Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook
Abstract
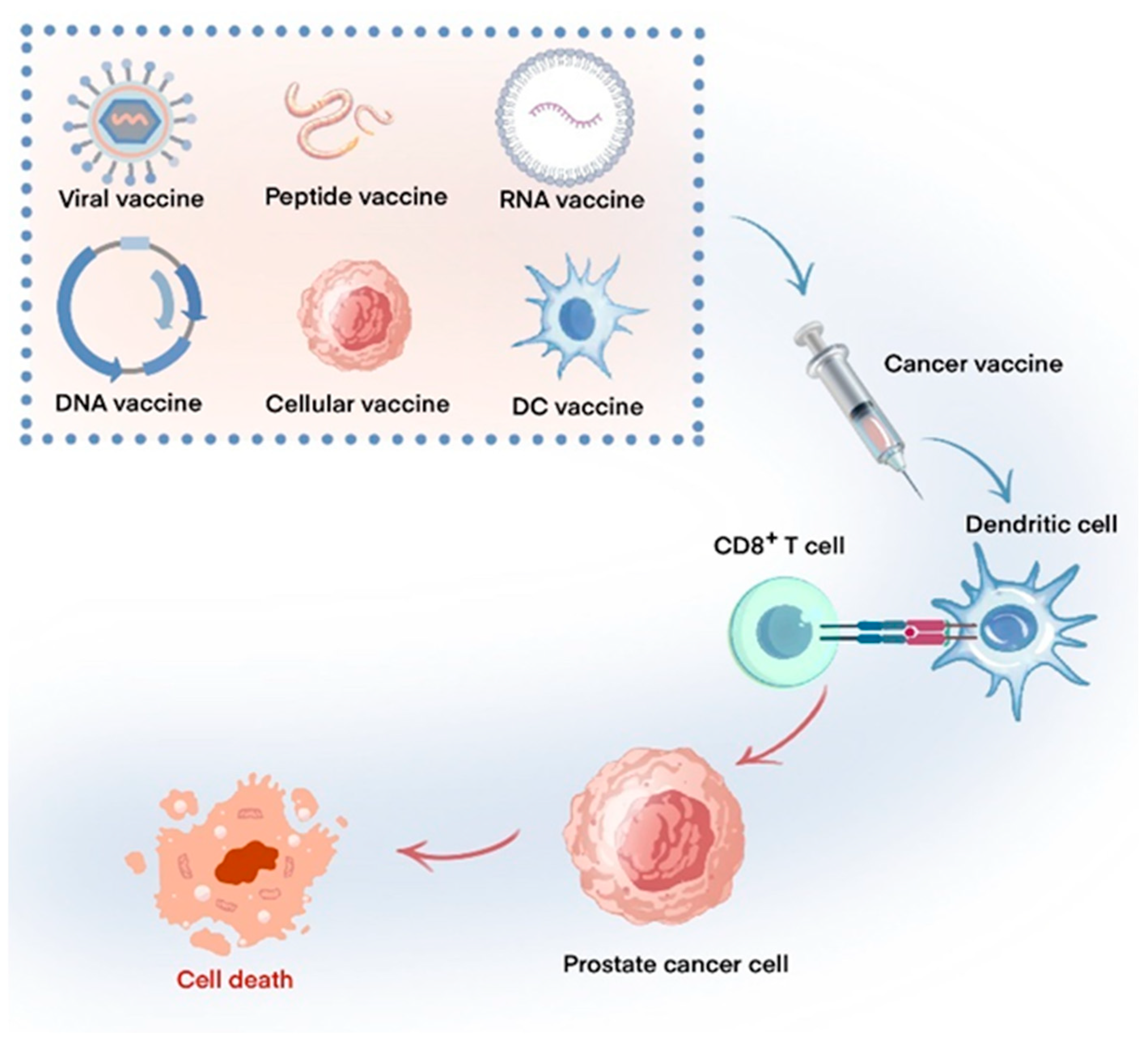
1. Introduction
2. Dendritic Cell Vaccines
3. Cellular Vaccines
4. Peptide Vaccines
5. Nucleic Acid Vaccines
5.1. DNA Vaccine
5.2. RNA Vaccine
6. Viral and Bacterial Vaccines
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Merseburger, A.S.; Alcaraz, A.; von Klot, C.A. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther. 2016, 9, 7263–7274. [Google Scholar] [CrossRef]
- Garcia, J.A.; Rini, B.I. Castration-resistant prostate cancer: Many treatments, many options, many challenges ahead. Cancer 2012, 118, 2583–2593. [Google Scholar] [CrossRef]
- Gillessen, S.; Armstrong, A.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Bulbul, M.; et al. Management of Patients with Advanced Prostate Cancer: Report from the Advanced Prostate Cancer Consensus Conference 2021. Eur. Urol. 2022, 82, 115–141. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Sellars, M.C.; Wu, C.J.; Fritsch, E.F. Cancer vaccines: Building a bridge over troubled waters. Cell 2022, 185, 2770–2788. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Pe’er, D.; Ogawa, S.; Elhanani, O.; Keren, L.; Oliver, T.G.; Wedge, D. Tumor heterogeneity. Cancer Cell 2021, 39, 1015–1017. [Google Scholar] [CrossRef]
- Sridaran, D.; Bradshaw, E.; DeSelm, C.; Pachynski, R.; Mahajan, K.; Mahajan, N.P. Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach. Cell Rep. Med. 2023, 4, 101199. [Google Scholar] [CrossRef]
- Sumanasuriya, S.; De Bono, J. Treatment of Advanced Prostate Cancer-A Review of Current Therapies and Future Promise. Cold Spring Harb Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol 2019, 10, 603. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Runcie, K.D.; Dallos, M.C. Prostate Cancer Immunotherapy-Finally in From the Cold? Curr. Oncol. Rep. 2021, 23, 88. [Google Scholar] [CrossRef]
- Claps, M.; Mennitto, A.; Guadalupi, V.; Sepe, P.; Stellato, M.; Zattarin, E.; Gillessen, S.S.; Sternberg, C.N.; Berruti, A.; De Braud, F.G.M.; et al. Immune-checkpoint inhibitors and metastatic prostate cancer therapy: Learning by making mistakes. Cancer Treat. Rev. 2020, 88, 102057. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- McKay, R.R.; Hafron, J.M.; Ferro, C.; Wilfehrt, H.M.; Fitch, K.; Flanders, S.C.; Fabrizio, M.D.; Schweizer, M.T. A Retrospective Observational Analysis of Overall Survival with Sipuleucel-T in Medicare Beneficiaries Treated for Advanced Prostate Cancer. Adv. Ther. 2020, 37, 4910–4929. [Google Scholar] [CrossRef]
- Schellhammer, P.F.; Chodak, G.; Whitmore, J.B.; Sims, R.; Frohlich, M.W.; Kantoff, P.W. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013, 81, 1297–1302. [Google Scholar] [CrossRef]
- Steele, K.E.; Tan, T.H.; Korn, R.; Dacosta, K.; Brown, C.; Kuziora, M.; Zimmermann, J.; Laffin, B.; Widmaier, M.; Rognoni, L.; et al. Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J. Immunother. Cancer 2018, 6, 20. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Kibel, A.S.; Yu, E.Y.; Karsh, L.I.; Elfiky, A.; Shore, N.D.; Vogelzang, N.J.; Corman, J.M.; Millard, F.E.; Maher, J.C.; et al. Sequencing of Sipuleucel-T and Androgen Deprivation Therapy in Men with Hormone-Sensitive Biochemically Recurrent Prostate Cancer: A Phase II Randomized Trial. Clin. Cancer Res. 2017, 23, 2451–2459. [Google Scholar] [CrossRef]
- Small, E.J.; Lance, R.S.; Gardner, T.A.; Karsh, L.I.; Fong, L.; McCoy, C.; DeVries, T.; Sheikh, N.A.; GuhaThakurta, D.; Chang, N.; et al. A Randomized Phase II Trial of Sipuleucel-T with Concurrent versus Sequential Abiraterone Acetate plus Prednisone in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2015, 21, 3862–3869. [Google Scholar] [CrossRef] [PubMed]
- Arina, A.; Gutiontov, S.I.; Weichselbaum, R.R. Radiotherapy and Immunotherapy for Cancer: From “Systemic” to “Multisite”. Clin. Cancer Res. 2020, 26, 2777–2782. [Google Scholar] [CrossRef]
- Twardowski, P.; Wong, J.Y.C.; Pal, S.K.; Maughan, B.L.; Frankel, P.H.; Franklin, K.; Junqueira, M.; Prajapati, M.R.; Nachaegari, G.; Harwood, D.; et al. Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat. Res. Commun. 2019, 19, 100116. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Park, J.C.; DeWeese, T.L.; Tran, P.T.; Song, D.Y.; King, S.; Afful, M.; Hurrelbrink, J.; et al. Randomized Phase II Trial of Sipuleucel-T with or without Radium-223 in Men with Bone-metastatic Castration-resistant Prostate Cancer. Clin. Cancer Res. 2021, 27, 1623–1630. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Morishima, C.; Szmulewitz, R.; Harshman, L.; Appleman, L.; Monk, P.; Bitting, R.L.; Kucuk, O.; Millard, F.; Seigne, J.D.; et al. IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 2021, 9, e002903. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Zhang, L.; Subudhi, S.; Chen, B.; Marquez, J.; Liu, E.V.; Allaire, K.; Cheung, A.; Ng, S.; Nguyen, C.; et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J. Immunother. Cancer 2021, 9, e002254. [Google Scholar] [CrossRef]
- Dorff, T.; Hirasawa, Y.; Acoba, J.; Pagano, I.; Tamura, D.; Pal, S.; Zhang, M.; Waitz, R.; Dhal, A.; Haynes, W.; et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J. Immunother. Cancer 2021, 9, e002931. [Google Scholar] [CrossRef]
- Saad, F.; Shore, N.; Zhang, T.; Sharma, S.; Cho, H.K.; Jacobs, I.A. Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat. Rev. 2019, 76, 1–9. [Google Scholar] [CrossRef]
- Podrazil, M.; Horvath, R.; Becht, E.; Rozkova, D.; Bilkova, P.; Sochorova, K.; Hromadkova, H.; Kayserova, J.; Vavrova, K.; Lastovicka, J.; et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015, 6, 18192–18205. [Google Scholar] [CrossRef]
- Fucikova, J.; Podrazil, M.; Jarolim, L.; Bilkova, P.; Hensler, M.; Becht, E.; Gasova, Z.; Klouckova, J.; Kayserova, J.; Horvath, R.; et al. Phase I/II trial of dendritic cell-based active cellular immunotherapy with DCVAC/PCa in patients with rising PSA after primary prostatectomy or salvage radiotherapy for the treatment of prostate cancer. Cancer Immunol. Immunother. 2018, 67, 89–100. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Beer, T.M.; Gerritsen, W.; Oudard, S.; Wiechno, P.; Kukielka-Budny, B.; Samal, V.; Hajek, J.; Feyerabend, S.; Khoo, V.; et al. Efficacy and Safety of Autologous Dendritic Cell-Based Immunotherapy, Docetaxel, and Prednisone vs Placebo in Patients With Metastatic Castration-Resistant Prostate Cancer: The VIABLE Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022, 8, 546–552. [Google Scholar] [CrossRef]
- Pérez-Baños, A.; Gleisner, M.A.; Flores, I.; Pereda, C.; Navarrete, M.; Araya, J.P.; Navarro, G.; Quezada-Monrás, C.; Tittarelli, A.; Salazar-Onfray, F. Whole tumour cell-based vaccines: Tuning the instruments to orchestrate an optimal antitumour immune response. Br. J. Cancer 2023, 129, 572–585. [Google Scholar] [CrossRef]
- Comiskey, M.C.; Dallos, M.C.; Drake, C.G. Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr. Oncol. Rep. 2018, 20, 75. [Google Scholar] [CrossRef]
- Simons, J.W.; Carducci, M.A.; Mikhak, B.; Lim, M.; Biedrzycki, B.; Borellini, F.; Clift, S.M.; Hege, K.M.; Ando, D.G.; Piantadosi, S.; et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin. Cancer Res. 2006, 12, 3394–3401. [Google Scholar] [CrossRef]
- Higano, C.S.; Corman, J.M.; Smith, D.C.; Centeno, A.S.; Steidle, C.P.; Gittleman, M.; Simons, J.W.; Sacks, N.; Aimi, J.; Small, E.J. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 2008, 113, 975–984. [Google Scholar] [CrossRef]
- Ward, J.E.; McNeel, D.G. GVAX: An allogeneic, whole-cell, GM-CSF-secreting cellular immunotherapy for the treatment of prostate cancer. Expert Opin. Biol. Ther. 2007, 7, 1893–1902. [Google Scholar] [CrossRef]
- Sonpavde, G.; Slawin, K.M.; Spencer, D.M.; Levitt, J.M. Emerging vaccine therapy approaches for prostate cancer. Rev. Urol. 2010, 12, 25–34. [Google Scholar] [PubMed]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation With or Without Vaccination in Localized Prostate Cancer. Clin. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef]
- Du, J.J.; Su, Z.; Yu, H.; Qin, S.; Wang, D. From design to clinic: Engineered peptide nanomaterials for cancer immunotherapy. Front. Chem. 2022, 10, 1107600. [Google Scholar] [CrossRef]
- Madan, R.A.; Gulley, J.L. Personalized peptide vaccine in prostate cancer: Capitalizing on existing immunity. Transl. Cancer Res. 2016, 5, S1333–S1335. [Google Scholar] [CrossRef]
- Noguchi, M.; Fujimoto, K.; Arai, G.; Uemura, H.; Hashine, K.; Matsumoto, H.; Fukasawa, S.; Kohjimoto, Y.; Nakatsu, H.; Takenaka, A.; et al. A randomized phase III trial of personalized peptide vaccination for castration-resistant prostate cancer progressing after docetaxel. Oncol. Rep. 2021, 45, 159–168. [Google Scholar] [CrossRef]
- Noguchi, M.; Moriya, F.; Koga, N.; Matsueda, S.; Sasada, T.; Yamada, A.; Kakuma, T.; Itoh, K. A randomized phase II clinical trial of personalized peptide vaccination with metronomic low-dose cyclophosphamide in patients with metastatic castration-resistant prostate cancer. Cancer Immunol. Immunother. 2016, 65, 151–160. [Google Scholar] [CrossRef]
- Noguchi, M.; Arai, G.; Egawa, S.; Ohyama, C.; Naito, S.; Matsumoto, K.; Uemura, H.; Nakagawa, M.; Nasu, Y.; Eto, M.; et al. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: A randomized phase II trial. Cancer Immunol. Immunother. 2020, 69, 847–857. [Google Scholar] [CrossRef]
- Calvo Tardón, M.; Allard, M.; Dutoit, V.; Dietrich, P.Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A. DNA vaccines: An historical perspective and view to the future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef]
- Rezaei, T.; Davoudian, E.; Khalili, S.; Amini, M.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Strategies in DNA vaccine for melanoma cancer. Pigment. Cell Melanoma Res. 2021, 34, 869–891. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Larregina, A.T.; Morelli, A.E. Impact of extracellular vesicles on innate immunity. Curr. Opin. Organ. Transpl. 2019, 24, 670–678. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, T.; Wei, X.; Zhou, Y.; Li, J. CpG DNA-triggered upregulation of TLR9 expression affects apoptosis and immune responses in human plasmacytoid dendritic cells isolated from chronic hepatitis B patients. Arch. Physiol. Biochem. 2023, 129, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Zahm, C.D.; Colluru, V.T.; McNeel, D.G. DNA vaccines for prostate cancer. Pharmacol. Ther. 2017, 174, 27–42. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G. Phase II Trial of a DNA Vaccine Encoding Prostatic Acid Phosphatase (pTVG-HP [MVI-816]) in Patients With Progressive, Nonmetastatic, Castration-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 3507–3517. [Google Scholar] [CrossRef]
- Wargowski, E.; Johnson, L.E.; Eickhoff, J.C.; Delmastro, L.; Staab, M.J.; Liu, G.; McNeel, D.G. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J. Immunother. Cancer 2018, 6, 21. [Google Scholar] [CrossRef]
- McNeel, D.G.; Dunphy, E.J.; Davies, J.G.; Frye, T.P.; Johnson, L.E.; Staab, M.J.; Horvath, D.L.; Straus, J.; Alberti, D.; Marnocha, R.; et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J. Clin. Oncol. 2009, 27, 4047–4054. [Google Scholar] [CrossRef]
- Pavlenko, M.; Roos, A.K.; Lundqvist, A.; Palmborg, A.; Miller, A.M.; Ozenci, V.; Bergman, B.; Egevad, L.; Hellström, M.; Kiessling, R.; et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer 2004, 91, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, F.; Tötterman, T.; Maltais, A.K.; Pisa, P.; Yachnin, J. DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 2013, 31, 3843–3848. [Google Scholar] [CrossRef]
- Olson, B.M.; Bradley, E.S.; Sawicki, T.; Zhong, W.; Ranheim, E.A.; Bloom, J.E.; Colluru, V.T.; Johnson, L.E.; Rekoske, B.T.; Eickhoff, J.C.; et al. Safety and Immunological Efficacy of a DNA Vaccine Encoding the Androgen Receptor Ligand-Binding Domain (AR-LBD). Prostate 2017, 77, 812–821. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Eickhoff, J.C.; Ferrari, A.C.; Schweizer, M.T.; Wargowski, E.; Olson, B.M.; McNeel, D.G. Multicenter Phase I Trial of a DNA Vaccine Encoding the Androgen Receptor Ligand-binding Domain (pTVG-AR, MVI-118) in Patients with Metastatic Prostate Cancer. Clin. Cancer Res. 2020, 26, 5162–5171. [Google Scholar] [CrossRef]
- Santoro, S.P.; Kim, S.; Motz, G.T.; Alatzoglou, D.; Li, C.; Irving, M.; Powell, D.J., Jr.; Coukos, G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol. Res. 2015, 3, 68–84. [Google Scholar] [CrossRef]
- Maes, J.; Gesquière, S.; De Spiegeleer, A.; Maes, A.; Van de Wiele, C. Prostate-Specific Membrane Antigen Biology and Pathophysiology in Prostate Carcinoma, an Update: Potential Implications for Targeted Imaging and Therapy. Int. J. Mol. Sci. 2024, 25, 9755. [Google Scholar] [CrossRef]
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170. [Google Scholar] [CrossRef]
- Teplensky, M.H.; Dittmar, J.W.; Qin, L.; Wang, S.; Evangelopoulos, M.; Zhang, B.; Mirkin, C.A. Spherical Nucleic Acid Vaccine Structure Markedly Influences Adaptive Immune Responses of Clinically Utilized Prostate Cancer Targets. Adv. Heal. Mater. 2021, 10, e2101262. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, L.; Li, Z.; Peng, X.; Li, H. mRNA vaccines in tumor targeted therapy: Mechanism, clinical application, and development trends. Biomark. Res. 2024, 12, 93. [Google Scholar] [CrossRef]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.; Moreno, A.C.R.; Aps, L.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Kübler, H.; Scheel, B.; Gnad-Vogt, U.; Miller, K.; Schultze-Seemann, W.; Vom Dorp, F.; Parmiani, G.; Hampel, C.; Wedel, S.; Trojan, L.; et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in-man phase I/IIa study. J. Immunother. Cancer 2015, 3, 26. [Google Scholar] [CrossRef]
- Stenzl, A.; Feyerabend, S.; Syndikus, I.; Sarosiek, T.; Kübler, H.; Heidenreich, A.; Cathomas, R.; Grüllich, C.; Loriot, Y.; Perez Gracia, S.L.; et al. Results of the randomized, placebo-controlled phase I/IIB trial of CV9104, an mRNA based cancer immunotherapy, in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2017, 28, v408–v409. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pal, S.K.; Alex, A.; Agarwal, N. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 2015, 11, 2137–2148. [Google Scholar] [CrossRef]
- Sharp, D.W.; Lattime, E.C. Recombinant Poxvirus and the Tumor Microenvironment: Oncolysis, Immune Regulation and Immunization. Biomedicines 2016, 4, 19. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Gulley, J.L.; Pico-Navarro, C. Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men With Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 124–125. [Google Scholar] [CrossRef]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Ng, S.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Abdul Sater, H.; Marté, J.L.; Donahue, R.N.; Walter-Rodriguez, B.; Heery, C.R.; Steinberg, S.M.; Cordes, L.M.; Chun, G.; Karzai, F.; Bilusic, M.; et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J. Immunother. Cancer 2020, 8, e000655. [Google Scholar] [CrossRef]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021, 21, e6. [Google Scholar] [CrossRef]
- Bilusic, M.; McMahon, S.; Madan, R.A.; Karzai, F.; Tsai, Y.T.; Donahue, R.N.; Palena, C.; Jochems, C.; Marté, J.L.; Floudas, C.; et al. Phase I study of a multitargeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 2021, 9, e002374. [Google Scholar] [CrossRef]
- Johnson, L.E.; Brockstedt, D.; Leong, M.; Lauer, P.; Theisen, E.; Sauer, J.D.; McNeel, D.G. Heterologous vaccination targeting prostatic acid phosphatase (PAP) using DNA and Listeria vaccines elicits superior anti-tumor immunity dependent on CD4+ T cells elicited by DNA priming. Oncoimmunology 2018, 7, e1456603. [Google Scholar] [CrossRef]
- Stein, M.N.; Fong, L.; Tutrone, R.; Mega, A.; Lam, E.T.; Parsi, M.; Vangala, S.; Gutierrez, A.A.; Haas, N.B. ADXS31142 Immunotherapy ± Pembrolizumab Treatment for Metastatic Castration-Resistant Prostate Cancer: Open-Label Phase I/II KEYNOTE-046 Study. Oncologist 2022, 27, 453–461. [Google Scholar] [CrossRef]
- Beer, T.M.; Bernstein, G.T.; Corman, J.M.; Glode, L.M.; Hall, S.J.; Poll, W.L.; Schellhammer, P.F.; Jones, L.A.; Xu, Y.; Kylstra, J.W.; et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin. Cancer Res. 2011, 17, 4558–4567. [Google Scholar] [CrossRef]
| Vaccine Type | Vaccine | Combination | Disease | N | Trail Phase | Results or Comments | Clinical Trial ID |
|---|---|---|---|---|---|---|---|
| Dendritic cell vaccines | Sipuleucel-T | NA | mCRPC | 512 (341 vs. 171) | III | The extended OS observed in patients administered the vaccine, in contrast to those receiving a placebo, resulted in the approval of the vaccine by the FDA in 2010 [16] | NCT00065442 |
| Sipuleucel-T | Androgen receptor signaling pathway inhibitors (ASPIs) | mCRPC | 6044 (906 vs. 5092) | Retrospective | The administration of Sipuleucel-T at any point in time was correlated with enhanced OS when compared to the use of ASPI alone [17] | ||
| Sipuleucel-T | NA | Androgen-dependent prostate cancer | 176 (117 vs. 59) | III | No significant difference in the time to biochemical failure was detected [80] | NCT00779402 | |
| Sipuleucel-T | ADT | Biochemically recurrent prostate cancer | 68 (34 vs. 34) | II | The administration of Sipuleucel-T prior to ADT resulted in a significantly enhanced specific T cell response compared to ADT alone [20] | NCT01431391 | |
| Sipuleucel-T | Abiraterone | mCRPC | 69 (35 vs. 34) | II | The combination of Sipuleucel-T and abiraterone was found to be well tolerated, with no new safety concerns identified [21] | NCT01487863 | |
| Sipuleucel-T | Radiation therapy | mCRPC | 51 (24 vs. 25) | II | Radiation therapy did not improve the humoral and cellular immune responses linked to Sipuleucel-T treatment [23] | NCT01807065 | |
| Sipuleucel-T | IL-7 | mCRPC | 56 (26 vs. 28) | II | Augmented immune responses observed in patients undergoing combination therapy [25] | NCT01881867 | |
| Sipuleucel-T | Ipilimumab | mCRPC | 50 (26 vs. 24) | II | The combination of ipilimumab and Sipuleucel-T demonstrated limited clinical efficacy and did not significantly modify antigen-specific responses [26] | NCT01804465 | |
| DCVAC/PCa | Docetaxel and prednisone | mCRPC | 1182 (787 vs. 395) | III | No improvement in OS of patients with mCRPC [31] | NCT02111577 | |
| Cellular vaccines | GVAX | NA | mCRPC | 600 | III | Terminated [36] | NA |
| GVAX | Degarelix | Localized prostate cancer | 29 (15 vs. 14) | NA | GVAX demonstrated a modest enhancement of the immunological effects associated with ADT [38] | NA | |
| Peptide vaccines | Personalized peptide vaccines | NA | CRPC | 310 (207 vs. 103) | III | No improvement in OS [41] | UMIN000011308 |
| Personalized peptide vaccines | Cyclophosphamide | mCRPC | 310 (35 vs. 35) | II | No improvement in OS and PFS [42] | UMIN000005329 | |
| KRM-20 | Docetaxel and dexamethasone | CRPC | 51 (25 vs. 26) | II | No improvement in OS and PFS [43] | UMIN000011028 | |
| DNA vaccines | pTVG-HP | NA | nmCSPC | 99 (48 vs. 49) | II | Two-year MFS was not different overall between study arms [53] | NCT01341652 |
| pTVG-HP | sipuleucel-T | mCRPC | 18 (9 vs. 9) | II | Median time to progression was not significantly different [54] | NCT01706458 | |
| pVAX/PSA | NA | CRPC | 8 | I | At maximum dose (900 µg), PSA-specific cellular and humoral immunity detected [56] | NA | |
| pTVG-AR | NA | mCSPC | 40 | I | Patients exhibiting T cell immunity demonstrated a marked extension of PFS [59] | NCT02411786 | |
| pDOM-PSMA27 | NA | Biochemically recurrent prostate cancer | 64 (32 vs. 32) | I/II | A notable improvement in PSA doubling time was observed among patients who received the vaccine [62] | NA | |
| RNA vaccines | CV9103 | NA | CRPC | 44 | I/IIa | There was no significant correlation between immune response and OS [68] | 2008-003967-37 |
| Viral and bacterial vaccines | PROSTVAC | GM-CSF | mCRPC | 1297 (429 vs. 429 vs. 428) | III | PROSTVAC demonstrated a favorable safety profile and was well tolerated; however, no significant enhancement in overall survival rates was observed [74] | NCT01322490 |
| Ad5-PSA | NA | mCRPC | 18 | I | This vaccine demonstrates a favorable tolerability and an acceptable safety profile [77] | NCT03481816 | |
| ADXS31-142 | pembrolizumab | mCRPC | 50 (37 vs. 13) | I/II | The combination of ADXS31-142 with pembrolizumab has been shown to enhance the median OS [79] | NCT02325557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Lu, X.; Tian, F.; Luo, Q.; Zhou, W.; Yang, S.; Li, W.; Yang, Y.; Shi, M.; Zhou, T. Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook. Vaccines 2024, 12, 1384. https://doi.org/10.3390/vaccines12121384
Zhou W, Lu X, Tian F, Luo Q, Zhou W, Yang S, Li W, Yang Y, Shi M, Zhou T. Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook. Vaccines. 2024; 12(12):1384. https://doi.org/10.3390/vaccines12121384
Chicago/Turabian StyleZhou, Wenhao, Xiaojun Lu, Feng Tian, Qianming Luo, Weihang Zhou, Siyuan Yang, Wenxuan Li, Yongjun Yang, Minfeng Shi, and Tie Zhou. 2024. "Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook" Vaccines 12, no. 12: 1384. https://doi.org/10.3390/vaccines12121384
APA StyleZhou, W., Lu, X., Tian, F., Luo, Q., Zhou, W., Yang, S., Li, W., Yang, Y., Shi, M., & Zhou, T. (2024). Vaccine Therapies for Prostate Cancer: Current Status and Future Outlook. Vaccines, 12(12), 1384. https://doi.org/10.3390/vaccines12121384





