Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques
Abstract
1. Introduction
2. Materials and Methods
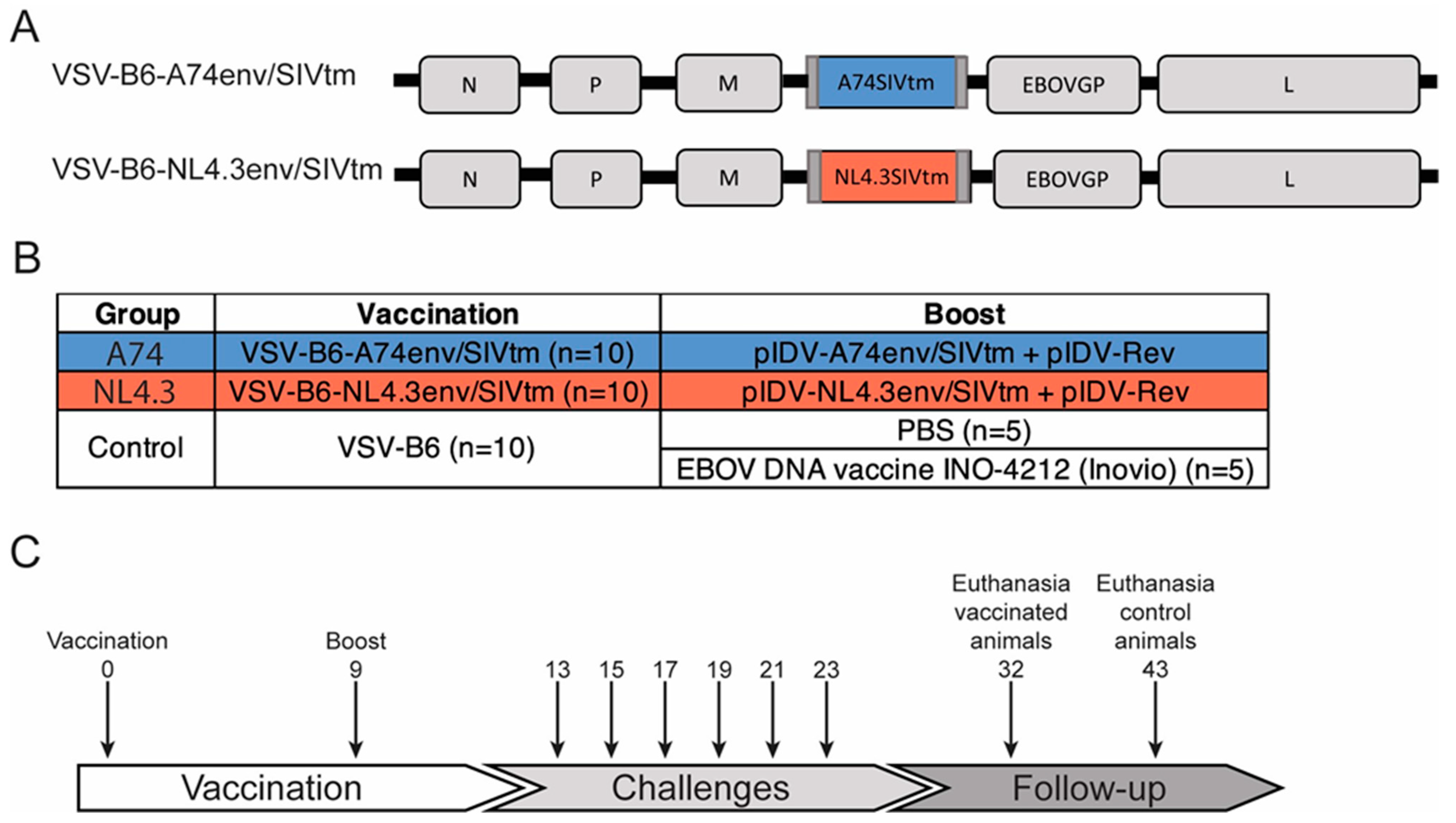
2.1. Vaccine Candidates
2.2. Animals, Vaccinations, and Challenges
2.3. ELISA
2.4. Virus Neutralization Assay
2.5. Plasma Viral Load and Viral Rectal Shedding
2.6. T-Cell Responses
2.7. Statistical Analysis
3. Results
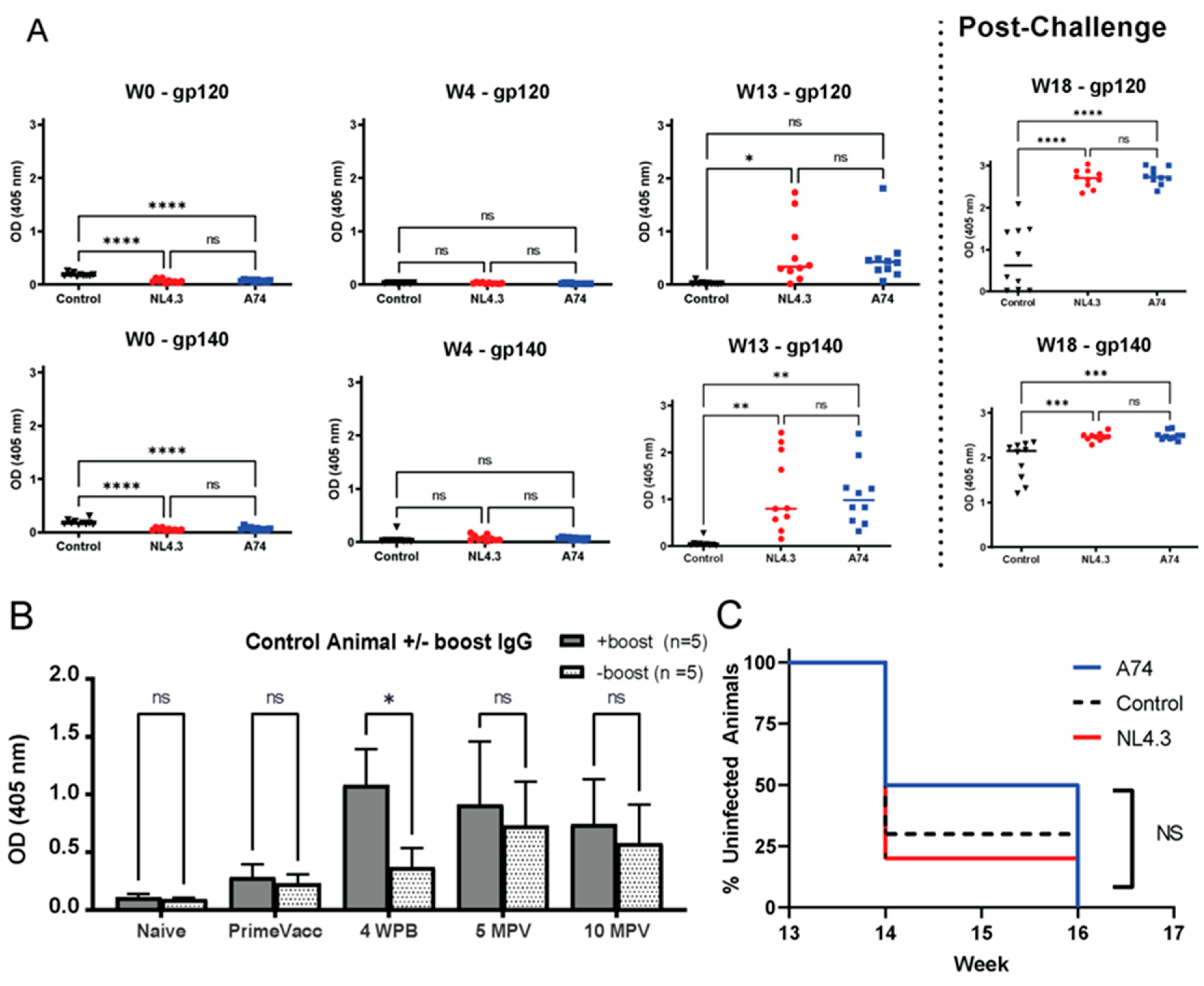
3.1. Recombinant VSV-HIV Vaccines Induce Strong Humoral Responses
3.2. Plasma Viral Load Trends and Correlation with Rectal Viral Shedding
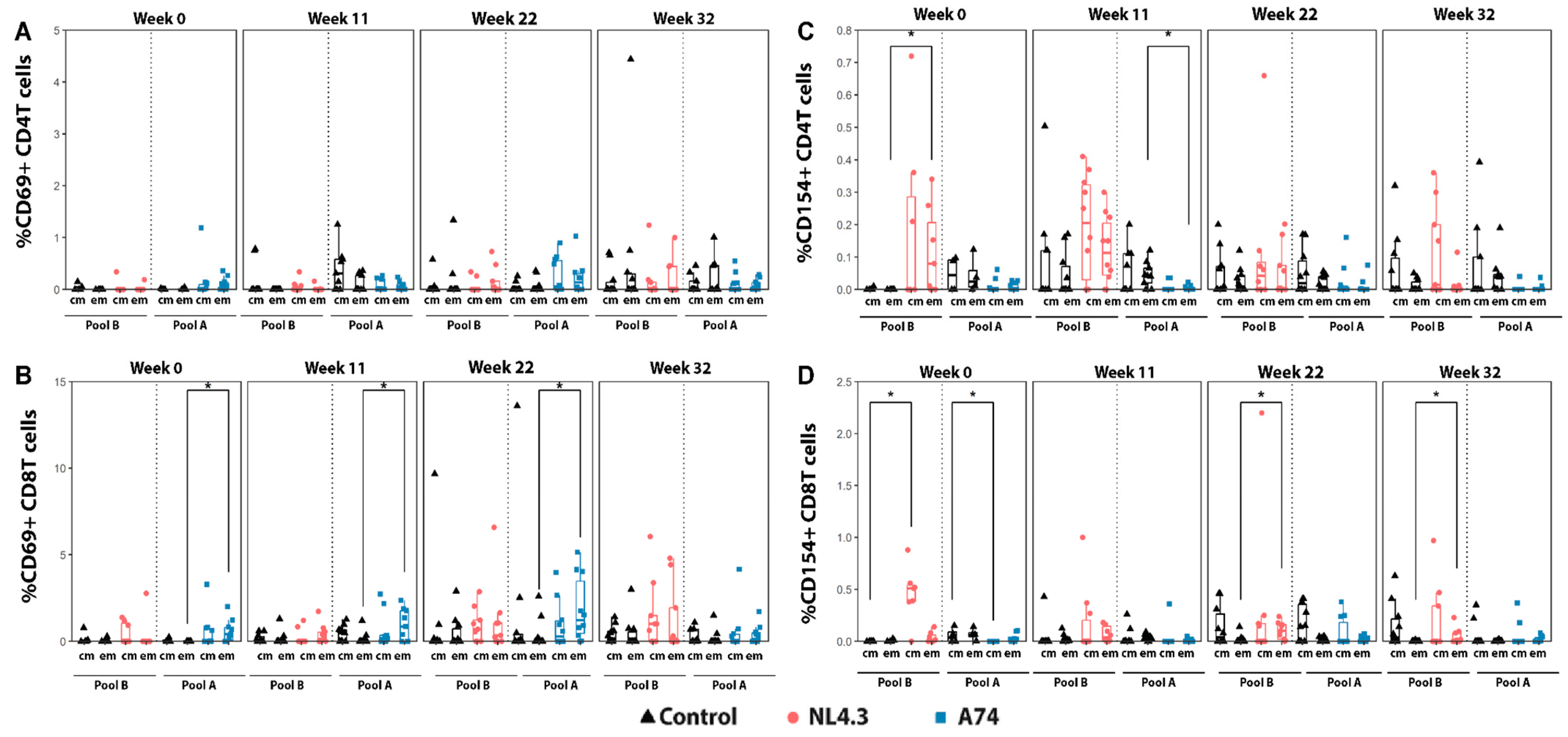
3.3. Cytokine Production and Activation Markers by Central and Effector Memory T-Cells
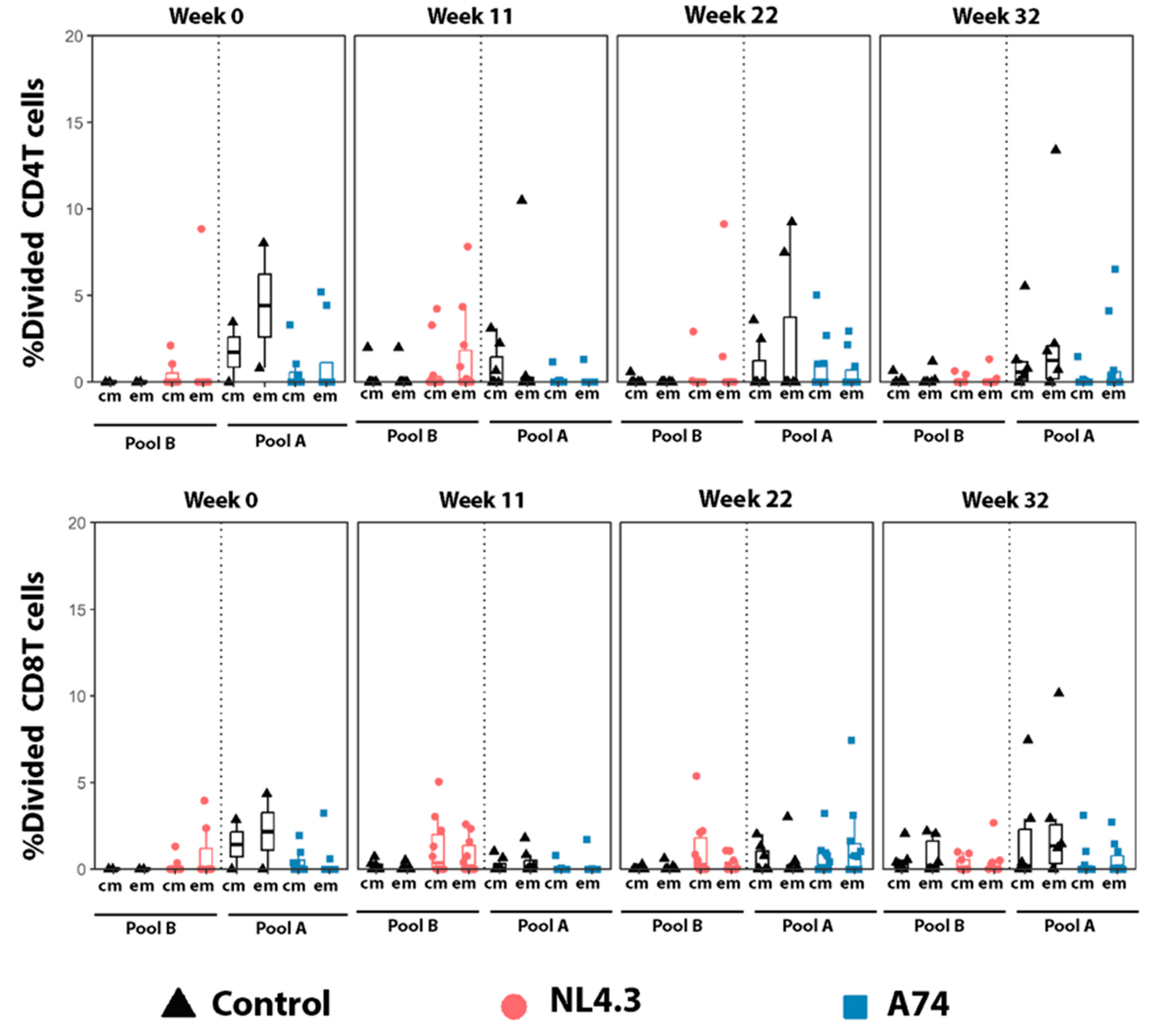
3.4. Proliferating Central- and Effector Memory T-Cells
3.5. Development of an AIDS-like Syndrome in a Control-Vaccinated Animal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, D.R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; De Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209. [Google Scholar] [CrossRef]
- Robinson, H.L. HIV/AIDS Vaccines: 2018. Clin. Pharmacol. Ther. 2018, 104, 1062. [Google Scholar] [CrossRef] [PubMed]
- Robb, M.L.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; Kunasol, P.; Khamboonruang, C.; Thongcharoen, P.; Morgan, P.; Kim, J.H.; et al. Ad Hoc Analysis of Behavior and Time as Co-Variates of the Thai Phase III Efficacy Trial: RV 144. Lancet Infect. Dis. 2012, 12, 531–537. [Google Scholar] [CrossRef]
- Barnett, S.W.; Burke, B.; Sun, Y.; Kan, E.; Legg, H.; Lian, Y.; Bost, K.; Zhou, F.; Goodsell, A.; zur Megede, J.; et al. Antibody-Mediated Protection against Mucosal Simian-Human Immunodeficiency Virus Challenge of Macaques Immunized with Alphavirus Replicon Particles and Boosted with Trimeric Envelope Glycoprotein in MF59 Adjuvant. J. Virol. 2010, 84, 5975–5985. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.B.; Rose, N.F.; Bahl, K.; Diller, K.; Buonocore, L.; Hunter, M.; Marx, P.A.; Gambhira, R.; Tang, H.; Montefiori, D.C.; et al. Significant Protection against High-Dose Simian Immunodeficiency Virus Challenge Conferred by a New Prime-Boost Vaccine Regimen. J. Virol. 2011, 85, 5764–5772. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Ford, J.C.; Lewis, M.S.; Ventura, A.B.; Hughes, C.M.; Coyne-Johnson, L.; Whizin, N.; Oswald, K.; Shoemaker, R.; Swanson, T.; et al. Profound Early Control of Highly Pathogenic SIV by an Effector Memory T-Cell Vaccine. Nature 2011, 473, 523–527. [Google Scholar] [CrossRef]
- Lai, L.; Kwa, S.; Kozlowski, P.A.; Montefiori, D.C.; Ferrari, G.; Johnson, W.E.; Hirsch, V.; Villinger, F.; Chennareddi, L.; Earl, P.L.; et al. Prevention of Infection by a Granulocyte-Macrophage Colony-Stimulating Factor Co-Expressing DNA/Modified Vaccinia Ankara Simian Immunodeficiency Virus Vaccine. J. Infect. Dis. 2011, 204, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Jalah, R.; Kulkarni, V.; Valentin, A.; Rosati, M.; Alicea, C.; von Gegerfelt, A.; Huang, W.; Guan, Y.; Keele, B.F.; et al. DNA and Virus Particle Vaccination Protects against Acquisition and Confers Control of Viremia upon Heterologous Simian Immunodeficiency Virus Challenge. Proc. Natl. Acad. Sci. USA 2013, 110, 2975–2980. [Google Scholar] [CrossRef]
- Barouch, D.H.; Liu, J.; Li, H.; Maxfield, L.F.; Abbink, P.; Lynch, D.M.; Iampietro, M.J.; SanMiguel, A.; Seaman, M.S.; Ferrari, G.; et al. Vaccine Protection against Acquisition of Neutralization-Resistant SIV Challenges in Rhesus Monkeys. Nature 2012, 482, 89–93. [Google Scholar] [CrossRef]
- Flatz, L.; Cheng, C.; Wang, L.; Foulds, K.E.; Ko, S.-Y.; Kong, W.-P.; Roychoudhuri, R.; Shi, W.; Bao, S.; Todd, J.-P.; et al. Gene-Based Vaccination with a Mismatched Envelope Protects against Simian Immunodeficiency Virus Infection in Nonhuman Primates. J. Virol. 2012, 86, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a Mosaic HIV-1 Vaccine in a Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 1/2a Clinical Trial (APPROACH) and in Rhesus Monkeys (NHP 13-19). Lancet 2018, 392, 232. [Google Scholar] [CrossRef] [PubMed]
- Pauthner, M.G.; Nkolola, J.P.; Havenar-Daughton, C.; Murrell, B.; Reiss, S.M.; Bastidas, R.; Prévost, J.; Nedellec, R.; von Bredow, B.; Abbink, P.; et al. Vaccine-Induced Protection from Homologous Tier 2 SHIV Challenge in Nonhuman Primates Depends on Serum-Neutralizing Antibody Titers. Immunity 2019, 50, 241–252.e6. [Google Scholar] [CrossRef] [PubMed]
- Felber, B.K.; Lu, Z.; Hu, X.; Valentin, A.; Rosati, M.; Remmel, C.A.; Weiner, J.A.; Carpenter, M.C.; Faircloth, K.; Stanfield-Oakley, S.; et al. Co-Immunization of DNA and Protein in the Same Anatomical Sites Induces Superior Protective Immune Responses against SHIV Challenge. Cell Rep. 2020, 31, 107624. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Alter, G.; Broge, T.; Linde, C.; Ackerman, M.E.; Brown, E.P.; Borducchi, E.N.; Smith, K.M.; Nkolola, J.P.; Liu, J.; et al. Protective Efficacy of Adenovirus/Protein Vaccines against SIV Challenges in Rhesus Monkeys. Science 2015, 349, 320. [Google Scholar] [CrossRef] [PubMed]
- Hessell, A.J.; Poignard, P.; Hunter, M.; Hangartner, L.; Tehrani, D.M.; Bleeker, W.K.; Parren, P.W.H.I.; Marx, P.A.; Burton, D.R. Effective, Low-Titer Antibody Protection against Low-Dose Repeated Mucosal SHIV Challenge in Macaques. Nat. Med. 2009, 15, 951. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-Correlates Analysis of an HIV-1 Vaccine Efficacy Trial. New Engl. J. Med. 2012, 366, 1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Finak, G.; Ushey, K.; Seshadri, C.; Hawn, T.R.; Frahm, N.; Scriba, T.J.; Mahomed, H.; Hanekom, W.; Bart, P.-A.; et al. COMPASS Identifies T-Cell Subsets Correlated with Clinical Outcomes. Nat. Biotechnol. 2015, 33, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and Effectiveness of an RVSV-Vectored Vaccine in Preventing Ebola Virus Disease: Final Results from the Guinea Ring Vaccination, Open-Label, Cluster-Randomised Trial (Ebola Ça Suffit!). Lancet 2017, 389, 505. [Google Scholar] [CrossRef]
- Clarke, D.K.; Xu, R.; Matassov, D.; Latham, T.E.; Ota-Setlik, A.; Gerardi, C.S.; Luckay, A.; Witko, S.E.; Hermida, L.; Higgins, T.; et al. Safety and Immunogenicity of a Highly Attenuated RVSVN4CT1-EBOVGP1 Ebola Virus Vaccine: A Randomised, Double-Blind, Placebo-Controlled, Phase 1 Clinical Trial. Lancet Infect. Dis. 2020, 20, 455. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Huttner, A.; Zinser, M.E.; Njuguna, P.; Dahlke, C.; Fernandes, J.F.; Yerly, S.; Dayer, J.-A.; Kraehling, V.; Kasonta, R.; et al. Phase 1 Trials of RVSV Ebola Vaccine in Africa and Europe. N. Engl. J. Med. 2016, 374, 1647–1660. [Google Scholar] [CrossRef]
- Huttner, A.; Dayer, J.-A.; Yerly, S.; Combescure, C.; Auderset, F.; Desmeules, J.; Eickmann, M.; Finckh, A.; Goncalves, A.R.; Hooper, J.W.; et al. The Effect of Dose on the Safety and Immunogenicity of the VSV Ebola Candidate Vaccine: A Randomised Double-Blind, Placebo-Controlled Phase 1/2 Trial. Lancet Infect. Dis. 2015, 15, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Fast, P.E.; Modjarrad, K.; Clarke, D.K.; Martin, B.K.; Fusco, J.; Nichols, R.; Heppner, D.G.; Simon, J.K.; Dubey, S.; et al. RVSVΔG-ZEBOV-GP (Also Designated V920) Recombinant Vesicular Stomatitis Virus Pseudotyped with Ebola Zaire Glycoprotein: Standardized Template with Key Considerations for a Risk/Benefit Assessment. Vaccine X 2019, 1, 100009. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, I.C.; Nguyen, H.T.; Kemelman, M.; Lindsay, R.W.; Yuan, M.; Wright, K.J.; Arendt, H.; Back, J.W.; DeStefano, J.; Hoffenberg, S.; et al. The Stem of Vesicular Stomatitis Virus G Can Be Replaced with the HIV-1 Env Membrane-Proximal External Region without Loss of g Function or Membrane-Proximal External Region Antigenic Properties. AIDS Res. Hum. Retroviruses 2014, 30, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Azizi, H.; Knapp, J.P.; Li, Y.; Berger, A.; Lafrance, M.-A.; Pedersen, J.; de la Vega, M.-A.; Racine, T.; Kang, C.-Y.; Mann, J.F.S.; et al. Optimal Expression, Function, and Immunogenicity of an HIV-1 Vaccine Derived from the Approved Ebola Vaccine, RVSV-ZEBOV. Vaccines 2023, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.; Yuan, M.; Coleman, J.; Destefano, J.; Zhang, X.; Yates, N.; Barouch, D.; Ackerman, M.; Decamp, A.; Alter, G.; et al. Protection from Rectal SHIV Infection Induced by Mucosal Vaccination with a Replication-Competent VSV-HIV Chimera Delivering Env Trimers. In AIDS Research and Human Retroviruses; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2016. [Google Scholar]
- Egan, M.A.; Chong, S.Y.; Rose, N.F.; Megathi, S.; Lopez, K.J.; Schadeck, E.B.; Johnson, J.E.; Masood, A.; Piacente, P.; Druilhet, R.E.; et al. Immunogenicity of Attenuated Vesicular Stomatitis Virus Vectors Expressing HIV Type 1 Env and SIV Gag Proteins: Comparison of Intranasal and Intramuscular Vaccination Routes. AIDS Res. Hum. Retroviruses 2004, 20, 989. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.F.; Marx, P.A.; Luckay, A.; Nixon, D.F.; Moretto, W.J.; Donahoe, S.M.; Montefiori, D.; Roberts, A.; Buonocore, L.; Rose, J.K. An Effective AIDS Vaccine Based on Live Attenuated Vesicular Stomatitis Virus Recombinants. Cell 2001, 106, 539. [Google Scholar] [CrossRef]
- Bresk, C.A.; Hofer, T.; Wilmschen, S.; Krismer, M.; Beierfuß, A.; Effantin, G.; Weissenhorn, W.; Hogan, M.J.; Jordan, A.P.; Gelman, R.S.; et al. Induction of Tier 1 HIV Neutralizing Antibodies by Envelope Trimers Incorporated into a Replication Competent Vesicular Stomatitis Virus Vector. Viruses 2019, 11, 159. [Google Scholar] [CrossRef]
- Wong, G.; Qiu, X.; Ebihara, H.; Feldmann, H.; Kobinger, G.P. Characterization of a Bivalent Vaccine Capable of Inducing Protection Against Both Ebola and Cross-Clade H5N1 Influenza in Mice. J. Infect. Dis. 2015, 212, S435. [Google Scholar] [CrossRef]
- Jelinski, J.; Kowatsch, M.M.; Lafrance, M.-A.; Berger, A.; Pedersen, J.; Azizi, H.; Li, Y.; Scholte, F.; Gomez, A.; Hollett, N.; et al. Rhesus Macaques Show Increased Resistance to Repeated SHIV Intrarectal Exposure Following a Heterologous Regimen of RVSV Vector Vaccine Expressing HIV Antigen. Emerg. Microbes Infect. 2023, 12, 2251595. [Google Scholar] [CrossRef]
- Mangion, M.; Gélinas, J.-F.; Gashti, A.B.Z.; Azizi, H.; Kiesslich, S.; Nassoury, N.; Chahal, P.S.; Kobinger, G.; Gilbert, R.; Garnier, A.; et al. Evaluation of Novel HIV Vaccine Candidates Using Recombinant Vesicular Stomatitis Virus Vector Produced in Serum-Free Vero Cell Cultures. Vaccine 2020, 38, 7949–7955. [Google Scholar] [CrossRef]
- Ratcliff, A.N.; Shi, W.; Arts, E.J. HIV-1 Resistance to Maraviroc Conferred by a CD4 Binding Site Mutation in the Envelope Glycoprotein Gp120. J. Virol. 2013, 87, 923–934. [Google Scholar] [CrossRef]
- Adachi, A.; Gendelman, H.E.; Koenig, S.; Folks, T.; Willey, R.; Rabson, A.; Martin, M.A. Production of Acquired Immunodeficiency Syndrome-Associated Retrovirus in Human and Nonhuman Cells Transfected with an Infectious Molecular Clone. J. Virol. 1986, 59, 284. [Google Scholar] [CrossRef]
- Urbanowicz, R.A.; McClure, C.P.; Sakuntabhai, A.; Sall, A.A.; Kobinger, G.; Müller, M.A.; Holmes, E.C.; Rey, F.A.; Simon-Loriere, E.; Ball, J.K. Human Adaptation of Ebola Virus during the West African Outbreak. Cell 2016, 167, 1079. [Google Scholar] [CrossRef]
- Garbutt, M.; Liebscher, R.; Wahl-Jensen, V.M.; Jones, S.M.; Wagner, R.; Volchkov, V.E.; Klenk, H. Properties of Replication-Competent Vesicular Stomatitis Virus Vectors Expressing Glycoproteins of Filoviruses and Arenaviruses Properties of Replication-Competent Vesicular Stomatitis Virus Vectors Expressing Glycoproteins of Filoviruses and Arenaviruses. J. Virol. 2004, 78, 5458. [Google Scholar] [CrossRef]
- Babuadze, G.G.; Echanove, J.; Lamarre, C.; deLaVega, M.-A.; Fausther-Bovendo, H.; Racine, T.; Gomez, A.M.; Azizi, H.; Wade, M.; Kozak, R.; et al. A Novel DNA Platform Designed for Vaccine Use with High Transgene Expression and Immunogenicity. Vaccine 2021, 39, 7175–7181. [Google Scholar] [CrossRef]
- Gomez, A.M.; Babuadze, G.; Plourde-Campagna, M.A.; Azizi, H.; Berger, A.; Kozak, R.; de La Vega, M.A.; Xiii, A.; Naghibosadat, M.; Nepveu-Traversy, M.E.; et al. A Novel Intradermal Tattoo-Based Injection Device Enhances the Immunogenicity of Plasmid DNA Vaccines. NPJ Vaccines 2022, 7, 172. [Google Scholar] [CrossRef]
- Seaman, M.S.; Janes, H.; Hawkins, N.; Grandpre, L.E.; Devoy, C.; Giri, A.; Coffey, R.T.; Harris, L.; Wood, B.; Daniels, M.G.; et al. Tiered Categorization of a Diverse Panel of HIV-1 Env Pseudoviruses for Assessment of Neutralizing Antibodies. J. Virol. 2010, 84, 1439. [Google Scholar] [CrossRef]
- Thermofisher, BestProtocols: Staining Intracellular Antigens for Flow Cytometry. Available online: https://www.thermofisher.com/us/en/home/references/protocols/cell-and-tissue-analysis/protocols/staining-intracellular-antigens-flow-cytometry.html (accessed on 30 September 2020).
- Murphy, K. Janeway’s Immuno Biology, 8th ed.; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Ratcliff, A.N.; Venner, C.M.; Olabode, A.S.; Knapp, J.; Pankrac, J.; Derecichei, I.; Gibson, R.M.; Finzi, A.; Li, Y.; Mann, J.F.S.; et al. Enhancement of CD4 Binding, Host Cell Entry, and Sensitivity to CD4bs Antibody Inhibition Conferred by a Natural but Rare Polymorphism in the HIV-1 Envelope. J. Virol. 2022, 96, e0185121. [Google Scholar] [CrossRef]
- McGettigan, J.P.; Sarma, S.; Orenstein, J.M.; Pomerantz, R.J.; Schnell, M.J. Expression and Immunogenicity of Human Immunodeficiency Virus Type 1 Gag Expressed by a Replication-Competent Rhabdovirus-Based Vaccine Vector. J. Virol. 2001, 75, 8724–8732. [Google Scholar] [CrossRef]
- Temchura, V.; Überla, K. Intrastructural Help: Improving the HIV-1 Envelope Antibody Response Induced by Virus-like Particle Vaccines. Curr. Opin. HIV AIDS 2017, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Marasini, B.; Vyas, H.K.; Lakhashe, S.K.; Hariraju, D.; Akhtar, A.; Ratcliffe, S.J.; Ruprecht, R.M. Mucosal AIDS Virus Transmission Is Enhanced by Antiviral IgG Isolated Early in Infection. AIDS 2021, 35, 2423. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Fernández, M.; de la Fuente, H.; Martín, P.; Cibrián, D.; Sánchez-Madrid, F. Unraveling Cd69 Signaling Pathways, Ligands And Laterally Associated Molecules. EXCLI J. 2023, 22, 334. [Google Scholar] [PubMed]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Chou, M.; Cyster, J.G.; Li, X. Transmembrane Protein CD69 Acts as an S1PR1 Agonist. Elife 2023, 12, e88204. [Google Scholar] [CrossRef] [PubMed]
- Kahle, E.M.; Bolton, M.; Hughes, J.P.; Donnell, D.; Celum, C.; Lingappa, J.R.; Ronald, A.; Cohen, C.R.; de Bruyn, G.; Fong, Y.; et al. Plasma Cytokine Levels and Risk of HIV Type 1 (HIV-1) Transmission and Acquisition: A Nested Case-Control Study among HIV-1-Serodiscordant Couples. J. Infect. Dis. 2015, 211, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Karim, S.S.A.; Altfeld, M.; Samsunder, N.; Durgiah, R.; Sibeko, S.; Karim, Q.A.; Carr, W.H. Innate Immune Activation Enhances HIV Acquisition in Women, Diminishing the Effectiveness of Tenofovir Microbicide Gel. J. Infect. Dis. 2012, 206, 993–1001. [Google Scholar] [CrossRef]
- Van Kooten, G.; Banchereau, J. CD40-CD40 Ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef]
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in Cell-Mediated Immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [CrossRef]
- Kopycinski, J.; Yang, H.; Hancock, G.; Pace, M.; Kim, E.; Frater, J.; Stöhr, W.; Hanke, T.; Fidler, S.; Dorrell, L. Therapeutic Vaccination Following Early Antiretroviral Therapy Elicits Highly Functional T Cell Responses against Conserved HIV-1 Regions. Sci. Rep. 2023, 13, 17155. [Google Scholar] [CrossRef]
- Llano, A.; Parera, M.; Lopez, M.; Oriol-Tordera, B.; Ruiz-Riol, M.; Coll, J.; Perez, F.; Leselbaum, A.R.; McGowan, I.; Sengupta, D.; et al. Safety, Immunogenicity and Effect on Viral Rebound of HTI Vaccines in Early Treated HIV-1 Infection: A Randomized, Placebo-Controlled Phase 1 Trial. Nat. Med. 2022, 28, 2611–2621. [Google Scholar]
- Weaver, E.A.; Nehete, P.N.; Nehete, B.P.; Yang, G.; Buchl, S.J.; Hanley, P.W.; Palmer, D.; Montefiori, D.C.; Ferrari, G.; Ng, P.; et al. Comparison of Systemic and Mucosal Immunization with Helper-Dependent Adenoviruses for Vaccination against Mucosal Challenge with SHIV. PLoS ONE 2013, 8, e67574. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.B.; Bahl, K.; Folta-Stogniew, E.; Rose, N.; Buonocore, L.; Marx, P.A.; Gambhira, R.; Rose, J.K. Antigenic Requirement for Gag in a Vaccine That Protects against High-Dose Mucosal Challenge with Simian Immunodeficiency Virus. Virology 2015, 476, 405–412. [Google Scholar] [CrossRef]
- Gautam, R.; Iyer, A.; Hunter, M.; Das, A.; Williams, T.; Dufour, J.; Apetrei, C.; Kousoulas, K.G.; Marx, P.A. Vesicular Stomatitis Virus-Simian Retrovirus Type 2 Vaccine Protects Macaques from Detectable Infection and B-Cell Destruction. J. Virol. 2011, 85, 5889–5896. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, A.; Pedersen, J.; Kowatsch, M.M.; Scholte, F.; Lafrance, M.-A.; Azizi, H.; Li, Y.; Gomez, A.; Wade, M.; Fausther-Bovendo, H.; et al. Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques. Vaccines 2024, 12, 369. https://doi.org/10.3390/vaccines12040369
Berger A, Pedersen J, Kowatsch MM, Scholte F, Lafrance M-A, Azizi H, Li Y, Gomez A, Wade M, Fausther-Bovendo H, et al. Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques. Vaccines. 2024; 12(4):369. https://doi.org/10.3390/vaccines12040369
Chicago/Turabian StyleBerger, Alice, Jannie Pedersen, Monika M. Kowatsch, Florine Scholte, Marc-Alexandre Lafrance, Hiva Azizi, Yue Li, Alejandro Gomez, Matthew Wade, Hugues Fausther-Bovendo, and et al. 2024. "Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques" Vaccines 12, no. 4: 369. https://doi.org/10.3390/vaccines12040369
APA StyleBerger, A., Pedersen, J., Kowatsch, M. M., Scholte, F., Lafrance, M.-A., Azizi, H., Li, Y., Gomez, A., Wade, M., Fausther-Bovendo, H., de La Vega, M.-A., Jelinski, J., Babuadze, G., Nepveu-Traversy, M.-E., Lamarre, C., Racine, T., Kang, C.-Y., Gaillet, B., Garnier, A., ... Kobinger, G. (2024). Impact of Recombinant VSV-HIV Prime, DNA-Boost Vaccine Candidates on Immunogenicity and Viremia on SHIV-Infected Rhesus Macaques. Vaccines, 12(4), 369. https://doi.org/10.3390/vaccines12040369







