The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viruses
2.3. Study Design
2.4. Sample Collection
- Blood: Erythrocytes were lysed using ACK buffer (KHCO3 0.01 M, NH4Cl 0.15 M, Na2EDTA 0.1 mM).
- Spleen: Spleens were homogenized, passed through 70 µm filters (BD biosciences), and washed with RPMI 1640 containing 10% FCS, 100 U/mL penicillin, streptomycin, and glutamate (P/S/G). Erythrocytes were lysed using ACK buffer.
- Lung: After collection, lungs were minced into 1 mm sized chunks using scissors and incubated in 3 mL 2.4 mg/mL collagenase A (Roche) and 1 mg/mL DNAse (Roche) in RPMI1640 suspension for 30 min at 37 °C. Subsequently, the tissue suspension was diluted with 7 mL washing medium (RPMI1640 + 1%FCS + 2 mM EDTA + 1× P/S/G) and mashed over a 70 µm cell strainer using a plunger. The resulting suspension was centrifuged for 5′ at 500× g to remove the collagenase, after which erythrocytes were shocked using ACK buffer. The cells were then washed, transferred over a 70 µm cell strainer, and resuspended in 1 mL of stimulation medium (RPMI1640 + 10% FCS + 1× P/S/G) for ELISpot, cytokine-FACS, and dextramer staining.
- BM: Before cutting the femurs at both ends, muscles and residue tissues surrounding the femur were removed. A 25-gauge needle and 10 cc syringe filled with ice-cold RPMI (10 mL) were used to flush the bone marrow out of the femur into a 70 µm nylon cell strainer placed in a 50 mL Falcon conical tube. Next, the tissue was smashed through the cell strainer and washed with medium (RPMI1640 + 10%FCS + 2 mM EDTA + 1× P/S/G).
2.5. ELISpot
2.6. Cytokine Responses Measured Using Flow Cytometry
2.7. Antigen-Specific CD8+ T Cells Measured Using Flow Cytometry
2.8. UMAP
2.9. Isolation of Dextramer-Specific T Cells for T-Cell-Receptor Analyses
2.10. Preparing TCRβ cDNA Libraries for Sequencing
2.11. TCRβ Clonotype Analysis
2.12. Statistics
3. Results
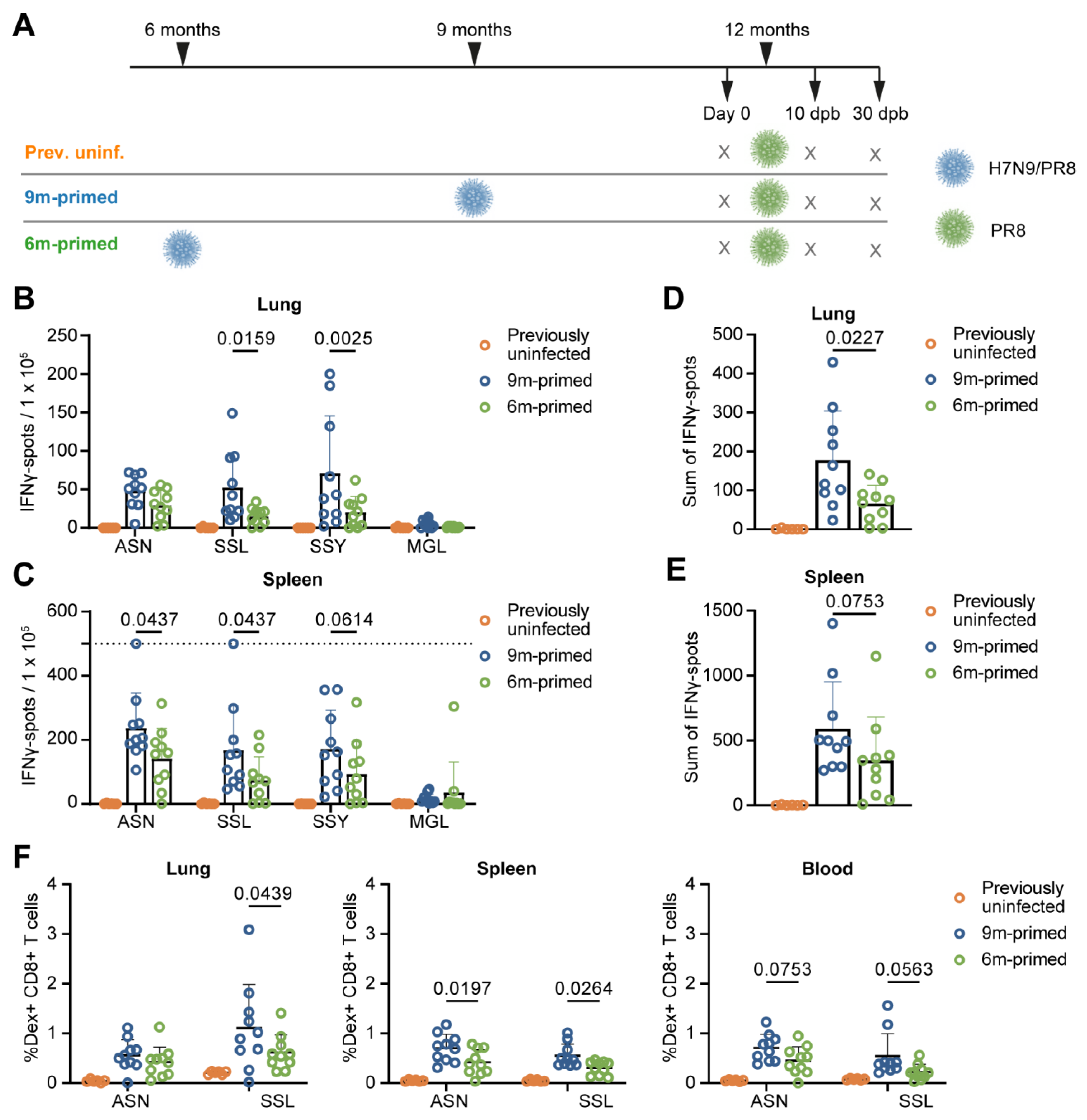
3.1. Study Design
3.2. Lower Baseline T-Cell Response in 6 m Primed Mice Compared to 9 m Primed Mice
3.3. Similar Differences in T-Cell Responses to Booster Infection in 6 m and 9 m Primed Mice
3.4. IAV-Specific T-Cell Responses Are Similarly Maintained in the Bone Marrow in 6 m Primed and 9 m Primed Mice
3.5. Lung-Derived T Cells of 6 m Primed and 9 m Primed Mice Express Similar Levels of TRM Markers after Booster
3.6. IAV-Specific TCR Repertoire Diversity Is Maintained between 3 and 6 Months after Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McElhaney, J.E.; Kuchel, G.A.; Zhou, X.; Swain, S.L.; Haynes, L. T-Cell Immunity to Influenza in Older Adults: A Pathophysiological Framework for Development of More Effective Vaccines. Front. Immunol. 2016, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.L.; Margolis, K.L.; Wuorenma, J.; von Sternberg, T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 1994, 331, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.O.; Angeletti, D.; Yewdell, J.W. Antibody Immunodominance: The Key to Understanding Influenza Virus Antigenic Drift. Viral Immunol. 2018, 31, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. A Perspective on the Structural and Functional Constraints for Immune Evasion: Insights from Influenza Virus. J. Mol. Biol. 2017, 429, 2694–2709. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, Y.; Qiu, C.; Quinones-Parra, S.; Zhu, Z.; Loh, L.; Tian, D.; Ren, Y.; Hu, Y.; Zhang, X.; et al. Recovery from severe H7N9 disease is associated with diverse response mechanisms dominated by CD8(+) T cells. Nat. Commun. 2015, 6, 6833. [Google Scholar] [CrossRef]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef]
- Savic, M.; Dembinski, J.L.; Kim, Y.; Tunheim, G.; Cox, R.J.; Oftung, F.; Peters, B.; Mjaaland, S. Epitope specific T-cell responses against influenza A in a healthy population. Immunology 2016, 147, 165–177. [Google Scholar] [CrossRef]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Isakova-Sivak, I.; Rudenko, L. Nucleoprotein as a Promising Antigen for Broadly Protective Influenza Vaccines. Vaccines 2023, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; Vilas Boas de Melo, C.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef] [PubMed]
- Boon, A.C.M.; de Mutsert, G.; Graus, Y.M.F.; Fouchier, R.A.M.; Sintnicolaas, K.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. The Magnitude and Specificity of Influenza A Virus-Specific Cytotoxic T-Lymphocyte Responses in Humans Is Related to HLA-A and -B Phenotype. J. Virol. 2002, 76, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.O.; Sant, S.; Bird, N.L.; Grant, E.J.; Clemens, E.B.; Koutsakos, M.; Valkenburg, S.A.; Gras, S.; Lappas, M.; Jaworowski, A.; et al. Perturbed CD8+ T cell immunity across universal influenza epitopes in the elderly. J. Leukoc. Biol. 2017, 103, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Grzelak, L.; Wang, Z.; Pizzolla, A.; Koutsakos, M.; Crowe, J.; Loudovaris, T.; Mannering, S.I.; Westall, G.P.; Wakim, L.M.; et al. Single-Cell Approach to Influenza-Specific CD8(+) T Cell Receptor Repertoires Across Different Age Groups, Tissues, and Following Influenza Virus Infection. Front. Immunol. 2018, 9, 1453. [Google Scholar] [CrossRef] [PubMed]
- Yager, E.J.; Ahmed, M.; Lanzer, K.; Randall, T.D.; Woodland, D.L.; Blackman, M.A. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008, 205, 711–723. [Google Scholar] [CrossRef]
- Valkenburg, S.A.; Venturi, V.; Dang, T.H.; Bird, N.L.; Doherty, P.C.; Turner, S.J.; Davenport, M.P.; Kedzierska, K. Early priming minimizes the age-related immune compromise of CD8(+) T cell diversity and function. PLoS Pathog. 2012, 8, e1002544. [Google Scholar] [CrossRef]
- Van Braeckel-Budimir, N.; Varga, S.M.; Badovinac, V.P.; Harty, J.T. Repeated Antigen Exposure Extends the Durability of Influenza-Specific Lung-Resident Memory CD8(+) T Cells and Heterosubtypic Immunity. Cell Rep. 2018, 24, 3374–3382.e3373. [Google Scholar] [CrossRef]
- Slütter, B.; Van Braeckel-Budimir, N.; Abboud, G.; Varga, S.M.; Salek-Ardakani, S.; Harty, J.T. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2017, 2, eaag2031. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, Y.; Lee, Y.T.; Bouchard, K.R.; Benechet, A.; Khanna, K.; Cauley, L.S. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014, 95, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Cookenham, T.; Lanzer, K.G.; Tighe, M.; Ward, J.M.; Reiley, W.W.; Blackman, M.A. Visualization of Resident Memory CD8 T Cells in the Lungs of Young and Aged Influenza Memory Mice and after Heterosubtypic Challenge. Immunohorizons 2021, 5, 543–556. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Crowell, H.; Chevrier, S.Z.V.; Robinson, M. CATALYST: Cytometry dATa anALYSis Tools. R Package Version 1.14.0. 2021. [Google Scholar]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, B.; Pandit, A.; Andeweg, A.C.; de Boer, R.J. RTCR: A pipeline for complete and accurate recovery of T cell repertoires from high throughput sequencing data. Bioinformatics 2016, 32, 3098–3106. [Google Scholar] [CrossRef] [PubMed]
- Venturi, V.; Kedzierska, K.; Turner, S.J.; Doherty, P.C.; Davenport, M.P. Methods for comparing the diversity of samples of the T cell receptor repertoire. J. Immunol. Methods 2007, 321, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Flurkey, K.; McUrrer, J.; Harrison, D. Mouse Models in Aging Research. In The Mouse in Biomedical Research; Academic Press: Cambridge, MA, USA, 2007; pp. 637–672. [Google Scholar]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Godec, J.; Odorizzi, P.M.; Brown, K.E.; Yates, K.B.; Ngiow, S.F.; Burke, K.P.; Maleri, S.; Grande, S.M.; Francisco, L.M.; et al. The PD-1 Pathway Regulates Development and Function of Memory CD8(+) T Cells following Respiratory Viral Infection. Cell Rep. 2020, 31, 107827. [Google Scholar] [CrossRef]
- Badovinac, V.P.; Porter, B.B.; Harty, J.T. Programmed contraction of CD8(+) T cells after infection. Nat. Immunol. 2002, 3, 619–626. [Google Scholar] [CrossRef]
- Sercan Alp, O.; Durlanik, S.; Schulz, D.; McGrath, M.; Grun, J.R.; Bardua, M.; Ikuta, K.; Sgouroudis, E.; Riedel, R.; Zehentmeier, S.; et al. Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur. J. Immunol. 2015, 45, 975–987. [Google Scholar] [CrossRef]
- Reilly, E.C.; Lambert Emo, K.; Buckley, P.M.; Reilly, N.S.; Smith, I.; Chaves, F.A.; Yang, H.; Oakes, P.W.; Topham, D.J. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.J.; Franki, S.N.; Pierce, R.H.; Dimitrova, S.; Koteliansky, V.; Sprague, A.G.; Doherty, P.C.; de Fougerolles, A.R.; Topham, D.J. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 2004, 20, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef] [PubMed]
- McMaster, S.R.; Wilson, J.J.; Wang, H.; Kohlmeier, J.E. Airway-Resident Memory CD8 T Cells Provide Antigen-Specific Protection against Respiratory Virus Challenge through Rapid IFN-gamma Production. J. Immunol. 2015, 195, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Yuan, L.; Chesnut, R.W.; Sidney, J.; Southwood, S.; Farness, P.; Jackson, M.R.; Peterson, P.A.; Sette, A. Immunodominance Analysis of CTL Responses to Influenza PR8 Reveals Two Dominant and Subdominant Kb-restricted Epitopes. J. Immunol. 1996, 157, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.T.; Stevenson, P.G.; Doherty, P.C. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 2000, 165, 2404–2409. [Google Scholar] [CrossRef]
- Belz, G.T.; Xie, W.; Doherty, P.C. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 2001, 166, 4627–4633. [Google Scholar] [CrossRef]
- Belz, G.T.; Xie, W.; Altman, J.D.; Doherty, P.C. A Previously Unrecognized H-2Db-Restricted Peptide Prominent in the Primary Influenza A Virus-specific CD8+ T-Cell Response Is Much Less Apparent following Secondary Challenge. J. Virol. 2000, 74, 3486–3493. [Google Scholar] [CrossRef]
- Crowe, S.R.; Turner, S.J.; Miller, S.C.; Roberts, A.D.; Rappolo, R.A.; Doherty, P.C.; Ely, K.H.; Woodland, D.L. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 2003, 198, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Belz, G.T.; Altman, J.D.; Ahmed, R.; Woodland, D.L.; Doherty, P.C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 1998, 8, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc. Natl. Acad. Sci. USA 2004, 101, 4942–4947. [Google Scholar] [CrossRef]
- Busch, D.H.; Pilip, I.; Pamer, E.G. Evolution of a Complex T Cell Receptor Repertoire during Primary and Recall Bacterial Infection. J. Exp. Med. 1998, 188, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, L.; Rimbert, M.; Echasserieau, K.; Saulquin, X.; Neveu, B.; Dechanet, J.; Cerundolo, V.; Bonneville, M. Selection of T Cell Clones Expressing High-Affinity Public TCRs within Human Cytomegalovirus-Specific CD8 T Cell Responses. J. Immunol. 2005, 175, 6123–6132. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Brenchley, J.M.; Ruff, L.E.; Betts, M.R.; Hill, B.J.; Roederer, M.; Koup, R.A.; Migueles, S.A.; Gostick, E.; Wooldridge, L.; et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005, 202, 1349–1361. [Google Scholar] [CrossRef]
- Cukalac, T.; Kan, W.T.; Dash, P.; Guan, J.; Quinn, K.M.; Gras, S.; Thomas, P.G.; La Gruta, N.L. Paired TCRalphabeta analysis of virus-specific CD8(+) T cells exposes diversity in a previously defined ‘narrow’ repertoire. Immunol. Cell Biol. 2015, 93, 804–814. [Google Scholar] [CrossRef]
- Kedzierska, K.; Day, E.B.; Pi, J.; Heard, S.B.; Doherty, P.C.; Turner, S.J.; Perlman, S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J. Immunol. 2006, 177, 6705–6712. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.J.; Diaz, G.; Cross, R.; Doherty, P.C. Analysis of Clonotype Distribution and Persistence for an Influenza Virus-Specific CD8 T cell response. Immunity 2003, 18, 549–559. [Google Scholar] [CrossRef]
- Slütter, B.; Pewe, L.L.; Kaech, S.M.; Harty, J.T. Lung airway-surveilling CXCR3(hi) memory CD8(+) T cells are critical for protection against influenza A virus. Immunity 2013, 39, 939–948. [Google Scholar] [CrossRef]
- Pizzolla, A.; Nguyen, T.H.O.; Smith, J.M.; Brooks, A.G.; Kedzieska, K.; Heath, W.R.; Reading, P.C.; Wakim, L.M. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2017, 2, eaam6970. [Google Scholar] [CrossRef]
- Cheuk, S.; Schlums, H.; Gallais Serezal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.C.; Sportiello, M.; Emo, K.L.; Amitrano, A.M.; Jha, R.; Kumar, A.B.R.; Laniewski, N.G.; Yang, H.; Kim, M.; Topham, D.J. CD49a Identifies Polyfunctional Memory CD8 T Cell Subsets that Persist in the Lungs After Influenza Infection. Front. Immunol. 2021, 12, 728669. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Anderson, K.G.; Mayer-Barber, K.; Sung, H.; Beura, L.; James, B.R.; Taylor, J.J.; Qunaj, L.; Griffith, T.S.; Vezys, V.; Barber, D.L.; et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 2014, 9, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Westera, L.; Drylewicz, J.; Den Braber, I.; Mugwagwa, T.; Van Der Maas, I.; Kwast, L.; Volman, T.; Van De Weg-Schrijver, E.H.R.; Bartha, I.; Spierenburg, G.; et al. Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood 2013, 122, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Lessler, J.; Read, J.M.; Zhu, H.; Jiang, C.Q.; Guan, Y.; Cummings, D.A.; Riley, S. Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data. PLoS Biol. 2015, 13, e1002082. [Google Scholar] [CrossRef] [PubMed]
- Somes, M.P.; Turner, R.M.; Dwyer, L.J.; Newall, A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine 2018, 36, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska, K.; Venturi, V.; Field, K.; Davenport, M.P.; Turner, S.J.; Doherty, P.C. Early establishment of diverse T cell receptor profiles for influenza-specific CD8+CD62Lhi memory T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 9184–9189. [Google Scholar] [CrossRef]
- van de Sandt, C.E.; Hillaire, M.L.; Geelhoed-Mieras, M.M.; Osterhaus, A.D.; Fouchier, R.A.; Rimmelzwaan, G.F. Human Influenza A Virus-Specific CD8+ T-Cell Response Is Long-lived. J. Infect. Dis. 2015, 212, 81–85. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Monto, A.S.; Longini, I.M., Jr. Estimates of the US health impact of influenza. Am. J. Public. Health 1993, 83, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.A.; Schenkel, J.M.; Jameson, S.C.; Vezys, V.; Masopust, D. Preexisting high frequencies of memory CD8+ T cells favor rapid memory differentiation and preservation of proliferative potential upon boosting. Immunity 2013, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Harty, J.T. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 2006, 203, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Ha, S.J.; Vezys, V.; Ahmed, R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J. Immunol. 2006, 177, 831–839. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanfermeijer, J.; van de Ven, K.; Hendriks, M.; van Dijken, H.; Lenz, S.; Vos, M.; Borghans, J.A.M.; van Baarle, D.; de Jonge, J. The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection. Vaccines 2024, 12, 419. https://doi.org/10.3390/vaccines12040419
Lanfermeijer J, van de Ven K, Hendriks M, van Dijken H, Lenz S, Vos M, Borghans JAM, van Baarle D, de Jonge J. The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection. Vaccines. 2024; 12(4):419. https://doi.org/10.3390/vaccines12040419
Chicago/Turabian StyleLanfermeijer, Josien, Koen van de Ven, Marion Hendriks, Harry van Dijken, Stefanie Lenz, Martijn Vos, José A. M. Borghans, Debbie van Baarle, and Jørgen de Jonge. 2024. "The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection" Vaccines 12, no. 4: 419. https://doi.org/10.3390/vaccines12040419
APA StyleLanfermeijer, J., van de Ven, K., Hendriks, M., van Dijken, H., Lenz, S., Vos, M., Borghans, J. A. M., van Baarle, D., & de Jonge, J. (2024). The Memory-CD8+-T-Cell Response to Conserved Influenza Virus Epitopes in Mice Is Not Influenced by Time Since Previous Infection. Vaccines, 12(4), 419. https://doi.org/10.3390/vaccines12040419





