Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza
Abstract
1. Introduction
2. Influenza Virus Diversity and Mechanisms of Variation
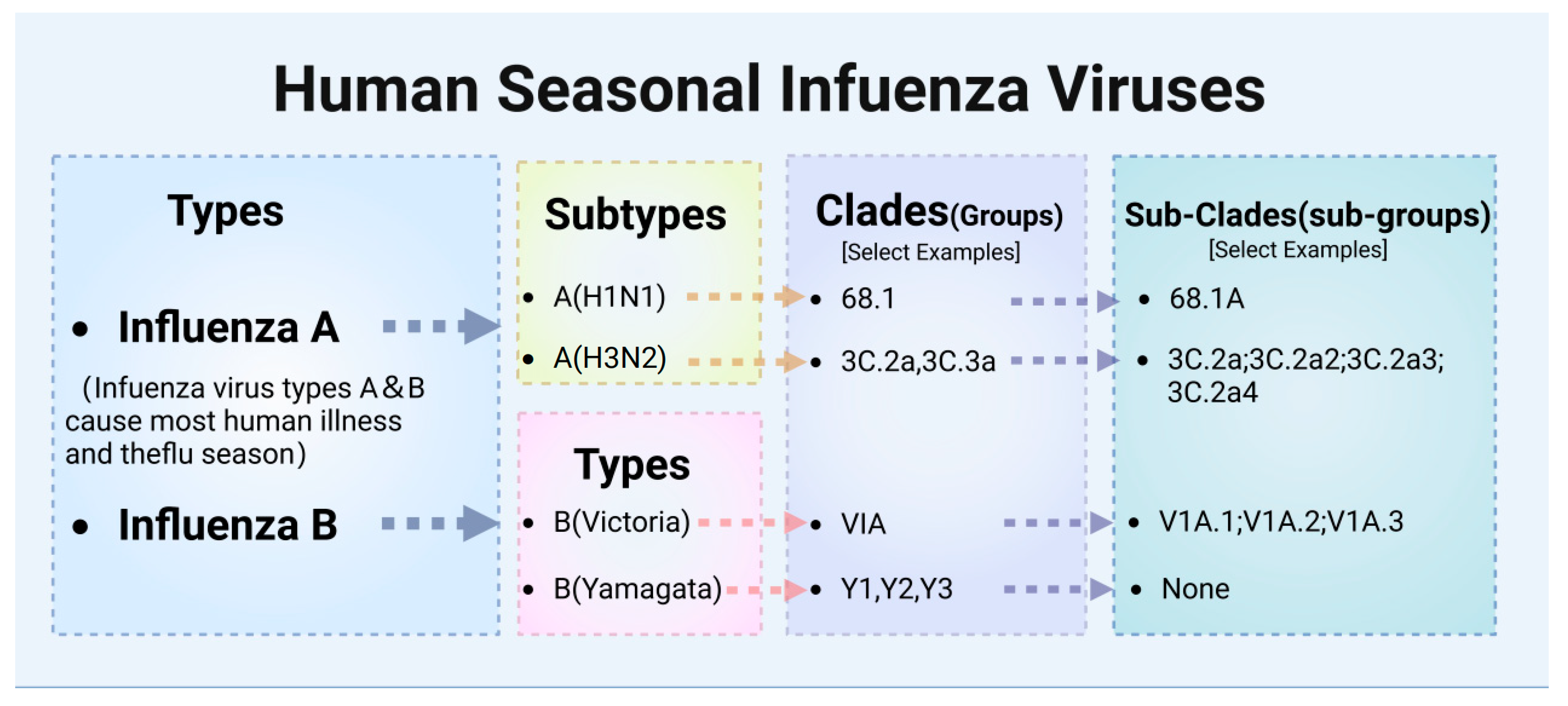
2.1. Classification of Influenza Viruses
2.2. Structure of Influenza A Viruses
2.3. Mechanisms of Antigenic Variation
2.3.1. Antigenic Drift
2.3.2. Antigenic Shift
2.3.3. Other Factors
2.4. Limitations of Classical Influenza Vaccines
| Vaccine Type | Synthesis | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|---|
| Inactivated Vaccines | Synthetic Long-Peptide Vaccine Inactivated | Peptides synthetically produced to match segments of viral proteins. | Specific immune response targeting, can be designed to enhance T-cell response, lower risk of autoimmunity. | Complex manufacturing process, may require adjuvants to enhance effectiveness, limited long-term data. | [88] |
| Whole-Virus Vaccine | Virus grown in culture, then killed using heat or chemicals to inactivate it. | Stable, well-understood, can provoke a strong immune response, long track record of safety. | Risk of incomplete virus inactivation, cold chain storage required, possible allergic reactions. | [77,89,90] | |
| Split-Virion Vaccine | Virus grown in culture, then physically broken up to remove genetic material, retaining only antigenic proteins. | Reduced risk of infection compared to whole-virus vaccines, better safety profile with high immunogenicity. | May require adjuvants to enhance immunogenicity, not as robust immune response as live vaccines. | [75,79,91,92] | |
| Subunit Vaccine | Only specific viral proteins or protein fragments used, excluding other viral components. | Highly specific immune response, less risk of side effects from viral proteins, can be used in immunocompromised individuals. | May require multiple doses and adjuvants, potential for lower immunogenicity compared to whole-virus vaccines. | [76,92,93,94] | |
| Live Attenuated Vaccines | Live Attenuated Virus Vaccine | Virus grown in culture and genetically modified to lose virulence but keep immunogenic properties. | Strong and long-lasting immune response, often requires fewer boosters, mimics natural infection. | Risk of causing disease in immunocompromised individuals, potential for reversion to virulence, cold chain storage required. | [78,79,80,81,89,95] |
| Viral Vector Vaccines | Virosome Vaccine | Viral antigens incorporated into lipid vesicles, mimicking virus structure without genetic material. | Targeted delivery system that can enhance immune response, reduced risk of infection compared to live vaccines. | Complex manufacturing process, cost may be higher than more traditional vaccines, limited data on long-term efficacy. | [82,83,84] |
| Nucleic Acid Vaccines | mRNA Vaccine | Produced synthetically using a DNA template to make mRNA that encodes a viral protein. | Rapid development, high efficacy, adjustable formulation, induces both humoral and cellular immunity. | Cold chain storage requirements, shorter track record, potential for immune reaction. | [96,97,98] |
| DNA Vaccine | Plasmids containing genes of interest synthesized; these are used to transfect cells and induce an immune response. | Can induce a broad range of immune responses, stable and relatively easy to manufacture, not temperature sensitive. | Concerns about integration into host DNA, variable immune response effectiveness, still under development. | [88,99] | |
| circRNA Vaccine | Circular RNA molecules synthesized to encode for antigens, leveraging the stability and expression efficiency of circRNA. | Can induce potent immune responses, versatile platform with potential for fewer side effects, stability advantages over mRNA. | Newer technology with limited data, manufacturing challenges, regulatory hurdles as a novel technology. | [100,101] | |
| Recombinant Virus Vaccines | Recombinant Vaccinia Virus Vaccine | Vaccinia virus engineered to express influenza virus proteins (e.g., NP, M2e). | Induces both humoral and cellular immune responses, can be engineered for multiple antigens, effective for cross-strain protection. | Risk of reversion to pathogenic forms in some cases, pre-existing immunity may reduce efficacy in certain populations, cold chain storage may be required. | [85,86] |
3. Overview of mRNA Vaccine Technology
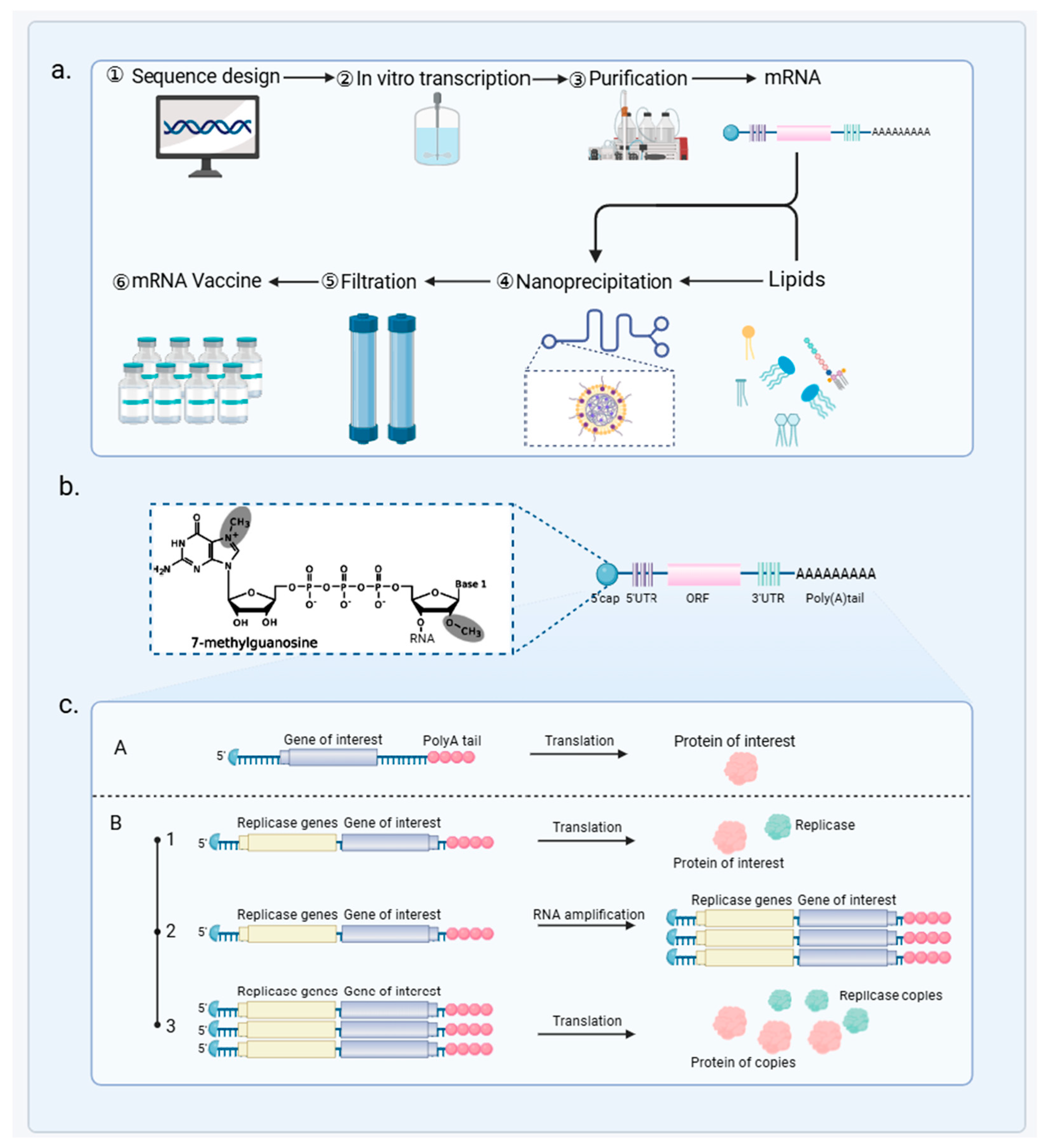
3.1. mRNA Vaccine Principles and Mechanisms
3.2. Advantages of mRNA Vaccines as Broad-Spectrum Influenza Vaccines
3.2.1. Rapid Vaccine Design and Production
3.2.2. Encoding Multiple Antigens
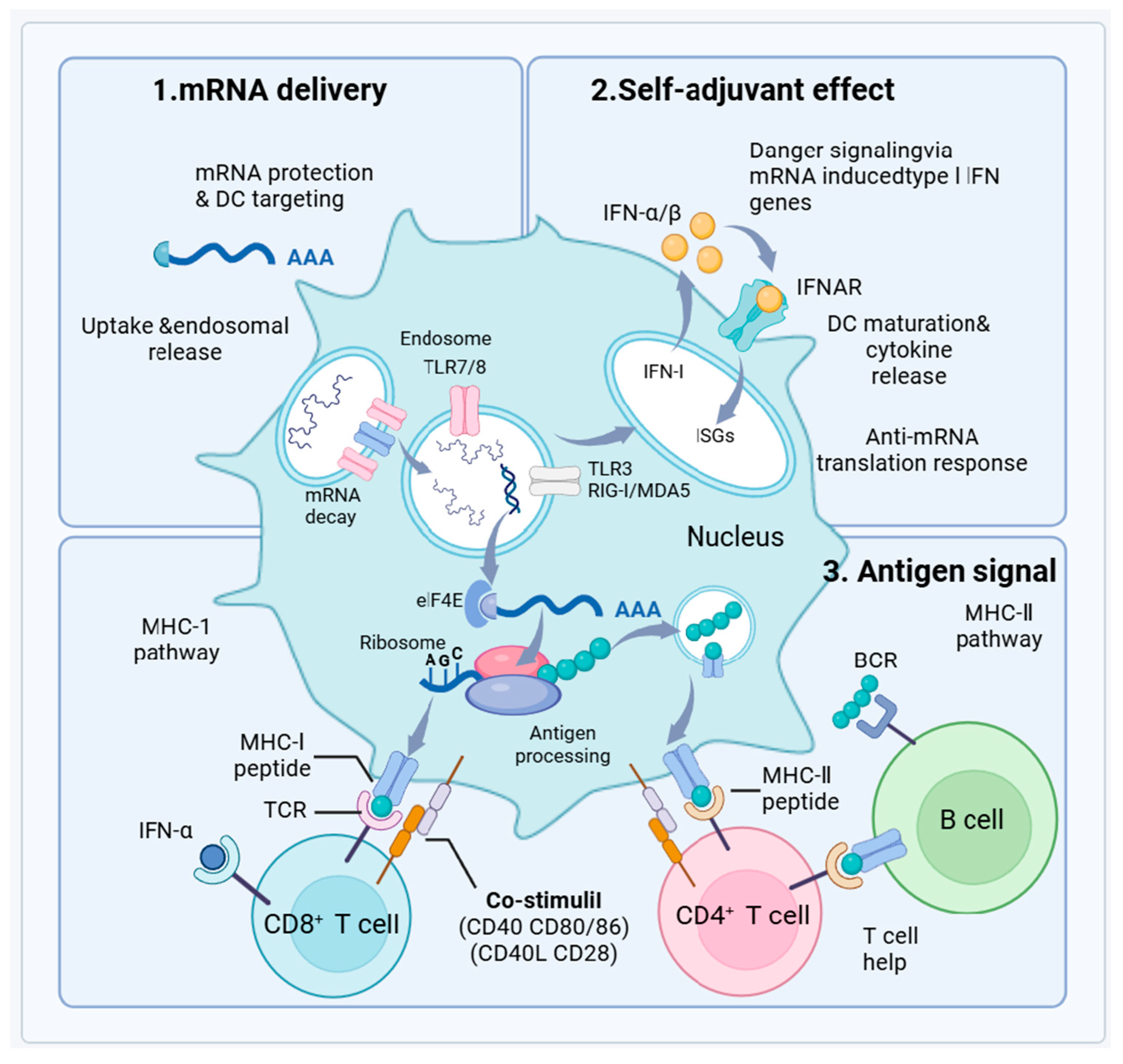
3.2.3. Enhanced Immunogenicity
3.2.4. Safety and Stability
3.3. Applications of mRNA Vaccines in Pulmonary Influenza
3.3.1. Animal Studies
3.3.2. Clinical Trials
4. Limitation and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Briand, S.; Mounts, A.; Chamberland, M. Challenges of global surveillance during an influenza pandemic. Public Health 2011, 125, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Keech, M.; Beardsworth, P. The impact of influenza on working days lost: A review of the literature. PharmacoEconomics 2008, 26, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; McGeer, A.; Uleryk, E.; Coleman, B.L. Burden of severe illness associated with laboratory confirmed influenza in adults aged 50–64 years: A rapid review. Influenza Other Respir. Viruses 2022, 16, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Welch, V.L.; Moran, M.M.; Cane, A.; Lopez, S.M.C.; Srivastava, A.; Enstone, A.L.; Sears, A.; Markus, K.J.; Heuser, M.; et al. High Clinical Burden of Influenza Disease in Adults Aged ≥ 65 Years: Can We Do Better? A Systematic Literature Review. Adv. Ther. 2023, 40, 1601–1627. [Google Scholar] [CrossRef]
- Near, A.M.; Tse, J.; Young-Xu, Y.; Hong, D.K.; Reyes, C.M. Burden of influenza hospitalization among high-risk groups in the United States. BMC Health Serv. Res. 2022, 22, 1209. [Google Scholar] [CrossRef]
- Putri, W.C.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef]
- Kiertiburanakul, S.; Phongsamart, W.; Tantawichien, T.; Manosuthi, W.; Kulchaitanaroaj, P. Economic Burden of Influenza in Thailand: A Systematic Review. Inq. J. Med. Care Organ. Provis. Financ. 2020, 57, 1–14. [Google Scholar] [CrossRef]
- Houser, K.; Subbarao, K. Influenza vaccines: Challenges and solutions. Cell Host Microbe 2015, 17, 295–300. [Google Scholar] [CrossRef]
- Jin, H.; Chen, Z. Production of live attenuated influenza vaccines against seasonal and potential pandemic influenza viruses. Curr. Opin. Virol. 2014, 6, 34–39. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, W.; Zhang, X.; Li, D.; Mei, M. Effectively Evaluating a Novel Consensus Subunit Vaccine Candidate to Prevent the H9N2 Avian Influenza Virus. Vaccines 2024, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.B.; Pardi, N. New Kids on the Block: RNA-Based Influenza Virus Vaccines. Vaccines 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing influenza vaccine efficacy against mismatched and matched strains: A systematic review and meta-analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.-L.; Dickinson, J.A.; Gubbay, J.B.; Drews, S.J.; Fonseca, K.; Charest, H.; et al. Vaccine Effectiveness Against Lineage-matched and -mismatched Influenza B Viruses Across 8 Seasons in Canada, 2010–2011 to 2017–2018. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 1754–1757. [Google Scholar] [CrossRef]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 2021, 6, 153. [Google Scholar] [CrossRef]
- Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 2003, 21, 1776–1779. [Google Scholar] [CrossRef]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Ghebrehewet, S.; MacPherson, P.; Ho, A. Influenza. BMJ (Clin. Res. Ed.) 2016, 355, i6258. [Google Scholar] [CrossRef]
- Blut, A. Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Yin, L.; Jiang, H.; Lu, X.; Bi, Y.; Zhang, W.; Shi, Y.; Burioni, R.; Tong, Z.; et al. Structural basis for a human broadly neutralizing influenza A hemagglutinin stem-specific antibody including H17/18 subtypes. Nat. Commun. 2022, 13, 7603. [Google Scholar] [CrossRef]
- Kandeil, A.; Patton, C.; Jones, J.C.; Jeevan, T.; Harrington, W.N.; Trifkovic, S.; Seiler, J.P.; Fabrizio, T.; Woodard, K.; Turner, J.C.; et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat. Commun. 2023, 14, 3082. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.; Zecchin, B.; Giussani, E.; Palumbo, E.; Agüero-García, M.; Bachofen, C.; Bálint, Á.; Banihashem, F.; Banyard, A.C.; Beerens, N.; et al. High pathogenic avian influenza A(H5) viruses of clade 2.3.4.4b in Europe—Why trends of virus evolution are more difficult to predict. Virus Evol. 2024, 10, veae027. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.W.; Wang, M.H.; Chen, Z.; Hui, D.S.C.; Kwok, A.K.; Yeung, A.C.M.; Liu, K.M.; Yeoh, Y.K.; Lee, N.; Chan, P.K.S. Frequent Genetic Mismatch between Vaccine Strains and Circulating Seasonal Influenza Viruses, Hong Kong, China, 1996–2012. Emerg. Infect. Dis. 2018, 24, 1825–1834. [Google Scholar] [CrossRef]
- Del Rosario, J.M.M.; da Costa, K.A.S.; Asbach, B.; Ferrara, F.; Ferrari, M.; Wells, D.A.; Mann, G.S.; Ameh, V.O.; Sabeta, C.T.; Banyard, A.C.; et al. Establishment of pan-Influenza A (H1–H18) and pan-Influenza B (pre-split, Vic/Yam) Pseudotype Libraries for efficient vaccine antigen selection. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jennings, L.; Huang, Q.S.; Barr, I.; Lee, P.; Kim, W.J.; Buchy, P.; Sanicas, M.; Mungall, B.A.; Chen, J. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia-Pacific region. Influenza Other Respir. Viruses 2018, 12, 383–411. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, R.; Durando, P.; Ansaldi, F.; Sticchi, L.; Banfi, F.; Amicizia, D.; Panatto, D.; Esposito, S.; Principi, N.; Icardi, G.; et al. Influenza and respiratory syncytial virus in infants and children: Relationship with attendance at a paediatric emergency unit and characteristics of the circulating strains. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 619–628. [Google Scholar] [CrossRef]
- Koutsakos, M.; Wheatley, A.K.; Laurie, K.; Kent, S.J.; Rockman, S. Influenza lineage extinction during the COVID-19 pandemic? Nat. Rev. Microbiol. 2021, 19, 741–742. [Google Scholar] [CrossRef]
- Chen, R.; Holmes, E.C. The evolutionary dynamics of human influenza B virus. J. Mol. Evol. 2008, 66, 655–663. [Google Scholar] [CrossRef]
- Suntronwong, N.; Klinfueng, S.; Korkong, S.; Vichaiwattana, P.; Thongmee, T.; Vongpunsawad, S.; Poovorawan, Y. Characterizing genetic and antigenic divergence from vaccine strain of influenza A and B viruses circulating in Thailand, 2017–2020. Sci. Rep. 2021, 11, 735. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, T.; Wan, X.-F. A computational framework for influenza antigenic cartography. PLoS Comput. Biol. 2010, 6, e1000949. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, S.; Sader, K.; Turner, J.; Calder, L.J.; Rosenthal, P.B. In situ structure and organization of the influenza C virus surface glycoprotein. Nat. Commun. 2021, 12, 1694. [Google Scholar] [CrossRef]
- Liu, R.; Sheng, Z.; Huang, C.; Wang, D.; Li, F. Influenza D virus. Curr. Opin. Virol. 2020, 44, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kargarfard, F.; Sami, A.; Mohammadi-Dehcheshmeh, M.; Ebrahimie, E. Novel approach for identification of influenza virus host range and zoonotic transmissible sequences by determination of host-related associative positions in viral genome segments. BMC Genom. 2016, 17, 925. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Andrews, R.; Groves, D.; Ghani, S.V.; Cordes, T.; Kapanidis, A.N.; Robb, N.C. High-throughput super-resolution analysis of influenza virus pleomorphism reveals insights into viral spatial organization. PLoS Pathog. 2023, 19, e1011484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fodor, E.; Keown, J.R. A structural understanding of influenza virus genome replication. Trends Microbiol. 2023, 31, 308–319. [Google Scholar] [CrossRef]
- Caton, A.J.; Brownlee, G.G.; Yewdell, J.W.; Gerhard, W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 1982, 31, 417–427. [Google Scholar] [CrossRef]
- Yang, J.; Liu, S.; Du, L.; Jiang, S. A new role of neuraminidase (NA) in the influenza virus life cycle: Implication for developing NA inhibitors with novel mechanism of action. Rev. Med. Virol. 2016, 26, 242–250. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- Boni, M.F. Vaccination and antigenic drift in influenza. Vaccine 2008, 26 (Suppl. 3), C8–C14. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bancej, C.; Lee, L.; Champredon, D. Antigenic drift and epidemiological severity of seasonal influenza in Canada. Sci. Rep. 2022, 12, 15625. [Google Scholar] [CrossRef] [PubMed]
- Hensley, S.E.; Das, S.R.; Bailey, A.L.; Schmidt, L.M.; Hickman, H.D.; Jayaraman, A.; Viswanathan, K.; Raman, R.; Sasisekharan, R.; Bennink, J.R.; et al. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science 2009, 326, 734–736. [Google Scholar] [CrossRef]
- Nelson, M.I.; Holmes, E.C. The evolution of epidemic influenza. Nat. Reviews. Genet. 2007, 8, 196–205. [Google Scholar] [CrossRef]
- Petrova, V.N.; Russell, C.A. The evolution of seasonal influenza viruses. Nat. Rev. Microbiol. 2018, 16, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Galvani, A.P.; Bush, R.M.; Ferguson, N.M.; Galvani, A.P.; Bush, R.M. Ecological and immunological determinants of influenza evolution. Nature 2003, 422, 428–433. [Google Scholar] [CrossRef]
- Boni, M.F.; Gog, J.R.; Andreasen, V.; Feldman, M.W. Epidemic dynamics and antigenic evolution in a single season of influenza A. Proc. R. Soc. B Biol. Sci. 2006, 273, 1307–1316. [Google Scholar] [CrossRef]
- Pavia, G.; Scarpa, F.; Ciccozzi, A.; Romano, C.; Branda, F.; Quirino, A.; Marascio, N.; Matera, G.; Sanna, D.; Ciccozzi, M. Changing and Evolution of Influenza Virus: Is It a Trivial Flu? Chemotherapy 2024, 69, 185–193. [Google Scholar] [CrossRef]
- Dove, A. Maurice Hilleman. Nat. Med. 2005, 11, S2. [Google Scholar] [CrossRef]
- Kurth, R. Obituary: Maurice R. Hilleman (1919–2005). Nature 2005, 434, 1083. [Google Scholar] [CrossRef]
- Fall, A.; Han, L.; Yunker, M.; Gong, Y.-N.; Li, T.-J.; Norton, J.M.; Abdullah, O.; Rothman, R.E.; Fenstermacher, K.Z.J.; Morris, C.P.; et al. Evolution of Influenza A(H3N2) Viruses in 2 Consecutive Seasons of Genomic Surveillance, 2021–2023. Open Forum Infect. Dis. 2023, 10, ofad577. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, J.; Wang, Q. Evolutionary Trends of A(H1N1) Influenza Virus Hemagglutinin Since 1918. PLoS ONE 2009, 4, e7789. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, J.A.; Kotturi, M.F.; Kim, Y.; Oseroff, C.; Vaughan, K.; Salimi, N.; Vita, R.; Ponomarenko, J.; Scheuermann, R.H.; Sette, A.; et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. USA 2009, 106, 20365–20370. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.M.; Burke, D.S. Historical perspective--Emergence of influenza A (H1N1) viruses. N. Engl. J. Med. 2009, 361, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F.; de Jong, M.D.; van Doorn, H.R.; Holmes, E.C. Guidelines for identifying homologous recombination events in influenza A virus. PLoS ONE 2010, 5, e10434. [Google Scholar] [CrossRef]
- Khaliq, Z.; Leijon, M.; Belák, S.; Komorowski, J. Identification of combinatorial host-specific signatures with a potential to affect host adaptation in influenza A H1N1 and H3N2 subtypes. BMC Genom. 2016, 17, 529. [Google Scholar] [CrossRef]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Vandegrift, K.J.; Sokolow, S.H.; Daszak, P.; Kilpatrick, A.M. Ecology of avian influenza viruses in a changing world. Ann. N. Y. Acad. Sci. 2010, 1195, 113–128. [Google Scholar] [CrossRef]
- Banyard, A.C.; Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; De Silva, D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. Nat. Commun. 2024, 15, 7433. [Google Scholar] [CrossRef]
- Han, X.; Bertzbach, L.D.; Veit, M. Mimicking the passage of avian influenza viruses through the gastrointestinal tract of chickens. Vet. Microbiol. 2019, 239, 108462. [Google Scholar] [CrossRef]
- Kurmi, B.; Murugkar, H.V.; Nagarajan, S.; Tosh, C.; Dubey, S.C.; Kumar, M. Survivability of Highly Pathogenic Avian Influenza H5N1 Virus in Poultry Faeces at Different Temperatures. Indian J. Virol. 2013, 24, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Marchi, J.; Lässig, M.; Mora, T.; Walczak, A.M. Multi-Lineage Evolution in Viral Populations Driven by Host Immune Systems. Pathogens 2019, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Sironi, M.; Cagliani, R.; Forni, D.; Clerici, M. Evolutionary insights into host–pathogen interactions from mammalian sequence data. Nat. Rev. Genet. 2015, 16, 224–236. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, M.; Tamuri, A.U.; Hay, A.J.; Goldstein, R.A. Charting the Host Adaptation of Influenza Viruses. Mol. Biol. Evol. 2011, 28, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wan, W.; Yu, K.; Lemey, P.; Pettersson, J.H.O.; Bi, Y.; Lu, M.; Li, X.; Chen, Z.; Zheng, M.; et al. Farmed fur animals harbour viruses with zoonotic spillover potential. Nature 2024, 634, 228–233. [Google Scholar] [CrossRef]
- Dadonaite, B.; Gilbertson, B.; Knight, M.L.; Trifkovic, S.; Rockman, S.; Laederach, A.; Brown, L.E.; Fodor, E.; Bauer, D.L.V. The structure of the influenza A virus genome. Nat. Microbiol. 2019, 4, 1781–1789. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef]
- Lyons, D.M.; Lauring, A.S. Mutation and Epistasis in Influenza Virus Evolution. Viruses 2018, 10, 407. [Google Scholar] [CrossRef]
- Duan, S.; Govorkova, E.A.; Bahl, J.; Zaraket, H.; Baranovich, T.; Seiler, P.; Prevost, K.; Webster, R.G.; Webby, R.J. Epistatic interactions between neuraminidase mutations facilitated the emergence of the oseltamivir-resistant H1N1 influenza viruses. Nat. Commun. 2014, 5, 5029. [Google Scholar] [CrossRef]
- Bolton, J.S.; Klim, H.; Wellens, J.; Edmans, M.; Obolski, U.; Thompson, C.P. An Antigenic Thrift-Based Approach to Influenza Vaccine Design. Vaccines 2021, 9, 657. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Pinto, M.V.; Sadarangani, M.; Plotkin, S.A. Whither vaccines? J. Infect. 2017, 74 (Suppl. 1), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Muraduzzaman, A.K.M.; Illing, P.T.; Mifsud, N.A.; Purcell, A.W. Understanding the Role of HLA Class I Molecules in the Immune Response to Influenza Infection and Rational Design of a Peptide-Based Vaccine. Viruses 2022, 14, 2578. [Google Scholar] [CrossRef] [PubMed]
- Aldeán, J.Á.; Salamanca, I.; Ocaña, D.; Barranco, J.L.; Walter, S. Effectiveness of cell culture-based influenza vaccines compared with egg-based vaccines: What does the literature say? Rev. Esp. Quimioter. 2022, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ortiz, J.R.; McLean, H.Q.; Fisher, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of Cell-Based Versus Egg-Based Quadrivalent Influenza Vaccines in Adults During the 2019–2020 Influenza Season in the United States. Open Forum Infect. Dis. 2022, 9, ofac532. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Zheng, X.; Tong, R.; Sun, X. Advanced subunit vaccine delivery technologies: From vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 2023, 13, 3321–3338. [Google Scholar] [CrossRef]
- Chua, B.Y.; Sekiya, T.; Koutsakos, M.; Nomura, N.; Rowntree, L.C.; Nguyen, T.H.O.; McQuilten, H.A.; Ohno, M.; Ohara, Y.; Nishimura, T.; et al. Immunization with inactivated whole virus particle influenza virus vaccines improves the humoral response landscape in cynomolgus macaques. PLoS Pathog. 2022, 18, e1010891. [Google Scholar] [CrossRef]
- Jang, Y.H.; Seong, B.-L. Principles underlying rational design of live attenuated influenza vaccines. Clin. Exp. Vaccine Res. 2012, 1, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Liu, Y.-M.; Tseng, Y.-C.; Ma, C. Better influenza vaccines: An industry perspective. J. Biomed. Sci. 2020, 27, 33. [Google Scholar] [CrossRef]
- Bull, J.J. Evolutionary reversion of live viral vaccines: Can genetic engineering subdue it? Virus Evol. 2015, 1, vev005. [Google Scholar] [CrossRef]
- Pérez-Losada, M.; Arenas, M.; Galán, J.C.; Palero, F.; González-Candelas, F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2015, 30, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Akbar, M.; Iqbal, B.; Ali, F.; Sharma, N.K.; Kumar, N.; Najmi, A.; Albratty, M.; Alhazmi, H.A.; Madkhali, O.A.; et al. Virosome: An engineered virus for vaccine delivery. Saudi Pharm. J. Off. Publ. Saudi Pharm. Soc. 2023, 31, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Asadi, K.; Gholami, A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. Int. J. Biol. Macromol. 2021, 182, 648–658. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Haach, V.; Bellaver, F.V.; Bombassaro, G.; Gava, D.; da Silva, L.P.; Baron, L.F.; Simonelly, M.; Carvalho, W.A.; Schaefer, R.; et al. Immunological profile of mice immunized with a polyvalent virosome-based influenza vaccine. Virol. J. 2023, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Verardi, P.H.; Titong, A.; Hagen, C.J. A vaccinia virus renaissance: New vaccine and immunotherapeutic uses after smallpox eradication. Hum. Vaccines Immunother. 2012, 8, 961–970. [Google Scholar] [CrossRef]
- Slobod, K.S.; Lockey, T.D.; Howlett, N.; Srinivas, R.V.; Rencher, S.D.; Freiden, P.J.; Doherty, P.C.; Hurwitz, J.L. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2004, 23, 106–110. [Google Scholar] [CrossRef]
- Kumari, R.; Sharma, S.D.; Kumar, A.; Ende, Z.; Mishina, M.; Wang, Y.; Falls, Z.; Samudrala, R.; Pohl, J.; Knight, P.R.; et al. Antiviral Approaches against Influenza Virus. Clin. Microbiol. Rev. 2023, 36, e0004022. [Google Scholar] [CrossRef]
- Rabu, C.; Rangan, L.; Florenceau, L.; Fortun, A.; Charpentier, M.; Dupré, E.; Paolini, L.; Beauvillain, C.; Dupel, E.; Latouche, J.-B.; et al. Cancer vaccines: Designing artificial synthetic long peptides to improve presentation of class I and class II T cell epitopes by dendritic cells. Oncoimmunology 2019, 8, e1560919. [Google Scholar] [CrossRef]
- Nogales, A.; Martínez-Sobrido, L. Reverse Genetics Approaches for the Development of Influenza Vaccines. Int. J. Mol. Sci. 2016, 18, 20. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef]
- Talbot, H.K.; Nian, H.; Zhu, Y.; Chen, Q.; Williams, J.V.; Griffin, M.R. Clinical effectiveness of split-virion versus subunit trivalent influenza vaccines in older adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Talbot, H.K.; Nian, H.; Zhu, Y.; Chen, Q.; Williams, J.; Griffin, M. 531: Split-virion compared to Subunit Influenza Trivalent Influenza Vaccines Has Greater Effectiveness in Older Adults. Open Forum Infect. Dis. 2014, 1, S18. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Vartak, A.; Sucheck, S.J. Recent Advances in Subunit Vaccine Carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. The Integrated Consideration of Vaccine Platforms, Adjuvants, and Delivery Routes for Successful Vaccine Development. Vaccines 2023, 11, 695. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Lu, Y. Tactics targeting circular mRNA biosynthesis. Biotechnol. Bioeng. 2023, 120, 1975–1985. [Google Scholar] [CrossRef]
- Kim, H.; Cha, H.; Cheong, T. Analyzing economic effect on mRNA vaccine inventory management with redistribution policy. Sci. Rep. 2024, 14, 20425. [Google Scholar] [CrossRef]
- Costa, G.L.; Sautto, G.A. Exploring T-Cell Immunity to Hepatitis C Virus: Insights from Different Vaccine and Antigen Presentation Strategies. Vaccines 2024, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.M.; Ebdah, S.; Al Haj Mahmoud, S.A.; Abu-Qatouseh, L.; Jaber, Y.H. Comparison of T cells mediated immunity and side effects of mRNA vaccine and conventional COVID-19 vaccines administrated in Jordan. Hum. Vaccines Immunother. 2024, 20, 2333104. [Google Scholar] [CrossRef]
- Wang, F.; Cai, G.; Wang, Y.; Zhuang, Q.; Cai, Z.; Li, Y.; Gao, S.; Li, F.; Zhang, C.; Zhao, B.; et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. MedComm 2024, 5, e667. [Google Scholar] [CrossRef]
- Lokras, A.G.; Bobak, T.R.; Baghel, S.S.; Sebastiani, F.; Foged, C. Advances in the design and delivery of RNA vaccines for infectious diseases. Adv. Drug Deliv. Rev. 2024, 213, 115419. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, B.; Yang, Y.; Jiang, Y.; Wang, R.; Wei, Q.; Pan, Y.; Chen, Y.; Wang, H.; Fan, J.; et al. Low-Dose Mildronate-Derived Lipidoids for Efficient mRNA Vaccine Delivery with Minimal Inflammation Side Effects. ACS Nano 2024, 18, 23289–23300. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarinodehi, N.; Niederkofler, S.; Emilsson, G.; Parkkila, P.; Olsén, E.; Jing, Y.; Sjöberg, M.; Agnarsson, B.; Lindfors, L.; Höök, F. Time-Resolved Inspection of Ionizable Lipid-Facilitated Lipid Nanoparticle Disintegration and Cargo Release at an Early Endosomal Membrane Mimic. ACS Nano 2024, 18, 22989–23000. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, D.; Huang, Y.; Deng, Y.; Wang, Y.; Mao, J.; Zhou, Y.; Xiong, Y.; Gao, X. Revolutionizing viral disease vaccination: The promising clinical advancements of non-replicating mRNA vaccines. Virol. J. 2023, 20, 64. [Google Scholar] [CrossRef]
- Tusup, M.; French, L.E.; De Matos, M.; Gatfield, D.; Kundig, T.; Pascolo, S. Design of in vitro Transcribed mRNA Vectors for Research and Therapy. Chimia 2019, 73, 391–394. [Google Scholar] [CrossRef]
- Oladipo, E.K.; Oyelakin, O.D.; Aiyelabegan, A.O.; Olajide, E.O.; Olatayo, V.O.; Owolabi, K.P.; Shittu, Y.B.; Olugbodi, R.O.; Ajala, H.A.; Rukayat, R.A.; et al. Exploring computational approaches to design mRNA Vaccine against vaccinia and Mpox viruses. Immun. Inflamm. Dis. 2024, 12, e1360. [Google Scholar] [CrossRef]
- He, W.; Zhang, X.; Zou, Y.; Li, J.; Wang, C.; He, Y.; Jin, Q.; Ye, J. Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription. Molecules 2024, 29, 2461. [Google Scholar] [CrossRef]
- Jani, B.; Fuchs, R. In vitro transcription and capping of Gaussia luciferase mRNA followed by HeLa cell transfection. J. Vis. Exp. JoVE 2012, e3702. [Google Scholar] [CrossRef]
- Hick, T.A.H.; Geertsema, C.; Nijland, R.; Pijlman, G.P. Packaging of alphavirus-based self-amplifying mRNA yields replication-competent virus through a mechanism of aberrant homologous RNA recombination. mBio 2024, 15, e0249424. [Google Scholar] [CrossRef]
- Savar, N.S.; Shengjuler, D.; Doroudian, F.; Vallet, T.; Mac Kain, A.; Arashkia, A.; Khamesipour, A.; Lundstrom, K.; Vignuzzi, M.; Niknam, H.M. An alphavirus-derived self-amplifying mRNA encoding PpSP15-LmSTI1 fusion protein for the design of a vaccine against leishmaniasis. Parasitol. Int. 2022, 89, 102577. [Google Scholar] [CrossRef] [PubMed]
- Papukashvili, D.; Rcheulishvili, N.; Liu, C.; Ji, Y.; He, Y.; Wang, P.G. Self-Amplifying RNA Approach for Protein Replacement Therapy. Int. J. Mol. Sci. 2022, 23, 12884. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.C.; Milona, P.; Thomann-Harwood, L.; Démoulins, T.; Englezou, P.; Suter, R.; Ruggli, N. Self-Amplifying Replicon RNA Vaccine Delivery to Dendritic Cells by Synthetic Nanoparticles. Vaccines 2014, 2, 735. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qiu, Z.; Cho, W.C.S.; Liu, Z.; Chen, S.; Li, H.; Chen, K.; Li, Y.; Zuo, C.; Qiu, M. Synthetic circRNA therapeutics: Innovations, strategies, and future horizons. MedComm 2024, 5, e720. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ye, F.; Deng, X.; Tang, Y.; Liang, J.-Y.; Huang, X.; Sun, Y.; Tang, H.; Lei, J.; Zheng, S.; et al. Circular RNA: A promising new star of vaccine. J. Transl. Intern. Med. 2023, 11, 372–381. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA Vaccines-How Far From Clinical Use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef]
- Sicca, F.; Martinuzzi, D.; Montomoli, E.; Huckriede, A. Comparison of influenza-specific neutralizing antibody titers determined using different assay readouts and hemagglutination inhibition titers: Good correlation but poor agreement. Vaccine 2020, 38, 2527–2541. [Google Scholar] [CrossRef]
- Troncoso-Bravo, T.; Ramírez, M.A.; Loaiza, R.A.; Román-Cárdenas, C.; Papazisis, G.; Garrido, D.; González, P.A.; Bueno, S.M.; Kalergis, A.M. Advancement in the development of mRNA-based vaccines for respiratory viruses. Immunology 2024, 173, 481–496. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef]
- Ananworanich, J.; Lee, I.T.; Ensz, D.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Choi, A.; Pucci, A.; McGrath, S.; et al. Safety and Immunogenicity of mRNA-1010, an Investigational Seasonal Influenza Vaccine, in Healthy Adults: Final Results From a Phase 1/2 Randomized Trial. J. Infect. Dis. 2024, jiae329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Kumari, M.; Chen, G.-H.; Hong, M.-H.; Yuan, J.P.-Y.; Tsai, J.-L.; Wu, H.-C. mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef] [PubMed]
- Kackos, C.M.; DeBeauchamp, J.; Davitt, C.J.H.; Lonzaric, J.; Sealy, R.E.; Hurwitz, J.L.; Samsa, M.M.; Webby, R.J. Seasonal quadrivalent mRNA vaccine prevents and mitigates influenza infection. NPJ Vaccines 2023, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Redit, C.; Ha, C.; Amy, L.; Ho, P. Big mRNA players focus on flu vaccines. Nat. Biotechnol. 2022, 40, 1706. [Google Scholar] [CrossRef]
- Parveen, A.; Elkordy, A.A. Brief Insights into mRNA Vaccines: Their Successful Production and Nanoformulation for Effective Response against COVID-19 and Their Potential Success for Influenza A and B. Pathogens 2024, 13, 500. [Google Scholar] [CrossRef]
- Schmid, A. Regulatory Considerations for Producing mRNA Vaccines for Clinical Trials. Methods Mol. Biol. 2024, 2786, 321–337. [Google Scholar] [CrossRef]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef]
- Pecetta, S.; Rappuoli, R. mRNA, the beginning of a new influenza vaccine game. Proc. Natl. Acad. Sci. USA 2022, 119, e2217533119. [Google Scholar] [CrossRef]
- Russell, C.A.; Fouchier, R.A.M.; Ghaswalla, P.; Park, Y.; Vicic, N.; Ananworanich, J.; Nachbagauer, R.; Rudin, D. Seasonal influenza vaccine performance and the potential benefits of mRNA vaccines. Hum. Vaccines Immunother. 2024, 20, 2336357. [Google Scholar] [CrossRef]
- Han, X.; Pan, H.; Jin, P.; Wei, M.; Jia, S.; Wang, W.; Chu, K.; Gao, S.; Zhou, L.; Li, J.; et al. A head-to-head comparison of humoral and cellular immune responses of five COVID-19 vaccines in adults in China. Front. Immunol. 2024, 15, 1455730. [Google Scholar] [CrossRef]
- Tadic, S.; Martínez, A. Nucleic acid cancer vaccines targeting tumor related angiogenesis. Could mRNA vaccines constitute a game changer? Front. Immunol. 2024, 15, 1433185. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lei, K.; Tang, L. Neoantigen Vaccine Delivery for Personalized Anticancer Immunotherapy. Front. Immunol. 2018, 9, 1499. [Google Scholar] [CrossRef]
- Uno, N.; Ross, T.M. Multivalent next generation influenza virus vaccines protect against seasonal and pre-pandemic viruses. Sci. Rep. 2024, 14, 1440. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, S.; Prigent, A.; Garraud, O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunol. 2015, 16, 31. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71–110. [Google Scholar] [CrossRef]
- Mazunina, E.P.; Gushchin, V.A.; Kleymenov, D.A.; Siniavin, A.E.; Burtseva, E.I.; Shmarov, M.M.; Mukasheva, E.A.; Bykonia, E.N.; Kozlova, S.R.; Evgrafova, E.A.; et al. Trivalent mRNA vaccine-candidate against seasonal flu with cross-specific humoral immune response. Front. Immunol. 2024, 15, 1381508. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg. Microbes Infect. 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Kallen, K.-J.; Heidenreich, R.; Schnee, M.; Petsch, B.; Schlake, T.; Thess, A.; Baumhof, P.; Scheel, B.; Koch, S.D.; Fotin-Mleczek, M. A novel, disruptive vaccination technology: Self-adjuvanted RNActive(®) vaccines. Hum. Vaccines Immunother. 2013, 9, 2263–2276. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y.; Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Clemente, B.; Denis, M.; Silveira, C.P.; Schiavetti, F.; Brazzoli, M.; Stranges, D. Straight to the point: Targeted mRNA-delivery to immune cells for improved vaccine design. Front. Immunol. 2023, 14, 1294929. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.-H. Influenza Viruses: Innate Immunity and mRNA Vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef]
- Nam, M.; Yun, S.G.; Kim, S.-W.; Kim, C.G.; Cha, J.H.; Lee, C.; Kang, S.; Park, S.G.; Kim, S.B.; Lee, K.-B.; et al. Humoral and Cellular Immune Responses to Vector, Mix-and-Match, or mRNA Vaccines against SARS-CoV-2 and the Relationship between the Two Immune Responses. Microbiol. Spectr. 2022, 10, e0249521. [Google Scholar] [CrossRef] [PubMed]
- Nogimori, T.; Nagatsuka, Y.; Kobayashi, S.; Murakami, H.; Masuta, Y.; Suzuki, K.; Tomimaru, Y.; Noda, T.; Akita, H.; Takahama, S.; et al. Humoral and cellular immune responses to COVID-19 mRNA vaccines in immunosuppressed liver transplant recipients. Commun. Med. 2024, 4, 30. [Google Scholar] [CrossRef]
- Reina, J. The new generation of messenger RNA (mRNA) vaccines against influenza. Enfermedades Infecc. Y Microbiol. Clin. (Engl. Ed.) 2023, 41, 301–304. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G.; Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Reneer, Z.B.; Bergeron, H.C.; Reynolds, S.; Thornhill-Wadolowski, E.; Feng, L.; Bugno, M.; Truax, A.D.; Tripp, R.A. mRNA vaccines encoding influenza virus hemagglutinin (HA) elicits immunity in mice from influenza A virus challenge. PLoS ONE 2024, 19, e0297833. [Google Scholar] [CrossRef]
- van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; Vilas Boas de Melo, C.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A universal influenza mRNA vaccine candidate boosts T cell responses and reduces zoonotic influenza virus disease in ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef]
- Pardi, N.; Carreño, J.M.; O’Dell, G.; Tan, J.; Bajusz, C.; Muramatsu, H.; Rijnink, W.; Strohmeier, S.; Loganathan, M.; Bielak, D.; et al. Development of a pentavalent broadly protective nucleoside-modified mRNA vaccine against influenza B viruses. Nat. Commun. 2022, 13, 4677. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Hum. Vaccines Immunother. 2018, 14, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Wang, H.-B.; Zhang, Y.-S.; Zhao, J.-H.; Zhong, Y.; Wang, Y.-J.; Huang, L.-Y.; Ma, J.-X.; Sun, Q.; Yang, L.; et al. Molecular evolution of influenza B virus during 2011–2017 in Chaoyang, Beijing, suggesting the free influenza vaccine policy. Sci. Rep. 2019, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Reina, J. [The Victoria and Yamagata Lineages of Influenza B Viruses, unknown and undervalued]. Rev. Esp. Quimioter. 2022, 35, 231–235. [Google Scholar] [CrossRef]
- Park, H.-J.; Bang, Y.-J.; Kwon, S.P.; Kwak, W.; Park, S.-I.; Roh, G.; Bae, S.-H.; Kim, J.-Y.; Kwak, H.W.; Kim, Y.; et al. Analyzing immune responses to varied mRNA and protein vaccine sequences. npj Vaccines 2023, 8, 84. [Google Scholar] [CrossRef]
- Beans, C. Researchers getting closer to a “universal” flu vaccine. Proc. Natl. Acad. Sci. USA 2022, 119, e2123477119. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Cheung, M.; Chang, C.; Rathnasinghe, R.; Rossignol, E.; Zhang, Y.; Ferrari, A.; Patel, H.; Huang, Y.; Sanchez Guillen, M.; Scalzo, T.; et al. Self-amplifying mRNA seasonal influenza vaccines elicit mouse neutralizing antibody and cell-mediated immunity and protect ferrets. npj Vaccines 2023, 8, 150. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Meng, L.; Li, F.; Yu, C. Comparison of Immune Responses Elicited by SARS-CoV-2 mRNA and Recombinant Protein Vaccine Candidates. Front. Immunol. 2022, 13, 906457. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Anastassopoulou, C.; Lazaros, G.; Vasileiou, K.; Tsioufis, C.; Tsakris, A. Comparative assessment of myocarditis and pericarditis reporting rates related to mRNA COVID-19 vaccines in Europe and the United States. Expert Rev. Vaccines 2022, 21, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Block, J.P.; Boehmer, T.K.; Forrest, C.B.; Carton, T.W.; Lee, G.M.; Ajani, U.A.; Christakis, D.A.; Cowell, L.G.; Draper, C.; Ghildayal, N.; et al. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination—PCORnet, United States, January 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S.; et al. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H.; Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The Science Underlying COVID-19. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef]
- Fraser, E. Long term respiratory complications of covid-19. BMJ 2020, 370, m3001. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir. Viruses 2010, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Thess, A.; Grund, S.; Mui, B.L.; Hope, M.J.; Baumhof, P.; Fotin-Mleczek, M.; Schlake, T. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 1456–1464. [Google Scholar] [CrossRef] [PubMed]

| Vaccine | Antigen | Delivery Method | Trial Status | Effects | Sponsor/Collaborators | References |
|---|---|---|---|---|---|---|
| mRNA-1440 | pre-membrane and E protein of H10N8 influenza virus | LNP | Completed Phase 1 clinical trials | Inducing strong humoral immune response against the H10N8 influenza virus | Moderna | [117,118] |
| mRNA-1851 | HA of H10N8 influenza virus | LNP | Completed Phase 1 clinical trials | Inducing strong humoral immune response against the H10N8 influenza virus | Moderna | [119] |
| mRNA-1010 | HA of H1N1, H3N2, B/Victoria, B/Yamagata * | LNP | Currently in Phase 3 clinical trials | Achieving higher antibody responses for A/H3N2 and A/H1N1; less effective for B strains. More efficacy data expected | Moderna | [120,121] |
| mRNA-1083 | HA of H1N1, H3N2, B/Victoria, and SARS-CoV-2 | LNP | Currently in Phase 3 clinical trials | Combines mRNA-1010 with mRNA-1283 achieving higher antibody responses for three targeted strains and SARS-CoV-2 virus | Moderna | [122] |
| Influenza modRNA | HA of H1N1, H3N2, B/Victoria, B/Yamagata * | LNP | Completed 3 Phase 2 trials | Inducing protective antibody titers against four targeted strains | Pfizer | [123,124] |
| GSK4382276A | HA of H1N1, H3N2, B/Victoria, B/Yamagata * | LNP | Currently in a second Phase 2 trial | Specific clinical results have not yet been released | GSK | [125] |
| Sanofi Quadrivalent mRNA Vaccine | HA of H1N1, H3N2, B/Victoria, B/Yamagata * | LNP | Completed 7 Phase 1 clinical trials | Inducing protective antibody titers against four targeted strains | Sanofi | [15,123] |
| MRT5400 | HA of H1N1, H3N2, B/Victoria, B/Yamagata * | LNP | Currently in Phase 1 and Phase 2 clinical trials | Have not been publicly released | Sanofi | - |
| MRT5401 | HA of H3N2 | LNP | Currently in Phase 1 clinical trials | Have not been publicly released | Sanofi | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Ma, J.; Zhao, C. Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza. Vaccines 2024, 12, 1382. https://doi.org/10.3390/vaccines12121382
Cheng Z, Ma J, Zhao C. Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza. Vaccines. 2024; 12(12):1382. https://doi.org/10.3390/vaccines12121382
Chicago/Turabian StyleCheng, Ziqi, Junfeng Ma, and Chenyan Zhao. 2024. "Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza" Vaccines 12, no. 12: 1382. https://doi.org/10.3390/vaccines12121382
APA StyleCheng, Z., Ma, J., & Zhao, C. (2024). Advantages of Broad-Spectrum Influenza mRNA Vaccines and Their Impact on Pulmonary Influenza. Vaccines, 12(12), 1382. https://doi.org/10.3390/vaccines12121382





