Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting, Information Sources, and Variables
2.3. Statistical Analysis
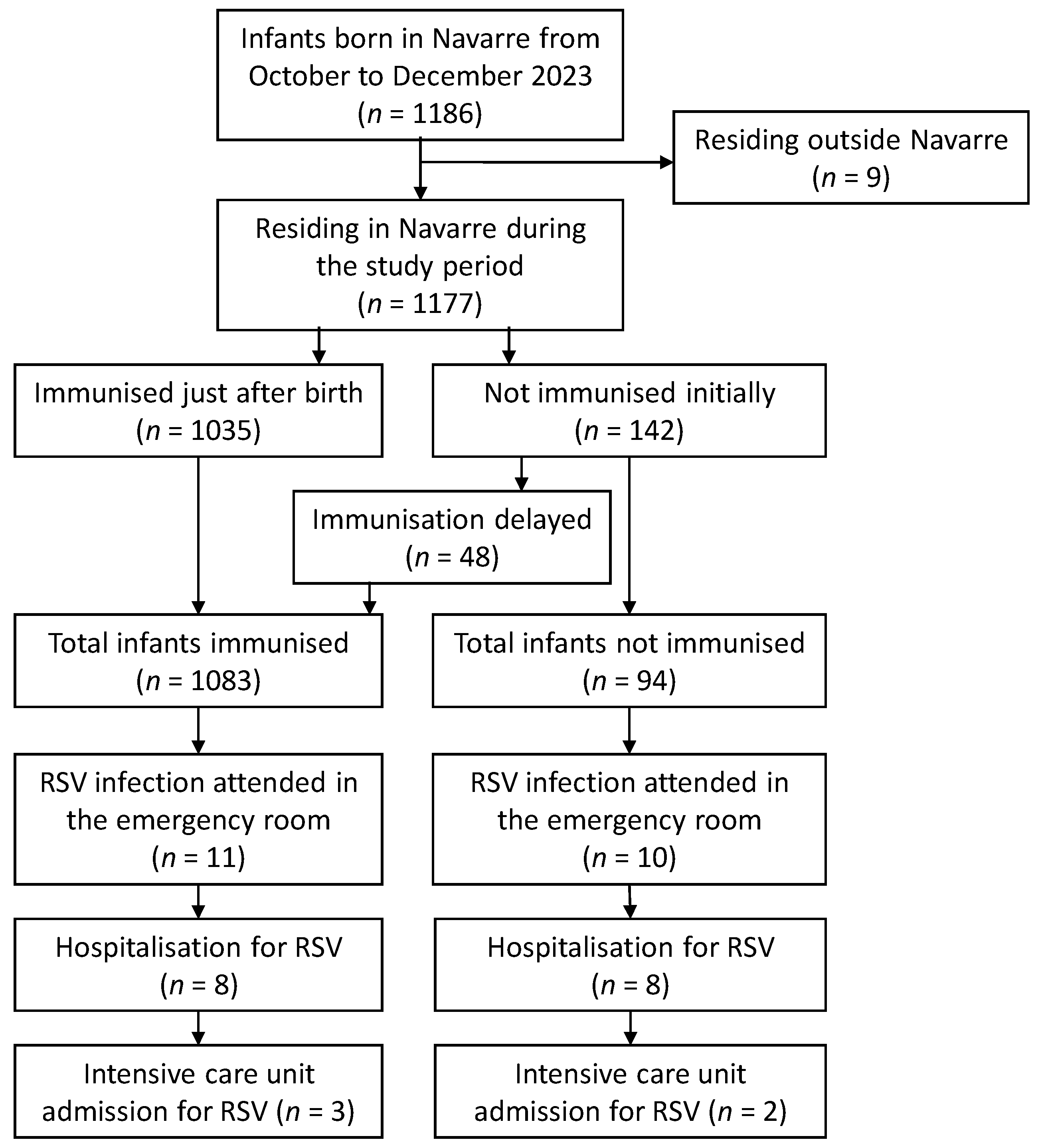
3. Results
3.1. Detections and Hospitalisations for RSV in the 2023–2024 Season
3.2. Nirsevimab Effectiveness in Preventing RSV-Related Hospitalisation
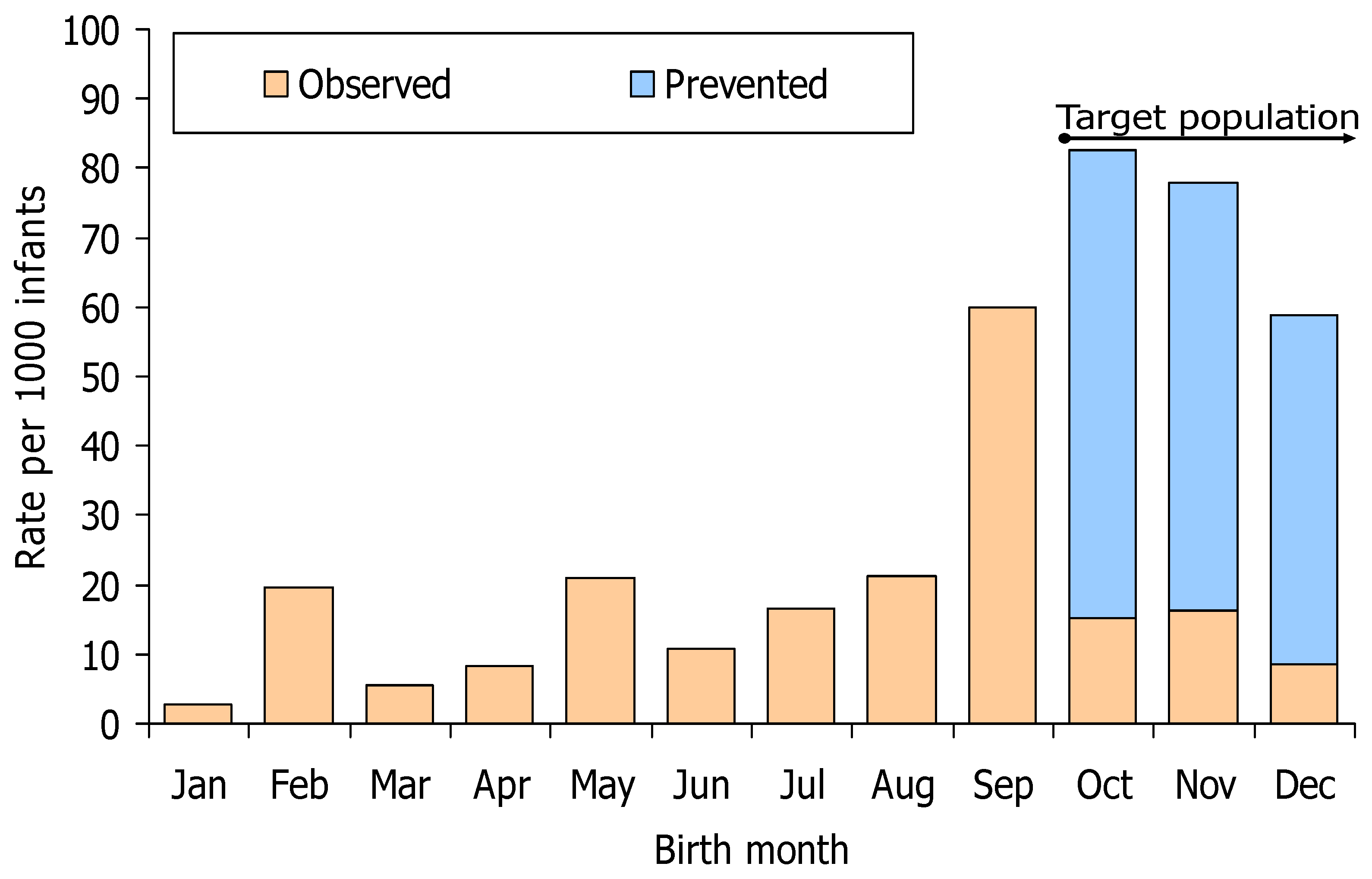
3.3. Evaluation of the Immunisation Strategy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Wildenbeest, J.G.; Billard, M.N.; Zuurbier, R.P.; Korsten, K.; Langedijk, A.C.; van de Ven, P.M.; Snape, M.D.; Drysdale, S.B.; Pollard, A.J.; Robinson, H.; et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: A prospective birth cohort study. Lancet Respir. Med. 2023, 11, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hartling, U.B.; Nielsen, J.; Vestergaard, L.S.; Dungu, K.H.S.; Nielsen, J.S.A.; Sellmer, A.; Matthesen, A.T.; Kristensen, K.; Holm, M. Hospital admissions and need for mechanical ventilation in children with respiratory syncytial virus before and during the COVID-19 pandemic: A Danish nationwide cohort study. Lancet Child Adolesc. Health 2023, 7, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.M.; Bianco, V.; Domachowske, J.B.; Madhi, S.A.; Stoszek, S.K.; Zaman, K.; Bueso, A.; Ceballos, A.; Cousin, L.; D’Andrea, U.; et al. Incidence of respiratory syncytial virus lower respiratory tract infections during the first 2 years of life: A Prospective Study Across Diverse Global Settings. J. Infect. Dis. 2022, 226, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Grupo de Trabajo Utilización de Nirsevimab Frente a Infección por Virus Respiratorio Sincitial de la Ponencia de Programa y Registro de Vacunaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud; Ministerio de Sanidad: Madrid, Spain, July 2023. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab.pdf (accessed on 22 February 2024).
- Viguria, N.; Martínez-Baz, I.; Moreno-Galarraga, L.; Sierrasesúmaga, L.; Salcedo, B.; Castilla, J. Respiratory syncytial virus hospitalization in children in northern Spain. PLoS ONE 2018, 13, e0206474. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Product Information Beyfortus® (Nirsevimab). Available online: https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_es.pdf (accessed on 22 February 2024).
- Simões, E.A.F.; Madhi, S.A.; Muller, W.J.; Atanasova, V.; Bosheva, M.; Cabañas, F.; Baca Cots, M.; Domachowske, J.B.; Garcia-Garcia, M.L.; Grantina, I.; et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child Adolesc. Health 2023, 7, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.J.; Madhi, S.A.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Dagan, R.; Hammitt, L.L.; Llapur, C.J.; Novoa, J.M.; Saez Llorens, X.; et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N. Engl. J. Med. 2023, 388, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Obando-Pacheco, P.; Justicia-Grande, A.J.; Rivero-Calle, I.; Rodríguez-Tenreiro, C.; Sly, P.; Ramilo, O.; Mejías, A.; Baraldi, E.; Papadopoulos, N.G.; Nair, H.; et al. Respiratory Syncytial Virus Seasonality: A Global Overview. J. Infect. Dis. 2018, 217, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.; Koltai, M.; Krauer, F.; Flasche, S.; Jit, M.; Atkins, K.E. Optimal Respiratory Syncytial Virus intervention programmes using Nirsevimab in England and Wales. Vaccine 2022, 40, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Voirin, N.; Virlogeux, V.; Demont, C.; Kieffer, A. Potential impact of nirsevimab on RSV transmission and medically attended lower respiratory tract illness caused by RSV: A disease transmission model. Infect. Dis. Ther. 2022, 11, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Winn, A.; Parikh, R.; Jones, J.M.; McMorrow, M.; Prill, M.M.; Silk, B.J.; Scobie, H.M.; Hall, A.J. Seasonality of Respiratory Syncytial Virus—United States, 2017–2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, M.; Morbey, R.A.; Hughes, H.E.; Beck, C.R.; Watson, C.H.; Zhao, H.; Ellis, J.; Smith, G.E.; Elliot, A.J. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: A retrospective observational study. Lancet Infect. Dis. 2023, 23, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, F.; Mirás-Carballal, S.; Durán-Parrondo, C. Early lessons from the implementation of universal respiratory syncytial virus prophylaxis in infants with long-acting monoclonal antibodies, Galicia, Spain, September and October 2023. Eurosurveillance 2023, 28, 2300606. [Google Scholar] [CrossRef] [PubMed]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; López-Labrador, F.X.; Mengual-Chuliá, B.; Fernández-García, C.; Carballido-Fernández, M.; Pineda-Caplliure, A.; Mollar-Maseres, J.; Shalabi Benavent, M.; et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Eurosurveillance 2024, 29, 2400046. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Tannis, A.; Toepfer, A.P.; Williams, J.V.; Boom, J.A.; Englund, J.A.; Halasa, N.B.; Staat, M.A.; Weinberg, G.A.; Selvarangan, R.; et al. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season—New Vaccine Surveillance Network, October 2023-February 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Bejko, D.; Gaasch, L.; Hannelas, E.; Kahn, I.; Pierron, C.; Del Lero, N.; Schalbar, C.; Do Carmo, E.; Kohnen, M.; et al. Impact of nirsevimab prophylaxis on paediatric respiratory syncytial virus (RSV)-related hospitalisations during the initial 2023/24 season in Luxembourg. Eurosurveillance 2024, 29, 2400033. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.; Madhi, S.A.; Simões, E.A.F.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of nirsevimab for RSV in infants with heart or lung disease or prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, Q.; Zhang, Y.; Yi, L.; Qian, C.; Lu, Y.; Shen, J.; Liu, X.; Jiang, M.; Wang, B.; et al. Modeling the optimal seasonal monoclonal antibody administration strategy for respiratory syncytial virus (RSV) prevention based on age-season specific hospitalization rate of RSV in Suzhou, China, 2016–2022. Vaccine 2024, 42, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Kopera, E.; Czajka, H.; Zapolnik, P.; Mazur, A. New Insights on Respiratory Syncytial Virus Prevention. Vaccines 2023, 11, 1797. [Google Scholar] [CrossRef] [PubMed]
- Ananworanich, J.; Heaton, P.M. Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities. Vaccines 2021, 9, 961. [Google Scholar] [CrossRef] [PubMed]


| Infants n | Person Days | Cases n | Risk % | Rate per 1000 Person Days | Crude Hazard Ratio | 95% Confidence Interval | Adjusted Hazard Ratio * | 95% Confidence Interval | |
|---|---|---|---|---|---|---|---|---|---|
| Hospitalisation | |||||||||
| Total | |||||||||
| Non-immunised | 94 | 9321 | 8 | 8.51 | 0.86 | 1 | 1 | ||
| Immunised | 1083 | 77,647 | 8 | 0.74 | 0.10 | 0.123 | 0.046–0.330 | 0.113 | 0.042–0.304 |
| Male | |||||||||
| Non-immunised | 49 | 4641 | 5 | 10.20 | 1.08 | 1 | 1 | ||
| Immunised | 583 | 41,055 | 4 | 0.69 | 0.10 | 0.096 | 0.026–0.357 | 0.085 | 0.022–0.332 |
| Female | |||||||||
| Non-immunised | 45 | 4680 | 3 | 6.67 | 0.64 | 1 | 1 | ||
| Immunised | 500 | 36,592 | 4 | 0.80 | 0.11 | 0.171 | 0.038–0.770 | 0.185 | 0.040–0.850 |
| Infants born in October | |||||||||
| Non-immunised | 33 | 4190 | 3 | 9.09 | 0.72 | 1 | 1 | ||
| Immunised | 361 | 36,399 | 3 | 0.83 | 0.08 | 0.112 | 0.023–0.555 | 0.118 | 0.023–0.598 |
| Infants born in November | |||||||||
| Non-immunised | 35 | 3523 | 4 | 11.43 | 1.14 | 1 | 1 | ||
| Immunised | 396 | 27,745 | 3 | 0.76 | 0.11 | 0.085 | 0.019–0.382 | 0.084 | 0.018–0.380 |
| Infants born in December | |||||||||
| Non-immunised | 26 | 1608 | 1 | 3.85 | 0.62 | 1 | 1 | ||
| Immunised | 326 | 13,503 | 2 | 0.61 | 0.15 | 0.241 | 0.022–2.677 | 0.194 | 0.015–2.430 |
| Emergency-room attended | |||||||||
| Non-immunised | 94 | 9321 | 9 | 9.57 | 0.97 | 1 | 1 | ||
| Immunised | 1083 | 77,647 | 11 | 1.02 | 0.14 | 0.141 | 0.058–0.341 | 0.121 | 0.049–0.297 |
| Intensive care unit admission | |||||||||
| Non-immunised | 94 | 9321 | 2 | 2.13 | 0.21 | 1 | 1 | ||
| Immunised | 1083 | 77,647 | 3 | 0.28 | 0.04 | 0.203 | 0.034–1.224 | 0.141 | 0.023–0.868 |
| Birth Cohorts | RSV-Related Hospitalisations in the Absence of Immunoprophylaxis | Suggested Moment and Site of Administration | Immunisation Coverage with Nirsevimab * | Estimated Rate of RSV-Related Hospitalisations That Were Prevented or Preventable | Immunisations to Prevent One RSV-Related Hospitalisation | ||
|---|---|---|---|---|---|---|---|
| N (%) | Rate per 1000 Person Days | % | N (%) | Rate per 1000 Person Days | N | ||
| Immunised cohorts in Navarre in 2023–2024 | |||||||
| October–December 2023 | 86.8 (56.8) | 73.7 | Just after birth in the hospital | 92.0 | 70.8 (59.4) | 60.2 | 15.3 |
| January 2024 ** | 4 (2.6) | 11.4 | Just after birth in the hospital | 92.0 | 4 (3.4) | 11.4 | 80.5 |
| Other birth cohorts of interest | |||||||
| September 2023 | 24 (15.7) | 60.0 | Just after birth in the hospital | 92.0 | 17.0 (14.3) | 49.0 | 18.8 |
| April–August 2023 | 29 (19.0) | 15.7 | Catch-up in primary healthcare | 81.4 | 20.9 (17.5) | 11.3 | 71.7 |
| January–March 2023 | 9 (5.9) | 8.8 | Catch-up in primary healthcare | 81.4 | 6.5 (5.5) | 6.3 | 128.8 |
| February–March 2024 | 0 (0) | 0 | Just after birth in the hospital | 92.0 | 0 (0) | 0 | >600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezpeleta, G.; Navascués, A.; Viguria, N.; Herranz-Aguirre, M.; Juan Belloc, S.E.; Gimeno Ballester, J.; Muruzábal, J.C.; García-Cenoz, M.; Trobajo-Sanmartín, C.; Echeverria, A.; et al. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines 2024, 12, 383. https://doi.org/10.3390/vaccines12040383
Ezpeleta G, Navascués A, Viguria N, Herranz-Aguirre M, Juan Belloc SE, Gimeno Ballester J, Muruzábal JC, García-Cenoz M, Trobajo-Sanmartín C, Echeverria A, et al. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines. 2024; 12(4):383. https://doi.org/10.3390/vaccines12040383
Chicago/Turabian StyleEzpeleta, Guillermo, Ana Navascués, Natividad Viguria, Mercedes Herranz-Aguirre, Sergio Enrique Juan Belloc, Juan Gimeno Ballester, Juan Carlos Muruzábal, Manuel García-Cenoz, Camino Trobajo-Sanmartín, Aitziber Echeverria, and et al. 2024. "Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study" Vaccines 12, no. 4: 383. https://doi.org/10.3390/vaccines12040383
APA StyleEzpeleta, G., Navascués, A., Viguria, N., Herranz-Aguirre, M., Juan Belloc, S. E., Gimeno Ballester, J., Muruzábal, J. C., García-Cenoz, M., Trobajo-Sanmartín, C., Echeverria, A., Martínez-Baz, I., Vera-Punzano, N., Casado, I., López-Mendoza, H., Ezpeleta, C., & Castilla, J. (2024). Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines, 12(4), 383. https://doi.org/10.3390/vaccines12040383






