84 | 0.51 | (0.47– 0.56) | <0.001 |
| Vaccine type | |||
| MM | 1.06 | (0.59–1.91) | 0.85 |
| MP | 0.98 | (0.79–1.21) | 0.84 |
| PM | 1.02 | (0.81–1.29) | 0.85 |
| PP | 1 (ref) | ||
| Weight at month 24 (kg) | 1.01 | (0.95–1.07) | 0.85 |
| Length at month 24 (cm) | 0.99 | (0.96–1.03) | 0.74 |
| Sex | |||
| Male | 1 (ref) | ||
| Female | 1.09 | (0.92–1.29) | 0.31 |
| Anti-rubella IgG | |||
| Study month | |||
| 36 | 1 (ref) | ||
| 84 | 0.51 | (0.48–0.55) | <0.001 |
| Vaccine type | |||
| MM | 1.50 | (0.86–2.61) | 0.16 |
| MP | 1.05 | (0.86–1.29) | 0.61 |
| PM | 1.06 | (0.85–1.32) | 0.58 |
| PP | 1 (ref) | ||
| Weight at month 24 (kg) | 0.99 | (0.93–1.05) | 0.75 |
| Length at month 24 (cm) | 0.99 | (0.96–1.02) | 0.42 |
| Sex | |||
| Male | 1 (ref) | ||
| Female | 1.21 | (1.03–1.42) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarawanangkoor, N.; Wanlapakorn, N.; Srimuan, D.; Thatsanathorn, T.; Thongmee, T.; Poovorawan, Y. Persistence of Antibodies against Measles, Mumps, and Rubella after the Two-Dose MMR Vaccination: A 7-Year Follow-Up Study. Vaccines 2024, 12, 744. https://doi.org/10.3390/vaccines12070744
Sarawanangkoor N, Wanlapakorn N, Srimuan D, Thatsanathorn T, Thongmee T, Poovorawan Y. Persistence of Antibodies against Measles, Mumps, and Rubella after the Two-Dose MMR Vaccination: A 7-Year Follow-Up Study. Vaccines. 2024; 12(7):744. https://doi.org/10.3390/vaccines12070744
Chicago/Turabian StyleSarawanangkoor, Nasiri, Nasamon Wanlapakorn, Donchida Srimuan, Thaksaporn Thatsanathorn, Thanunrat Thongmee, and Yong Poovorawan. 2024. "Persistence of Antibodies against Measles, Mumps, and Rubella after the Two-Dose MMR Vaccination: A 7-Year Follow-Up Study" Vaccines 12, no. 7: 744. https://doi.org/10.3390/vaccines12070744
APA StyleSarawanangkoor, N., Wanlapakorn, N., Srimuan, D., Thatsanathorn, T., Thongmee, T., & Poovorawan, Y. (2024). Persistence of Antibodies against Measles, Mumps, and Rubella after the Two-Dose MMR Vaccination: A 7-Year Follow-Up Study. Vaccines, 12(7), 744. https://doi.org/10.3390/vaccines12070744






