mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases
Abstract
1. Introduction
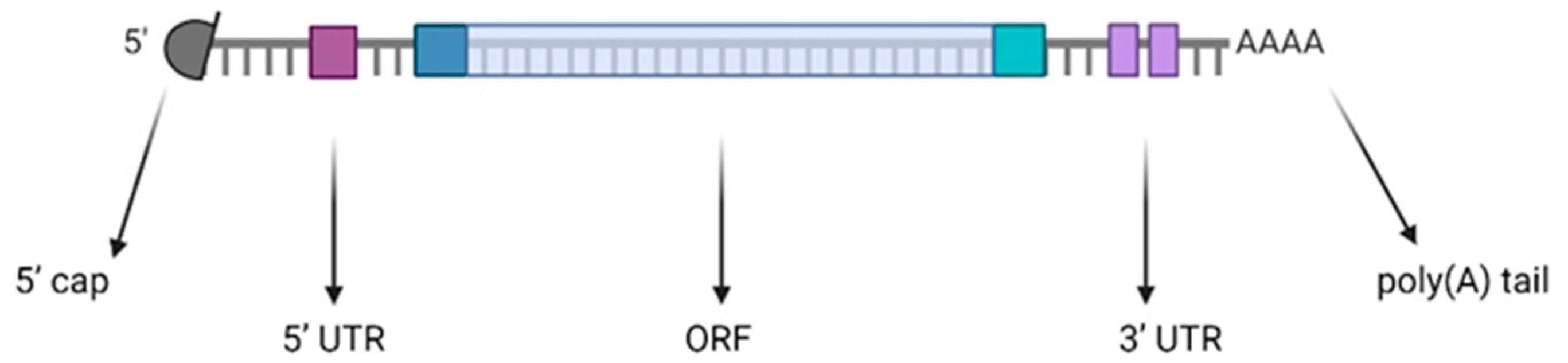
2. mRNA
3. Delivery Systems
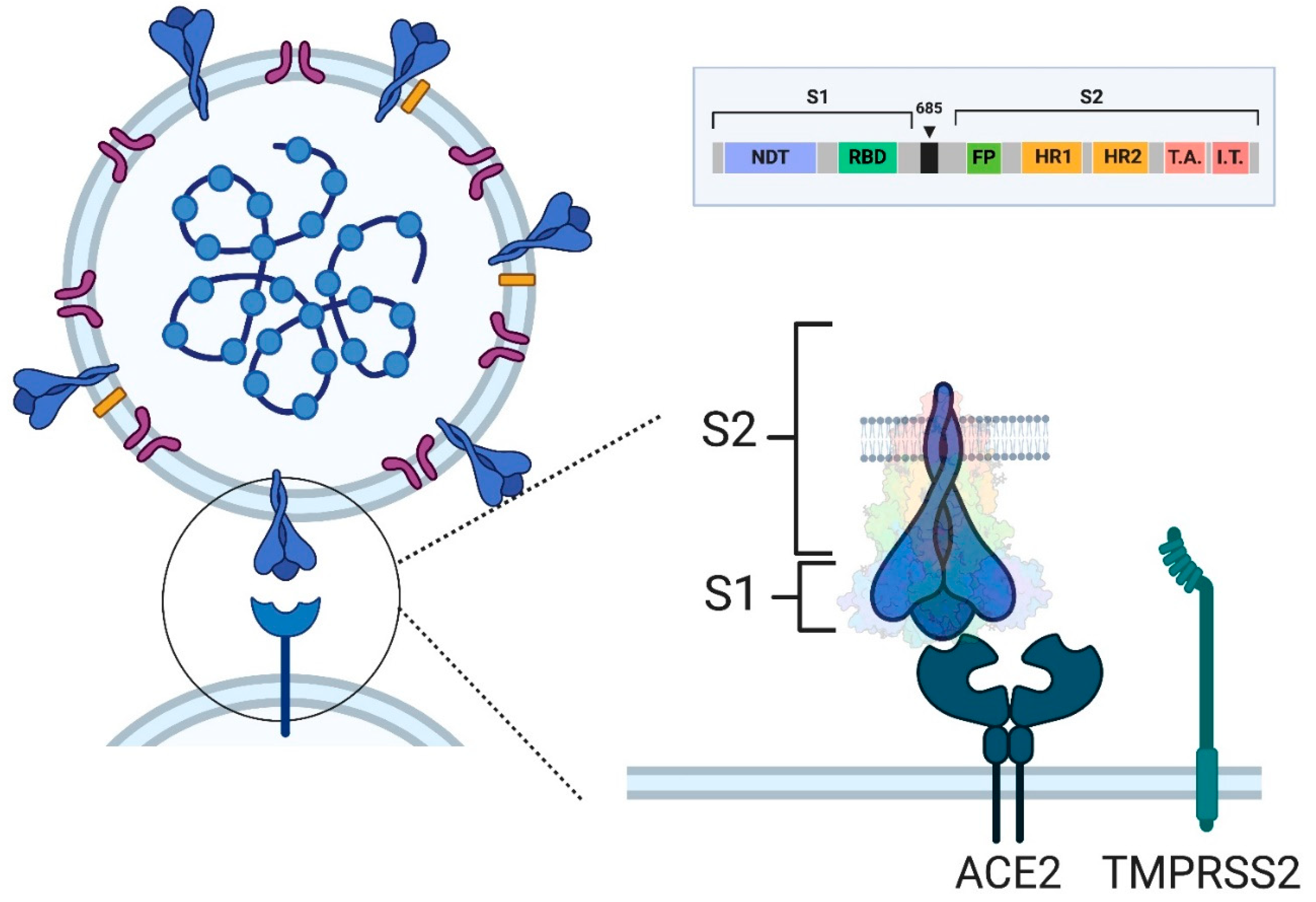
4. SARS-CoV-2
5. mRNA Vaccines Against COVID-19
COVID-19
6. Immune Response to SARS-CoV-2 and to COVID-19 Vaccines
7. Factors Influencing the Durability of Vaccine-Induced Protection: Immune Correlates of Protection
8. Real-World Experience with mRNA Vaccines
8.1. Safety
8.2. Effectiveness
8.3. mRNA Vaccines in Immunocompromised Patients
9. mRNA Vaccines Against Other Infectious Diseases
9.1. Respiratory Syncytial Virus
9.2. Influenza
9.3. Human Cytomegalovirus
9.4. Zika
9.5. HIV
9.6. Epstein–Barr Virus
9.7. Varicella Zoster Virus
9.8. Other Pathogens
9.9. Combined mRNA Vaccines
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Brenner, S.; Jacob, F.; Meselson, M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature 1961, 190, 576–581. [Google Scholar] [CrossRef]
- Melton, D.A.; Krieg, P.A.; Rebagliati, M.R.; Maniatis, T.; Zinn, K.; Green, M.R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984, 12, 7035–7056. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.A.; Melton, D.A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984, 12, 7057–7070. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Jirikowski, G.F.; Sanna, P.P.; Maciejewski-Lenoir, D.; Bloom, F.E. Reversal of diabetes insipidus in Brattleboro rats: Intrahypothalamic injection of vasopressin mRNA. Science 1992, 255, 996–998. [Google Scholar] [CrossRef]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1531. [Google Scholar] [CrossRef]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef]
- Le, T.; Sun, C.; Chang, J.; Zhang, G.; Yin, X. mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases. Viruses 2022, 14, 401. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Baiersdörfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Karikó, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Xie, C.; Xia, X. Advances in mRNA vaccines. Int. Rev. Cell Mol. Biol. 2022, 372, 295–316. [Google Scholar] [CrossRef]
- Weissman, D. mRNA transcript therapy. Expert. Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.Y.; Verrier, B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020, 26, 311–323. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Fauci, A.S. The story behind COVID-19 vaccines. Science 2021, 372, 109. [Google Scholar] [CrossRef]
- Lista, F.; Peragallo, M.S.; Biselli, R.; De Santis, R.; Mariotti, S.; Nisini, R.; D’Amelio, R. Have Diagnostics, Therapies, and Vaccines Made the Difference in the Pandemic Evolution of COVID-19 in Comparison with “Spanish Flu”? Pathogens 2023, 12, 868. [Google Scholar] [CrossRef]
- D’Amelio, E.; Salemi, S.; D’Amelio, R. Anti-Infectious Human Vaccination in Historical Perspective. Int. Rev. Immunol. 2016, 35, 260–290. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, F. A comprehensive comparison of DNA and RNA vaccines. Adv. Drug Deliv. Rev. 2024, 210, 115340. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744.e16. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520.e4. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef]
- Li, X.; Qi, J.; Wang, J.; Hu, W.; Zhou, W.; Wang, Y.; Li, T. Nanoparticle technology for mRNA: Delivery strategy, clinical application and developmental landscape. Theranostics 2024, 14, 738–760. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Matarazzo, L.; Bettencourt, P.J.G. mRNA vaccines: A new opportunity for malaria, tuberculosis and HIV. Front. Immunol. 2023, 14, 1172691. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P., 3rd; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Zabidi, N.Z.; Liew, H.L.; Farouk, I.A.; Puniyamurti, A.; Yip, A.J.W.; Wijesinghe, V.N.; Low, Z.Y.; Tang, J.W.; Chow, V.T.K.; Lal, S.K. Evolution of SARS-CoV-2 Variants: Implications on Immune Escape, Vaccination, Therapeutic and Diagnostic Strategies. Viruses 2023, 15, 944. [Google Scholar] [CrossRef] [PubMed]
- Statement on the Update of WHO’s Working Definitions and Tracking System for SARS-CoV-2 Variants of Concern and Variants of Interest. Available online: https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest (accessed on 20 February 2024).
- COVID-19. Available online: https://www.cdc.gov/covid/?CDC_AAref_Val (accessed on 1 September 2023).
- Gupta, P.; Gupta, V.; Singh, C.M.; Singhal, L. Emergence of COVID-19 Variants: An Update. Cureus 2023, 15, e41295. [Google Scholar] [CrossRef] [PubMed]
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 13 October 2024).
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef]
- Koyama, T.; Platt, D.; Parida, L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 2020, 98, 495–504. [Google Scholar] [CrossRef]
- Duchene, S.; Featherstone, L.; Haritopoulou-Sinanidou, M.; Rambaut, A.; Lemey, P.; Baele, G. Temporal signal and the phylodynamic threshold of SARS-CoV-2. Virus Evol. 2020, 6, veaa061. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Wei, C.; Li, S.; Zhao, J.; Zheng, Y.; Liu, X.; Zeng, X.; Yuan, W.; Peng, S. Molecular Evolutionary Characteristics of SARS-CoV-2 Emerging in the United States. J. Med. Virol. 2022, 94, 310–317. [Google Scholar] [CrossRef]
- Corey, L.; Beyrer, C.; Cohen, M.S.; Michael, N.L.; Bedford, T.; Rolland, M. SARS-CoV-2 Variants in Patients with Immunosuppression. N. Engl. J. Med. 2021, 385, 562–566. [Google Scholar] [CrossRef]
- Wilkinson, S.A.J.; Richter, A.; Casey, A.; Osman, H.; Mirza, J.D.; Stockton, J.; Quick, J.; Ratcliffe, L.; Sparks, N.; Cumley, N.; et al. Recurrent SARS-CoV-2 mutations in immunodeficient patients. Virus Evol. 2022, 8, veac050. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Holmes, E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef]
- Yewdell, J.W. Antigenic drift: Understanding COVID-19. Immunity 2021, 54, 2681–2687. [Google Scholar] [CrossRef]
- Gupta, R.K. Will SARS-CoV-2 variants of concern affect the promise of vaccines? Nat. Rev. Immunol. 2021, 21, 340–341. [Google Scholar] [CrossRef]
- Konings, F.; Perkins, M.D.; Kuhn, J.H.; Pallen, M.J.; Alm, E.J.; Archer, B.N.; Barakat, A.; Bedford, T.; Bhiman, J.N.; Caly, L.; et al. SARS-CoV-2 Variants of Interest and Concern naming scheme conducive for global discourse. Nat. Microbiol. 2021, 6, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; De Marco, A.; Ferreira, I.A.T.M.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Silacci-Fregni, C.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, Y.; Chu, M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front. Immunol. 2022, 13, 898192. [Google Scholar] [CrossRef]
- Cele, S.; Gazy, I.; Jackson, L.; Hwa, S.H.; Tegally, H.; Lustig, G.; Giandhari, J.; Pillay, S.; Wilkinson, E.; Naidoo, Y.; et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 2021, 593, 142–146. [Google Scholar] [CrossRef]
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill 2021, 26, 2100509. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat. Med. 2022, 28, 1933–1943. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.C.; Ng, K.C.; Ching, R.H.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Muik, A.; Lui, B.G.; Wallisch, A.K.; Bacher, M.; Mühl, J.; Reinholz, J.; Ozhelvaci, O.; Beckmann, N.; Güimil Garcia, R.C.; Poran, A.; et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022, 375, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Carreño, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Rouphael, N.G.; Diemert, D.J.; Falsey, A.R.; Losada, C.; Baden, L.R.; Frey, S.E.; Whitaker, J.A.; Little, S.J.; Anderson, E.J.; et al. Comparison of bivalent and monovalent SARS-CoV-2 variant vaccines: The phase 2 randomized open-label COVAIL trial. Nat. Med. 2023, 29, 2334–2346. [Google Scholar] [CrossRef]
- Offit, P.A. Bivalent COVID-19 Vaccines—A Cautionary Tale. N. Engl. J. Med. 2023, 388, 481–483. [Google Scholar] [CrossRef]
- Lin, D.Y.; Xu, Y.; Gu, Y.; Zeng, D.; Sunny, S.K.; Moore, Z. Durability of Bivalent Boosters against Omicron Subvariants. N. Engl. J. Med. 2023, 388, 1818–1820. [Google Scholar] [CrossRef]
- Pather, S.; Muik, A.; Rizzi, R.; Mensa, F. Clinical development of variant-adapted BNT162b2 COVID-19 vaccines: The early Omicron era. Expert. Rev. Vaccines 2023, 22, 650–661. [Google Scholar] [CrossRef]
- Offit, P.A. COVID-19 Boosters—Where from Here? N. Engl. J. Med. 2022, 386, 1661–1662. [Google Scholar] [CrossRef]
- Kampf, G. The epidemiological relevance of the COVID-19-vaccinated population is increasing. Lancet Reg. Health Eur. 2021, 11, 100272. [Google Scholar] [CrossRef]
- Lin, D.Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef]
- Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 16 June 2024).
- Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 16 June 2024).
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control Release 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R.; et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: A phase 1 randomized clinical trial. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Ahuad Guerrero, R.A.; Arana-Arri, E.; Aroca Martinez, G.J.; Bonten, M.; Chandler, R.; Corral, G.; De Block, E.J.L.; Ecker, L.; Gabor, J.J.; et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): A randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2022, 22, 329–340. [Google Scholar] [CrossRef]
- Gebre, M.S.; Rauch, S.; Roth, N.; Yu, J.; Chandrashekar, A.; Mercado, N.B.; He, X.; Liu, J.; McMahan, K.; Martinot, A.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 2022, 601, 410–414. [Google Scholar] [CrossRef]
- ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 23 November 2023).
- ANNEX I SUMMARY OF PRODUCT CHARACTE. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 23 November 2023).
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Moss, W.J.; Gostin, L.O.; Nuzzo, J.B. Pediatric COVID-19 Vaccines: What Parents, Practitioners, and Policy Makers Need to Know. JAMA 2021, 326, 2257–2258. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Melendez Baez, I.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef]
- Muñoz, F.M.; Sher, L.D.; Sabharwal, C.; Gurtman, A.; Xu, X.; Kitchin, N.; Lockhart, S.; Riesenberg, R.; Sexter, J.M.; Czajka, H.; et al. Evaluation of BNT162b2 COVID-19 Vaccine in Children Younger than 5 Years of Age. N. Engl. J. Med. 2023, 388, 621–634. [Google Scholar] [CrossRef]
- Anderson, E.J.; Creech, C.B.; Berthaud, V.; Piramzadian, A.; Johnson, K.A.; Zervos, M.; Garner, F.; Griffin, C.; Palanpurwala, K.; Turner, M.; et al. Evaluation of mRNA-1273 Vaccine in Children 6 Months to 5 Years of Age. N. Engl. J. Med. 2022, 387, 1673–1687. [Google Scholar] [CrossRef]
- Hause, A.M.; Marquez, P.; Zhang, B.; Myers, T.R.; Gee, J.; Su, J.R.; Parker, C.; Thompson, D.; Panchanathan, S.S.; Shimabukuro, T.T.; et al. COVID-19 mRNA Vaccine Safety Among Children Aged 6 Months-5 Years—United States, June 18, 2022–August 21, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1115–1120. [Google Scholar] [CrossRef]
- Dalapati, T.; Williams, C.A.; Giorgi, E.E.; Hurst, J.H.; Herbek, S.; Chen, J.L.; Kosman, C.; Rotta, A.T.; Turner, N.A.; Pulido, N.; et al. Immunogenicity of Monovalent mRNA-1273 and BNT162b2 Vaccines in Children <5 Years of Age. Pediatrics 2024, 153, e2024066190. [Google Scholar] [CrossRef]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Soe, P.; Vanderkooi, O.G.; Sadarangani, M.; Naus, M.; Muller, M.P.; Kellner, J.D.; Top, K.A.; Wong, H.; Isenor, J.E.; Marty, K.; et al. mRNA COVID-19 vaccine safety among children and adolescents: A Canadian National Vaccine Safety Network cohort study. Lancet Reg. Health Am. 2024, 40, 100949. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef]
- Mariotti, S.; Venturi, G.; Chiantore, M.V.; Teloni, R.; De Santis, R.; Amendola, A.; Fortuna, C.; Marsili, G.; Grilli, G.; Lia, M.S.; et al. Antibodies Induced by Smallpox Vaccination after at Least 45 Years Cross-React with and In Vitro Neutralize Mpox Virus: A Role for Polyclonal B Cell Activation? Viruses 2024, 16, 620. [Google Scholar] [CrossRef]
- Matz, H.C.; McIntire, K.M.; Ellebedy, A.H. ‘Persistent germinal center responses: Slow-growing trees bear the best fruits’. Curr. Opin. Immunol. 2023, 83, 102332. [Google Scholar] [CrossRef]
- Berek, C.; Berger, A.; Apel, M. Maturation of the immune response in germinal centers. Cell 1991, 67, 1121–1129. [Google Scholar] [CrossRef]
- Schwickert, T.A.; Lindquist, R.L.; Shakhar, G.; Livshits, G.; Skokos, D.; Kosco-Vilbois, M.H.; Dustin, M.L.; Nussenzweig, M.C. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 2007, 446, 83–87. [Google Scholar] [CrossRef]
- Tipton, C.M.; Fucile, C.F.; Darce, J.; Chida, A.; Ichikawa, T.; Gregoretti, I.; Schieferl, S.; Hom, J.; Jenks, S.; Feldman, R.J.; et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 2015, 16, 755–765. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Haddad, N.S.; Anam, F.A.; Rudolph, M.E.; Walker, T.A.; Truong, A.D.; Dixit, A.N.; Han, J.E.; Cabrera-Mora, M.; et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 2022, 611, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157.e13. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Woodruff, M.C.; Kim, E.H.; Nam, J.H. Knife’s edge: Balancing immunogenicity and reactogenicity in mRNA vaccines. Exp. Mol. Med. 2023, 55, 1305–1313. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Guerrera, G.; Picozza, M.; D’Orso, S.; Placido, R.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Giannessi, F.; Sambucci, M.; et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021, 6, eabl5344. [Google Scholar] [CrossRef]
- Underwood, A.P.; Sølund, C.; Jacobsen, K.; Binderup, A.; Fernandez-Antunez, C.; Mikkelsen, L.S.; Inekci, D.; Villadsen, S.L.; Castruita, J.A.S.; Pinholt, M.; et al. Neutralizing antibody and CD8+ T cell responses following BA.4/5 bivalent COVID-19 booster vaccination in adults with and without prior exposure to SARS-CoV-2. Front. Immunol. 2024, 15, 1353353. [Google Scholar] [CrossRef]
- Liu, Y.; Soh, W.T.; Kishikawa, J.I.; Hirose, M.; Nakayama, E.E.; Li, S.; Sasai, M.; Suzuki, T.; Tada, A.; Arakawa, A.; et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell 2021, 184, 3452–3466.e18. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kam, Y.W.; Kien, F.; Roberts, A.; Cheung, Y.C.; Lamirande, E.W.; Vogel, L.; Chu, S.L.; Tse, J.; Guarner, J.; Zaki, S.R.; et al. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcgammaRII-dependent entry into B cells in vitro. Vaccine 2007, 25, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Sun, S.; Tai, W.; Chen, J.; Geng, Q.; He, L.; Chen, Y.; Wu, J.; Shi, Z.; et al. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J. Virol. 2020, 94, e02015–e02019. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillon, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat. Med. 2024. [Google Scholar] [CrossRef]
- Tehrani, Z.R.; Habibzadeh, P.; Flinko, R.; Chen, H.; Abbasi, A.; Yared, J.A.; Ciupe, S.M.; Lewis, G.K.; Sajadi, M.M. Deficient Generation of Spike-Specific Long-Lived Plasma Cells in the Bone Marrow After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2024, 230, e30–e33. [Google Scholar] [CrossRef]
- Serwanga, J.; Ankunda, V.; Katende, J.S.; Baine, C.; Oluka, G.K.; Odoch, G.; Nantambi, H.; Mugaba, S.; Namuyanja, A.; Ssali, I.; et al. Sustained S-IgG and S-IgA antibodies to Moderna’s mRNA-1273 vaccine in a Sub-Saharan African cohort suggests need for booster timing reconsiderations. Front. Immunol. 2024, 15, 1348905. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e4. [Google Scholar] [CrossRef]
- Fumagalli, V.; Ravà, M.; Marotta, D.; Di Lucia, P.; Bono, E.B.; Giustini, L.; De Leo, F.; Casalgrandi, M.; Monteleone, E.; Mouro, V.; et al. Antibody-independent protection against heterologous SARS-CoV-2 challenge conferred by prior infection or vaccination. Nat. Immunol. 2024. ahead of print. [Google Scholar] [CrossRef]
- Soresina, A.; Moratto, D.; Chiarini, M.; Paolillo, C.; Baresi, G.; Focà, E.; Bezzi, M.; Baronio, B.; Giacomelli, M.; Badolato, R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020, 31, 565–569. [Google Scholar] [CrossRef]
- Mizera, D.; Dziedzic, R.; Drynda, A.; Gradzikiewicz, A.; Jakieła, B.; Celińska-Löwenhoff, M.; Padjas, A.; Matyja-Bednarczyk, A.; Zaręba, L.; Bazan-Socha, S. Cellular immune response to SARS-CoV-2 in patients with primary antibody deficiencies. Front. Immunol. 2023, 14, 1275892. [Google Scholar] [CrossRef] [PubMed]
- Montero-Escribano, P.; Matías-Guiu, J.; Gómez-Iglesias, P.; Porta-Etessam, J.; Pytel, V.; Matias-Guiu, J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord. 2020, 42, 102185. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- Brasu, N.; Elia, I.; Russo, V.; Montacchiesi, G.; Stabile, S.A.; De Intinis, C.; Fesi, F.; Gizzi, K.; Macagno, M.; Montone, M.; et al. Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat. Immunol. 2022, 23, 1445–1456. [Google Scholar] [CrossRef]
- Dan Jennifer, M.; Mateus, J.; Kato, Y.; Kathryn, M.H.; Esther, D.Y.; Caterina, E.F.; Grifoni, A.; Sydney, I.R.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Piano Mortari, E.; Russo, C.; Vinci, M.R.; Terreri, S.; Fernandez Salinas, A.; Piccioni, L.; Alteri, C.; Colagrossi, L.; Coltella, L.; Ranno, S.; et al. Highly Specific Memory B Cells Generation after the 2nd Dose of BNT162b2 Vaccine Compensate for the Decline of Serum Antibodies and Absence of Mucosal IgA. Cells 2021, 10, 2541. [Google Scholar] [CrossRef]
- Pieren, D.K.J.; Kuguel, S.G.; Rosado, J.; Robles, A.G.; Rey-Cano, J.; Mancebo, C.; Esperalba, J.; Falcó, V.; Buzón, M.J.; Genescà, M. Limited induction of polyfunctional lung-resident memory T cells against SARS-CoV-2 by mRNA vaccination compared to infection. Nat. Commun. 2023, 14, 1887. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Havervall, S.; Marking, U.; Svensson, J.; Greilert-Norin, N.; Bacchus, P.; Nilsson, P.; Hober, S.; Gordon, M.; Blom, K.; Klingström, J.; et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1333–1336. [Google Scholar] [CrossRef]
- Zuo, F.; Marcotte, H.; Hammarström, L.; Pan-Hammarström, Q. Mucosal IgA against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, e55. [Google Scholar] [CrossRef]
- Vashishtha, V.M.; Kumar, P. The durability of vaccine-induced protection: An overview. Expert. Rev. Vaccines 2024, 23, 389–408. [Google Scholar] [CrossRef]
- Le Moli, S.; Matricardi, P.M.; Quinti, I.; Stroffolini, T.; D’Amelio, R. Clonotypic analysis of human antibodies specific for Neisseria meningitidis polysaccharides A and C in adults. Clin. Exp. Immunol. 1991, 83, 460–465. [Google Scholar] [CrossRef]
- Kato, Y.; Abbott, R.K.; Freeman, B.L.; Haupt, S.; Groschel, B.; Silva, M.; Menis, S.; Irvine, D.J.; Schief, W.R.; Crotty, S. Multifaceted Effects of Antigen Valency on B Cell Response Composition and Differentiation In Vivo. Immunity 2020, 53, 548–563.e8. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Gilman, M.S.A.; McLellan, J.S. Structure-Based Vaccine Antigen Design. Annu. Rev. Med. 2019, 70, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Chuang, G.Y.; Lai, Y.T.; Boyington, J.C.; Cheng, C.; Geng, H.; Narpala, S.; Rawi, R.; Schmidt, S.D.; Tsybovsky, Y.; Verardi, R.; et al. Development of a 3Mut-Apex-Stabilized Envelope Trimer That Expands HIV-1 Neutralization Breadth When Used To Boost Fusion Peptide-Directed Vaccine-Elicited Responses. J. Virol. 2020, 94, e00074-20. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Roederer, M.; Morris, L.; Seaman, M.S. Neutralization tiers of HIV-1. Curr. Opin. HIV AIDS 2018, 13, 128–136. [Google Scholar] [CrossRef]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef]
- Orr, M.T.; Khandhar, A.P.; Seydoux, E.; Liang, H.; Gage, E.; Mikasa, T.; Beebe, E.L.; Rintala, N.D.; Persson, K.H.; Ahniyaz, A.; et al. Reprogramming the adjuvant properties of aluminum oxyhydroxide with nanoparticle technology. NPJ Vaccines 2019, 4, 1. [Google Scholar] [CrossRef]
- Billeskov, R.; Beikzadeh, B.; Berzofsky, J.A. The effect of antigen dose on T cell-targeting vaccine outcome. Hum. Vaccin. Immunother. 2019, 15, 407–411. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef]
- Styles, T.M.; Gangadhara, S.; Reddy, P.B.J.; Sahoo, A.; Shiferaw, A.; Welbourn, S.; Kozlowski, P.A.; Derdeyn, C.A.; Velu, V.; Amara, R.R. V2 hotspot optimized MVA vaccine expressing stabilized HIV-1 Clade C envelope Gp140 delays acquisition of heterologous Clade C Tier 2 challenges in Mamu-A*01 negative Rhesus Macaques. Front. Immunol. 2022, 13, 914969. [Google Scholar] [CrossRef]
- Palin, A.C.; Alter, G.; Crotty, S.; Ellebedy, A.H.; Lane, M.C.; Lee, F.E.; Locci, M.; Malaspina, A.; Mallia, C.; McElrath, M.J.; et al. The persistence of memory: Defining, engineering, and measuring vaccine durability. Nat. Immunol. 2022, 23, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandani, S.H.; Yang, L.; Lam, J.H.; Maiorino, L.; Ben-Akiva, E.; Rodrigues, K.A.; Romanov, A.; Suh, H.; Aung, A.; Wu, S.; et al. Two-dose priming immunization amplifies humoral immunity by synchronizing vaccine delivery with the germinal center response. Sci. Immunol. 2024, 9, eadl3755. [Google Scholar] [CrossRef]
- Cheung, F.; Apps, R.; Dropulic, L.; Kotliarov, Y.; Chen, J.; Jordan, T.; Langweiler, M.; Candia, J.; Biancotto, A.; Han, K.L.; et al. Sex and prior exposure jointly shape innate immune responses to a live herpesvirus vaccine. Elife 2023, 12, e80652. [Google Scholar] [CrossRef]
- Posteraro, B.; Pastorino, R.; Di Giannantonio, P.; Ianuale, C.; Amore, R.; Ricciardi, W.; Boccia, S. The link between genetic variation and variability in vaccine responses: Systematic review and meta-analyses. Vaccine 2014, 32, 1661–1669. [Google Scholar] [CrossRef]
- Jordan, A.; Carding, S.R.; Hall, L.J. The early-life gut microbiome and vaccine efficacy. Lancet Microbe. 2022, 3, e787–e794. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Donis, R.O.; Koup, R.A.; Fong, Y.; Plotkin, S.A.; Follmann, D. A COVID-19 Milestone Attained—A Correlate of Protection for Vaccines. N. Engl. J. Med. 2022, 387, 2203–2206. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): A prospective cohort study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef]
- Santoro, A.; Capri, A.; Petrone, D.; Colavita, F.; Meschi, S.; Matusali, G.; Mizzoni, K.; Notari, S.; Agrati, C.; Goletti, D.; et al. SARS-CoV-2 Breakthrough Infections According to the Immune Response Elicited after mRNA Third Dose Vaccination in COVID-19-Naïve Hospital Personnel. Biomedicines 2023, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.; Xu, H.; Polmear, J.; Kealy, L.; Szeto, C.; Pang, E.S.; Gupta, M.; Kirn, A.; Taylor, J.J.; Jackson, K.J.L.; et al. Type I interferons induce an epigenetically distinct memory B cell subset in chronic viral infection. Immunity 2024, 57, 1037–1055.e6. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Frank, F.; Besancon, L.; Samuel, A.; Saada, V.; Billy, E.; Al-Ahmad, A.; Florens, N.; Seitz-Polski, B.; Robert, J. Letter to Editor “Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs”: Important concerns on the validity of this article. Food Chem. Toxicol. 2023, 178, 113897. [Google Scholar] [CrossRef]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Brian, T.; Bremner, M.R.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef]
- Parry, P.I.; Lefringhausen, A.; Turni, C.; Neil, C.J.; Cosford, R.; Hudson, N.J.; Gillespie, J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines 2023, 11, 2287. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Jaycox, J.R.; Lucas, C.; Yildirim, I.; Dai, Y.; Wang, E.Y.; Monteiro, V.; Lord, S.; Carlin, J.; Kita, M.; Buckner, J.H.; et al. SARS-CoV-2 mRNA vaccines decouple anti-viral immunity from humoral autoimmunity. Nat. Commun. 2023, 14, 1299. [Google Scholar] [CrossRef]
- Jung, S.W.; Jeon, J.J.; Kim, Y.H.; Choe, S.J.; Lee, S. Long-term risk of autoimmune diseases after mRNA-based SARS-CoV2 vaccination in a Korean, nationwide, population-based cohort study. Nat. Commun. 2024, 15, 6181. [Google Scholar] [CrossRef]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of anaphylaxis after vaccination in children and adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Anastassopoulou, C.; Hatziantoniou, S.; Poland, G.A.; Tsakris, A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines. Vaccine 2022, 40, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Greenhawt, M.; Shaker, M.; Golden, D.B.K.; Abrams, E.M.; Blumenthal, K.G.; Wolfson, A.R.; Stone, C.A., Jr.; Krantz, M.S.; Chu, D.K.; Dwamena, B.A. Diagnostic accuracy of vaccine and vaccine excipient testing in the setting of allergic reactions to COVID-19 vaccines: A systematic review and meta-analysis. Allergy 2023, 78, 71–83. [Google Scholar] [CrossRef]
- Khalid, M.B.; Frischmeyer-Guerrerio, P.A. The conundrum of COVID-19 mRNA vaccine-induced anaphylaxis. J. Allergy Clin. Immunol. Glob. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Shah, M.M.; Layhadi, J.A.; Hourcade, D.E.; Fulton, W.T.; Tan, T.J.; Dunham, D.; Chang, I.; Vel, M.S.; Fernandes, A.; Lee, A.S.; et al. Elucidating allergic reaction mechanisms in response to SARS-CoV-2 mRNA vaccination in adults. Allergy 2024, 79, 2502–2523. [Google Scholar] [CrossRef]
- Simone, A.; Herald, J.; Chen, A.; Gulati, N.; Shen, A.Y.; Lewin, B.; Lee, M.S. Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern. Med. 2021, 181, 1668–1670. [Google Scholar] [CrossRef]
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Alami, A.; Villeneuve, P.J.; Farrell, P.J.; Mattison, D.; Farhat, N.; Haddad, N.; Wilson, K.; Gravel, C.A.; Crispo, J.A.G.; Perez-Lloret, S.; et al. Myocarditis and Pericarditis Post-mRNA COVID-19 Vaccination: Insights from a Pharmacovigilance Perspective. J. Clin. Med. 2023, 12, 4971. [Google Scholar] [CrossRef] [PubMed]
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis associated with COVID-19 vaccination. NPJ Vaccines 2024, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M’Barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat. Commun. 2022, 13, 25. [Google Scholar] [CrossRef]
- Eckart, R.E.; Love, S.S.; Atwood, J.E.; Arness, M.K.; Cassimatis, D.C.; Campbell, C.L.; Boyd, S.Y.; Murphy, J.G.; Swerdlow, D.L.; Collins, L.C.; et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J. Am. Coll. Cardiol. 2004, 44, 201–205. [Google Scholar] [CrossRef]
- Engler, R.J.; Nelson, M.R.; Collins, L.C., Jr.; Spooner, C.; Hemann, B.A.; Gibbs, B.T.; Atwood, J.E.; Howard, R.S.; Chang, A.S.; Cruser, D.L.; et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE 2015, 10, e0118283. [Google Scholar] [CrossRef]
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef]
- Frasca, L.; Ocone, G.; Palazzo, R. Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases, in Patients with Cardiac Issues, and in the Healthy Population. Pathogens 2023, 12, 233. [Google Scholar] [CrossRef]
- Semenzato, L.; Le Vu, S.; Botton, J.; Bertrand, M.; Jabagi, M.J.; Drouin, J.; Cuenot, F.; Zores, F.; Dray-Spira, R.; Weill, A.; et al. Long-Term Prognosis of Patients With Myocarditis Attributed to COVID-19 mRNA Vaccination, SARS-CoV-2 Infection, or Conventional Etiologies. JAMA 2024, 332, e2416380. [Google Scholar] [CrossRef]
- Jain, S.S.; Anderson, S.A.; Steele, J.M.; Wilson, H.C.; Muniz, J.C.; Soslow, J.H.; Beroukhim, R.S.; Maksymiuk, V.; Jacquemyn, X.; Frosch, O.H.; et al. Cardiac manifestations and outcomes of COVID-19 vaccine-associated myocarditis in the young in the USA: Longitudinal results from the Myocarditis After COVID Vaccination (MACiV) multicenter study. eClinicalMedicine 2024, 76, 102809. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Dahl, R.M.; Prill, M.M.; Meites, E.; Rodriguez-Barradas, M.C.; Marconi, V.C.; Beenhouwer, D.O.; Brown, S.T.; Holodniy, M.; Lucero-Obusan, C.; et al. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization—Five Veterans Affairs Medical Centers, United States, February 1–August 6, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions—United States, March-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Hammerman, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Peretz, A.; Netzer, D.; Yaron, S.; Arbel, R. Effectiveness of the BNT162b2 Vaccine after Recovery from COVID-19. N. Engl. J. Med. 2022, 386, 1221–1229. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.R.; Green, P.K.; Berry, K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: A target trial emulation study in the U.S. Veterans Affairs healthcare system. eClinicalMedicine 2022, 45, 101326. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Patel, M.M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; Gaglani, M.; McNeal, T.; et al. Effectiveness of Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccines for Preventing Coronavirus Disease 2019 Hospitalizations in the United States. Clin. Infect. Dis. 2022, 74, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Bruxvoort, K.J.; Sy, L.S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; Qiu, S.; et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat. Commun. 2023, 14, 189. [Google Scholar] [CrossRef]
- Lin, D.Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764–766. [Google Scholar] [CrossRef]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Uraki, R.; Ito, M.; Furusawa, Y.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Adachi, E.; Saito, M.; Koga, M.; Tsutsumi, T.; Yamamoto, S.; et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect. Dis. 2023, 23, 30–32. [Google Scholar] [CrossRef]
- Muik, A.; Lui, B.G.; Quandt, J.; Diao, H.; Fu, Y.; Bacher, M.; Gordon, J.; Toker, A.; Grosser, J.; Ozhelvaci, O.; et al. Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity. Cell Rep. 2023, 42, 112888. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Follmann, D.; Hachigian, G.; Strout, C.; Overcash, J.S.; Doblecki-Lewis, S.; Whitaker, J.A.; Anderson, E.J.; et al. Long-term safety and effectiveness of mRNA-1273 vaccine in adults: COVE trial open-label and booster phases. Nat. Commun. 2024, 15, 7469. [Google Scholar] [CrossRef]
- Hajnik, R.L.; Plante, J.A.; Reddy Bonam, S.; Rafael, G.H.; Liang, Y.; Hazell, N.C.; Walker, J.; Reyna, R.A.; Walker, D.H.; Alameh, M.G.; et al. Broad protection and respiratory immunity of dual mRNA vaccination against SARS-CoV-2 variants. NPJ Vaccines 2024, 9, 160. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of COVID-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Feng, Y.; Qiu, J.; Zhang, R.; So, H.C. Association of COVID-19 vaccination with risks of hospitalization due to cardiovascular and other diseases: A study using data from the UK Biobank. Int. J. Infect. Dis. 2024, 145, 107080. [Google Scholar] [CrossRef] [PubMed]
- Cezard, G.I.; Denholm, R.E.; Knight, R.; Wei, Y.; Teece, L.; Toms, R.; Forbes, H.J.; Walker, A.J.; Fisher, L.; Massey, J.; et al. Impact of vaccination on the association of COVID-19 with cardiovascular diseases: An OpenSAFELY cohort study. Nat. Commun. 2024, 15, 2173. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ryder, D.; Flavell, R.R.; Wang, Y.; Levi, J.; LaFranchi, B.H.; Deveau, T.M.; Buck, A.M.; Munter, S.E.; Asare, K.A.; et al. Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadk3295. [Google Scholar] [CrossRef]
- Ely, E.W.; Brown, L.M.; Fineberg, H.V.; National Academies of Sciences, Engineering, and Medicine Committee on Examining the Working Definition for Long Covid. Long Covid Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Hernán, M.A.; Lipsitch, M.; Hernandez-Diaz, S.; Reis, B.Y.; et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Simeone, R.M.; Zambrano, L.D.; Halasa, N.B.; Fleming-Dutra, K.E.; Newhams, M.M.; Wu, M.J.; Orzel-Lockwood, A.O.; Kamidani, S.; Pannaraj, P.S.; Irby, K.; et al. Effectiveness of Maternal mRNA COVID-19 Vaccination During Pregnancy Against COVID-19-Associated Hospitalizations in Infants Aged <6 Months During SARS-CoV-2 Omicron Predominance—20 States, March 9, 2022–May 31, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1057–1064. [Google Scholar] [CrossRef]
- D’Amelio, R.; Asero, R.; Cassatella, M.A.; Laganà, B.; Lunardi, C.; Migliorini, P.; Nisini, R.; Parronchi, P.; Quinti, I.; Racanelli, V.; et al. Anti-COVID-19 Vaccination in Patients with Autoimmune-Autoinflammatory Disorders and Primary/Secondary Immunodeficiencies: The Position of the Task Force on Behalf of the Italian Immunological Societies. Biomedicines 2021, 9, 1163. [Google Scholar] [CrossRef]
- Finckh, A.; Ciurea, A.; Raptis, C.E.; Rubbert-Roth, A. Susceptibility to COVID-19 and Immunologic Response to Vaccination in Patients With Immune-Mediated Inflammatory Diseases. J. Infect. Dis. 2023, 228 (Suppl. S1), S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Frommert, L.M.; Arumahandi de Silva, A.N.; Zernicke, J.; Scholz, V.; Braun, T.; Jeworowski, L.M.; Schwarz, T.; Tober-Lau, P.; Ten Hagen, A.; Habermann, E.; et al. Type of vaccine and immuno-suppressive therapy but not diagnosis critically influence antibody response after COVID-19 vaccination in patients with rheumatic disease. RMD Open 2022, 8, e002650. [Google Scholar] [CrossRef]
- Gerosa, M.; Schioppo, T.; Argolini, L.M.; Sciascia, S.; Ramirez, G.A.; Moroni, G.; Sinico, R.A.; Bonelli, G.; Alberici, F.; Mescia, F.; et al. The Impact of Anti-SARS-CoV-2 Vaccine in Patients with Systemic Lupus Erythematosus: A Multicentre Cohort Study. Vaccines 2022, 10, 663. [Google Scholar] [CrossRef]
- Pinte, L.; Negoi, F.; Ionescu, G.D.; Caraiola, S.; Balaban, D.V.; Badea, C.; Mazilu, D.; Dumitrescu, B.; Mateescu, B.; Ionescu, R.; et al. COVID-19 Vaccine Does Not Increase the Risk of Disease Flare-Ups among Patients with Autoimmune and Immune-Mediated Diseases. J. Pers. Med. 2021, 11, 1283. [Google Scholar] [CrossRef]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Kroger, A.; et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases: Version 5. Arthritis Rheumatol. 2023, 75, E1–E16. [Google Scholar] [CrossRef]
- Landewé, R.B.M.; Kroon, F.P.B.; Alunno, A.; Najm, A.; Bijlsma, J.W.; Burmester, G.R.; Caporali, R.; Combe, B.; Conway, R.; Curtis, J.R.; et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: The November 2021 update. Ann. Rheum. Dis. 2022, 81, 1628–1639. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Bieber, A.; Sagy, I.; Novack, L.; Brikman, S.; Abuhasira, R.; Ayalon, S.; Novofastovski, I.; Abu-Shakra, M.; Mader, R. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: A national cohort study. Ann. Rheum. Dis. 2022, 81, 1028–1035. [Google Scholar] [CrossRef]
- Kim, W.J.; Choi, S.H.; Park, J.Y.; Song, J.S.; Chung, J.W.; Choi, S.T. SARS-CoV-2 Omicron escapes mRNA vaccine booster-induced antibody neutralisation in patients with autoimmune rheumatic diseases: An observational cohort study. Ann. Rheum. Dis. 2022, 81, 1585–1593. [Google Scholar] [CrossRef]
- Mena-Vázquez, N.; García-Studer, A.; Rojas-Gimenez, M.; Romero-Barco, C.M.; Manrique-Arija, S.; Mucientes, A.; Velloso-Feijoo, M.L.; Godoy-Navarrete, F.J.; Morales-Garrido, P.; Redondo-Rodríguez, R.; et al. Importance of Vaccination against SARS-CoV-2 in Patients with Interstitial Lung Disease Associated with Systemic Autoimmune Disease. J. Clin. Med. 2022, 11, 2437. [Google Scholar] [CrossRef] [PubMed]
- Sieiro Santos, C.; Calleja Antolin, S.; Moriano Morales, C.; Garcia Herrero, J.; Diez Alvarez, E.; Ramos Ortega, F.; Ruiz de Morales, J.G. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open 2022, 8, e001898. [Google Scholar] [CrossRef] [PubMed]
- Syversen, S.W.; Jyssum, I.; Tveter, A.T.; Tran, T.T.; Sexton, J.; Provan, S.A.; Mjaaland, S.; Warren, D.J.; Kvien, T.K.; Grødeland, G.; et al. Immunogenicity and Safety of Standard and Third-Dose SARS-CoV-2 Vaccination in Patients Receiving Immunosuppressive Therapy. Arthritis Rheumatol. 2022, 74, 1321–1332. [Google Scholar] [CrossRef]
- Simon, D.; Tascilar, K.; Fagni, F.; Kleyer, A.; Krönke, G.; Meder, C.; Dietrich, P.; Orlemann, T.; Mößner, J.; Taubmann, J.; et al. Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: A prospective cohort study. Lancet Rheumatol. 2022, 4, e614–e625. [Google Scholar] [CrossRef]
- Lang, K. What do we know about covid in immunocompromised people? BMJ 2023, 383, 1612. [Google Scholar] [CrossRef]
- Fung, M.; Babik, J.M. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin. Infect. Dis. 2021, 72, 340–350. [Google Scholar] [CrossRef]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health Eur. 2023, 35, 100747. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Goodyear, C.S.; Patel, A.; Barnes, E.; Willicombe, M.; Siebert, S.; de Silva, T.I.; Snowden, J.A.; Lim, S.H.; Bowden, S.J.; Billingham, L.; et al. Immunogenicity of third dose COVID-19 vaccine strategies in patients who are immunocompromised with suboptimal immunity following two doses (OCTAVE-DUO): An open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Rheumatol. 2024, 6, e339–e351. [Google Scholar] [CrossRef]
- Kavikondala, S.; Haeussler, K.; Wang, X.; Spellman, A.; Bausch-Jurken, M.T.; Sharma, P.; Amiri, M.; Krivelyova, A.; Vats, S.; Nassim, M.; et al. Immunogenicity of mRNA-1273 and BNT162b2 in Immunocompromised Patients: Systematic Review and Meta-analysis Using GRADE. Infect. Dis. Ther. 2024, 13, 1419–1438. [Google Scholar] [CrossRef]
- Quek, A.M.L.; Wang, S.; Teng, O.; Shunmuganathan, B.; Er, B.G.C.; Mahmud, N.F.B.; Ng, I.X.Q.; Gupta, R.; Tan, I.S.L.; Tan, N.Y.; et al. Hybrid immunity augments cross-variant protection against COVID-19 among immunocompromised individuals. J. Infect. 2024, 89, 106238. [Google Scholar] [CrossRef] [PubMed]
- Gram, M.A.; Thiesson, E.M.; Pihlström, N.; Perälä, J.; Poukka, E.; Leino, T.; Ljung, R.; Andersson, N.W.; Hviid, A. Comparative effectiveness of bivalent BA.4-5 or BA.1 mRNA booster vaccines among immunocompromised individuals across three Nordic countries: A nationwide cohort study. J. Infect. 2024, 89, 106261. [Google Scholar] [CrossRef] [PubMed]
- Reeg, D.B.; Hofmann, M.; Neumann-Haefelin, C.; Thimme, R.; Luxenburger, H. SARS-CoV-2-Specific T Cell Responses in Immunocompromised Individuals with Cancer, HIV or Solid Organ Transplants. Pathogens 2023, 12, 244. [Google Scholar] [CrossRef]
- Sabbagh, S.E.; Haribhai, D.; Gershan, J.A.; Verbsky, J.; Nocton, J.; Yassai, M.; Naumova, E.N.; Hammelev, E.; Dasgupta, M.; Yan, K.; et al. Patients with juvenile idiopathic arthritis have decreased clonal diversity in the CD8+ T cell repertoire response to influenza vaccination. Front. Immunol. 2024, 15, 1306490. [Google Scholar] [CrossRef]
- Paganelli, R. When Cell-Mediated Immunity after Vaccination Is Important. Pathogens 2024, 13, 65. [Google Scholar] [CrossRef]
- Brook, B.; Duval, V.; Barman, S.; Speciner, L.; Sweitzer, C.; Khanmohammed, A.; Menon, M.; Foster, K.; Ghosh, P.; Abedi, K.; et al. Adjuvantation of a SARS-CoV-2 mRNA vaccine with controlled tissue-specific expression of an mRNA encoding IL-12p70. Sci. Transl. Med. 2024, 16, eadm8451. [Google Scholar] [CrossRef] [PubMed]
- Kopera, E.; Czajka, H.; Zapolnik, P.; Mazur, A. New Insights on Respiratory Syncytial Virus Prevention. Vaccines 2023, 11, 1797. [Google Scholar] [CrossRef]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222 (Suppl. S7), S577–S583. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults. J. Infect. Dis. 2024, 230, e647–e656. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)#:~:text=There%20are%20around%20a%20billion,650%20000%20respiratory%20deaths%20annually (accessed on 4 February 2024).
- Shtyrya, Y.A.; Mochalova, L.V.; Bovin, N.V. Influenza virus neuraminidase: Structure and function. Acta Naturae 2009, 1, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef]
- Do, T.H.T.; Wheatley, A.K.; Kent, S.J.; Koutsakos, M. Influenza B virus neuraminidase: A potential target for next-generation vaccines? Expert. Rev. Vaccines 2024, 23, 39–48. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Saha, S.; Chadha, M.; Shu, Y.; Group of Asian Researchers on Influenza (GARI). Divergent seasonal patterns of influenza types A and B across latitude gradient in Tropical Asia. Influenza Other Respir. Viruses 2016, 10, 176–184. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.G.; Lévy, J.P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Zhang, A.; Chaudhari, H.; Agung, Y.; D’Agostino, M.R.; Ang, J.C.; Tugg, Y.; Miller, M.S. Hemagglutinin stalk-binding antibodies enhance effectiveness of neuraminidase inhibitors against influenza via Fc-dependent effector functions. Cell Rep. Med. 2022, 3, 100718. [Google Scholar] [CrossRef]
- McMahon, M.; O’Dell, G.; Tan, J.; Sárközy, A.; Vadovics, M.; Carreño, J.M.; Puente-Massaguer, E.; Muramatsu, H.; Bajusz, C.; Rijnink, W.; et al. Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses. Proc. Natl. Acad. Sci. USA 2022, 119, e2206333119. [Google Scholar] [CrossRef]
- Pardi, N.; Carreño, J.M.; O’Dell, G.; Tan, J.; Bajusz, C.; Muramatsu, H.; Rijnink, W.; Strohmeier, S.; Loganathan, M.; Bielak, D.; et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 2022, 13, 4677. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef]
- Fierro, C.; Brune, D.; Shaw, M.; Schwartz, H.; Knightly, C.; Lin, J.; Carfi, A.; Natenshon, A.; Kalidindi, S.; Reuter, C.; et al. Safety and Immunogenicity of a Messenger RNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results From a Phase 1 Randomized Clinical Trial. J. Infect. Dis. 2024, 230, e668–e678. [Google Scholar] [CrossRef]
- Panther, L.; Basnet, S.; Fierro, C.; Brune, D.; Leggett, R.; Peterson, J.; Pickrell, P.; Lin, J.; Wu, K.; Lee, H.; et al. 2892. Safety and Immunogenicity of mRNA-1647, an mRNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results of a Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose-Finding Trial. Open Forum Infect Dis. 2023, 10 (Suppl. S2), ofad500.2475. [Google Scholar] [CrossRef]
- Hu, X.; Karthigeyan, K.P.; Herbek, S.; Valencia, S.M.; Jenks, J.A.; Webster, H.; Miller, I.G.; Connors, M.; Pollara, J.; Andy, C.; et al. Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine. J. Infect. Dis. 2024, 230, 455–466. [Google Scholar] [CrossRef]
- Poland, G.A.; Kennedy, R.B.; Ovsyannikova, I.G.; Palacios, R.; Ho, P.L.; Kalil, J. Development of vaccines against Zika virus. Lancet Infect. Dis. 2018, 18, e211–e219. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- CDC. Pneumocystis pneumonia—Los Angeles. MMWR 1981, 30, 250–252. [Google Scholar]
- Global HIV & AIDS Statistics Fact Sheet. UNAIDS. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 30 September 2024).
- Reardon, S. Third patient free of HIV after receiving virus-resistant cells. Nature 2023, 615, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Morbach, H.; Eichhorn, E.M.; Liese, J.G.; Girschick, H.J. Reference values for B cell subpopulations from infancy to adulthood. Clin. Exp. Immunol. 2010, 162, 271–279. [Google Scholar] [CrossRef]
- Jardine, J.G.; Kulp, D.W.; Havenar-Daughton, C.; Sarkar, A.; Briney, B.; Sok, D.; Sesterhenn, F.; Ereño-Orbea, J.; Kalyuzhniy, O.; Deresa, I.; et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016, 351, 1458–1463. [Google Scholar] [CrossRef]
- Leggat, D.J.; Cohen, K.W.; Willis, J.R.; Fulp, W.J.; decamp, A.C.; Kalyuzhniy, O.; Cottrell, C.A.; Menis, S.; Finak, G.; Ballweber-Fleming, L.; et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 2022, 378, eadd6502. [Google Scholar] [CrossRef]
- Cohen, K.W.; De Rosa, S.C.; Fulp, W.J.; decamp, A.C.; Fiore-Gartland, A.; Mahoney, C.R.; Furth, S.; Donahue, J.; Whaley, R.E.; Ballweber-Fleming, L.; et al. A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses. Sci. Transl. Med. 2023, 15, eadf3309. [Google Scholar] [CrossRef]
- Sanders, R.W.; Moore, J.P. Progress on priming HIV-1 immunity. Science 2024, 384, 738–739. [Google Scholar] [CrossRef]
- Ray, R.; Schiffner, T.; Wang, X.; Yan, Y.; Rantalainen, K.; Lee, C.D.; Parikh, S.; Reyes, R.A.; Dale, G.A.; Lin, Y.C.; et al. Affinity gaps among B cells in germinal centers drive the selection of MPER precursors. Nat. Immunol. 2024, 25, 1083–1096. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, Q.; Zeng, M.S.; Zhang, X. Prophylactic vaccines against Epstein-Barr virus. Lancet 2024, 404, 845. [Google Scholar] [CrossRef]
- Damania, B.; Kenney, S.C.; Raab-Traub, N. Epstein-Barr virus: Biology and clinical disease. Cell 2022, 185, 3652–3670. [Google Scholar] [CrossRef] [PubMed]
- Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec 2014, 89, 265–287.
- Oliver, S.L.; Yang, E.; Arvin, A.M. Varicella-Zoster Virus Glycoproteins: Entry, Replication, and Pathogenesis. Curr. Clin. Microbiol. Rep. 2016, 3, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Echeverria Proano, D.A.; Zhu, F.; Sun, X.; Zoco, J.; Soni, J.; Parmar, N.; Ali, S.O.; Zoster-076 Study Group. Efficacy, reactogenicity, and safety of the adjuvanted recombinant zoster vaccine for the prevention of herpes zoster in Chinese adults ≥ 50 years: A randomized, placebo-controlled trial. Hum. Vaccin. Immunother. 2024, 20, 2351584. [Google Scholar] [CrossRef]
- August, A.; Shaw, C.A.; Lee, H.; Knightly, C.; Kalidindia, S.; Chu, L.; Essink, B.J.; Seger, W.; Zaks, T.; Smolenov, I.; et al. Safety and Immunogenicity of an mRNA-Based Human Metapneumovirus and Parainfluenza Virus Type 3 Combined Vaccine in Healthy Adults. Open Forum Infect. Dis. 2022, 9, ofac206. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Deloria-Knoll, M.; Madhi, S.A.; Cohen, C.; Ali, A.; Basnet, S.; Bassat, Q.; Brooks, W.A.; Chittaganpitch, M.; et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2021, 9, e33–e43. [Google Scholar] [CrossRef]
- Shaw, C.A.; August, A.; Bart, S.; Booth, P.J.; Knightly, C.; Brasel, T.; Weaver, S.C.; Zhou, H.; Panther, L. A phase 1, randomized, placebo-controlled, dose-ranging study to evaluate the safety and immunogenicity of an mRNA-based chikungunya virus vaccine in healthy adults. Vaccine 2023, 41, 3898–3906. [Google Scholar] [CrossRef]
- Rodrigue, V.; Gravagna, K.; Yao, J.; Nafade, V.; Basta, N.E. Current progress towards prevention of Nipah and Hendra disease in humans: A scoping review of vaccine and monoclonal antibody candidates being evaluated in clinical trials. Trop. Med. Int. Health 2024, 29, 354–364. [Google Scholar] [CrossRef]
- Rabies. Available online: https://www.who.int/health-topics/rabies#tab=tab_1 (accessed on 29 January 2024).
- Aldrich, C.; Leroux-Roels, I.; Huang, K.B.; Bica, M.A.; Loeliger, E.; Schoenborn-Kellenberger, O.; Walz, L.; Leroux-Roels, G.; von Sonnenburg, F.; Oostvogels, L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef]
- Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 29 January 2024).
- Whitley, R.; Kimberlin, D.W.; Prober, C.G. Pathogenesis and Disease. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; Chapter 32. Available online: https://www.ncbi.nlm.nih.gov/books/NBK47449/ (accessed on 10 September 2024).
- Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 1 February 2024).
- Focosi, D. From Co-Administration to Co-Formulation: The Race for New Vaccines against COVID-19 and Other Respiratory Viruses. Vaccines 2023, 11, 109. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.-S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e411. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O.; Lee, E.; Parks, R.; Martinez, D.R.; Li, D.; Chen, H.; Edwards, R.J.; Gobeil, S.; Barr, M.; Mansouri, K.; et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature 2021, 594, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Peng, Y.; Zhang, L.; Mok, C.K.; Zhao, S.; Li, A.; Ching, J.Y.; Liu, Y.; Yan, S.; Chan, D.L.S.; et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 2022, 71, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Bladh, O.; Aguilera, K.; Marking, U.; Kihlgren, M.; Greilert Norin, N.; Smed-Sörensen, A.; Sällberg Chen, M.; Klingström, J.; Blom, K.; Russell, M.W.; et al. Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum. Front. Immunol. 2024, 15, 1346749. [Google Scholar] [CrossRef]
- Zhang, Y.; Chamblee, M.; Xu, J.; Qu, P.; Shamseldin, M.M.; Yoo, S.J.; Misny, J.; Thongpan, I.; Kc, M.; Hall, J.M.; et al. Three SARS-CoV-2 spike protein variants delivered intranasally by measles and mumps vaccines are broadly protective. Nat. Commun. 2024, 15, 5589. [Google Scholar] [CrossRef]


| Types of Vaccines | ||||
|---|---|---|---|---|
| Live, Attenuated | Inactivated | Subunits | mRNA | |
| Development time * | Years | Years | Years | Months |
| Production cost | High | High | High | Low |
| Infection risk in vaccinees | Yes | No | No | No |
| Infection risk during manufacturing | Yes | Yes | Yes | No |
| Risk of integration | NA | NA | NA | Very low |
| Induced immunity | C/H | H | H | C/H |
| Safety | Caution in ID | Satisfactory | Satisfactory | Satisfactory |
| Efficacy | Generally high | Variable | Variable | H/S |
| Need for adjuvants | No | Yes | Yes | No |
| WHO ° | Pango @ | GISAID # | Nextstrain ^ | Documented † | Designation ‡ | S mut ª | Infect. ˜ | Evasion ˢ | VOC ˪ |
|---|---|---|---|---|---|---|---|---|---|
| Alpha | B.1.1.7 | GRY * | 20I/501Y.V1 | Sep. 2020 UK ˫ | 18 Dec. 2020 | 8 | 29% | >2-fold | 9 March 22 |
| Beta | B.1.351 | GH/501Y.V2 | 20H/501Y.V2 | May 2020 SA ˹ | 18 Dec. 2020 | 8 | 25% | >8-fold | 9 March 22 |
| Gamma | P.1 | GR/501Y.V3 | 20J/501Y.V3 | Nov. 2020 BR ⊕ | 11 Jan. 2021 | 12 | 38% | NA | 9 March 22 |
| Delta | B.1.617.2 | G/452R.V3 | 21A/S:478K | Oct 2020 IN ⊗ | 11 May 2021 | 9 | 97% | >5-fold | 7 June 22 |
| Omicron | B.1.1.529 | GR/484A | 21K | Nov. 2021 | 26 Nov. 2021 | >30 | NA | 22-fold | 14 March 23 |
| Project/Company | Encoded Antigen | Composition | Delivery System | Efficacy | Safety |
|---|---|---|---|---|---|
| Pfizer-BNT162b2 | SARS-CoV2 Spike protein (S1 and S2) | mRNA 30 μg/dose | LNPs | 95% protection | Good; slight increase in anaphylaxis myo/pericarditis in real-world studies |
| Moderna mRNA-1273 | SARS-CoV2 Spike protein (S1 and S2) | mRNA 100 μg/dose | LNPs | 94.1% protection | Good; slight increase in anaphylaxis myo/pericarditis in real-world studies |
| Vaccine-Related | Type of Exerted Influence |
|---|---|
| Platform | Live attenuated, VLP, and mRNA are the most effective platforms |
| Molecular conformation | Proteins are more effective than polysaccharide antigens—particulate and prefusion antigens are highly stimulating. Antibodies to the HIV trimer enveloped in closed conformation are broadly protective—adjuvants |
| Dose of antigen | The dose of antigen may modulate the recruitment of high-affinity antibodies and T-cell subpopulations |
| Schedule | ID and IM may differently modulate humoral and cellular immunity—higher intervals and heterologous prime-boost more effectively |
| Age | Elderly people are less responsive |
| Gender | Males are less responsive |
| State of immunity | Primary and secondary immunodeficiencies may be less responsive |
| Genetics | Human leucocyte antigens may influence T-cell responses significantly |
| Gut microbiota | It may influence the immunological response to vaccines |
| Adverse Event | Rate in mRNA Vaccines | Rate in Traditional Vaccines | Characteristics in mRNA Vaccines |
|---|---|---|---|
| Anaphylaxis | 2.5–4.7/106 doses | 1.31/106 doses | Young females, first dose |
| Myopericarditis | 21.3 (106.9)/106 persons * | 124/106 persons for smallpox vaccine | Young males, second dose |
| Viral Strains | Effectiveness Early After II Dose | Reference |
|---|---|---|
| Ancestral and Alpha | 92–94% against infection; 87% against hospitalization; 92% against SD | [179] |
| Ancestral and Alpha | 90% against symptomatic/asymptomatic breakthrough infections | [180] |
| Alpha, Beta, Delta | 87% (80% ≥ 65 years—95% 18–64 years) against COVID-19 hospitalization | [181] |
| Alpha, Beta, Delta | 88% (BNT162b2) and 93% (mRNA-1273) against COVID-19 hospitalization | [182] |
| Beta and Delta | 77.5%, waning to 20% at 5–7 months; against SD 96% for 6 months | [183] |
| VOCs except Omicron | Delta: 93%, then 53% at 4th month; non-Delta: 97%, then 67% at 4–5 months | [184] |
| Omicron/subvariants | Specific humoral immunity: very reduced; specific cellular immunity: maintained | [191,195] |
| Project/ Company | Encoded Antigen | Composition | Delivery System | Phase | Efficacy/ Immunogenicity | Safety |
|---|---|---|---|---|---|---|
| mRNA-1345 Moderna | RSV F prefusion glycoprotein | mRNA 50 μg | LNPs | FDA/EMA-approved | 82.4–83.7% | Good in Phase 1 |
| mRNA-1851 Moderna | Influenza A H7N9 | mRNA 25-50-75-100-400 μg | LNPs | Phase 1 | Robust humoral immune response | Good |
| mRNA-1440 Moderna | Influenza A H10N8 | mRNA 10-25-50 μg | LNPs | Phase 1 | Robust humoral immune response | Good |
| PF-07252220 Pfizer | Quadrivalent A-B Flu strains | NA | LNPs | Phase 3 | Documented specific CD4+ and CD8+ response | Good |
| CVSQIV GSK/CureVac | Quadrivalent A-B Flu strains | NA | NA | Phase 1 | NA | NA |
| SP0273/MRT5407 Sanofi- Pasteur | Quadrivalent A-B Flu strains | NA | NA | Phase 1 | NA | NA |
| mRNA-1647 Moderna | CMV 6 mRNA * | mRNA 50 μg | LNPs | Phase 3 | Good, humoral, and cellular, in Phases 1–2 | Good in Phases 1–2 |
| mRNA-1644 Moderna | eOD-GT8 60-mer | NA | LNPs | Phase 1 | NA | NA |
| mRNA-1893 Moderna | Zika prM-E | mRN 10-30-100-250 μg | LNPs | Phase 2 | Strong, persistent nAbs with all the doses in Phase 1 | Good in Phase 1 |
| mRNA-1189 Moderna | EBV gH, gL, gB, and gp42 | NA | LNPs | Phase 1 | NA | NA |
| VZV modRNA Pfizer/BioNTech | VZV glycoprotein E | NA | NA | Phase 1/2 | NA | NA |
| mRNA-1653 Moderna | hMPV/PIV3 | NA | LNPs | Phase 1 | NA | NA |
| mRNA-1388 Moderna | Chikungunya E3, E2, 6 k/TF, and E1 | mRNA 25-50-100 μg | LNPs | Phase 1 | High-titer nAbs lasting 1 year | Good |
| mRNA-1215 Moderna | Nipah | mRNA 25-50-100 μg | LNPs | Phase 1 | NA | NA |
| CV7202 CureVac | RABV-G | 1 or 2 μg/dose | LNPs | Phase 1 | Good—all vaccinees had nAbs ≥ 0.5 IU/ml | Good |
| BNT163 BioNTech | HSV 1-2 | NA | LNPs | Phase 1 | NA | NA |
| BNT164 BioNTech-BMGF | Tuberculosis | NA | LNPs | Phase 1 | NA | NA |
| BNT165b1 BioNTech | Malaria | mRNA PfCSP | LNPs | Phase 1 | NA | NA |
| mRNA-1075 Moderna | Quadrivalent Influenza/ SARS-CoV-2 | NA | LNPs | Phase 1/2 | NA | NA |
| mRNA-1045 mRNA-1230 Moderna | Influenza/RSV Influenza/RSV/ SARS-CoV-2 | NA NA | LNPs | Phase 1 | NA | NA |
| PF-07252220 Pfizer- BioNTech | Quadrivalent Influenza/ SARS-CoV-2 | NA | NA | Phase 1 | NA | NA |
| Sanofi Pasteur | RSV/hMPV | NA | LNPs | Phase 1 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandi, R.; Paganelli, A.; D’Amelio, R.; Giuliani, P.; Lista, F.; Salemi, S.; Paganelli, R. mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases. Vaccines 2024, 12, 1418. https://doi.org/10.3390/vaccines12121418
Brandi R, Paganelli A, D’Amelio R, Giuliani P, Lista F, Salemi S, Paganelli R. mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases. Vaccines. 2024; 12(12):1418. https://doi.org/10.3390/vaccines12121418
Chicago/Turabian StyleBrandi, Rossella, Alessia Paganelli, Raffaele D’Amelio, Paolo Giuliani, Florigio Lista, Simonetta Salemi, and Roberto Paganelli. 2024. "mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases" Vaccines 12, no. 12: 1418. https://doi.org/10.3390/vaccines12121418
APA StyleBrandi, R., Paganelli, A., D’Amelio, R., Giuliani, P., Lista, F., Salemi, S., & Paganelli, R. (2024). mRNA Vaccines Against COVID-19 as Trailblazers for Other Human Infectious Diseases. Vaccines, 12(12), 1418. https://doi.org/10.3390/vaccines12121418







