Lessons from Recent Measles Post-Campaign Coverage Surveys Worldwide
Abstract
1. Background
2. Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2017, 92, 205–227. [Google Scholar]
- World Health Organization. Planning and Implementing High-Quality Supplementary Immunization Activities for Injectable Vaccines: Using an Example of Measles-Rubella Vaccines—Field Guide; WHO: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241511254 (accessed on 15 October 2024).
- Strebel, P.; Grabowsky, M.; Hoekstra, E.; Gay, A.; Cochi, S. Evolution and Contribution of a Global Partnership against Measles and Rubella, 2001–2023. Vaccines 2024, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- De Quadros, C.A.; Hersh, B.S.; Nogueira, A.C.; A Carrasco, P.; Da Silveira, C.M. Measles eradication: Experience in the Americas. Bull. World Health Organ. 1998, 76 (Suppl. 2), 47–52. [Google Scholar] [PubMed]
- Verguet, S.; Johri, M.; Morris, S.K.; Gauvreau, C.L.; Jha, P.; Jit, M. Controlling measles using supplemental immunization activities: A mathematical model to inform optimal policy. Vaccine 2015, 33, 1291–1296. [Google Scholar] [CrossRef]
- Prada, J.; Metcalf, C.; Takahashi, S.; Lessler, J.; Tatem, A.; Ferrari, M. Demographics, epidemiology and the impact of vaccination campaigns in a measles-free world—Can elimination be maintained? Vaccine 2017, 35, 1488–1493. [Google Scholar] [CrossRef]
- Auzenbergs, M.; Fu, H.; Abbas, K.; Procter, S.R.; Cutts, F.T.; Jit, M. Health effects of routine measles vaccination and supplementary immunisation activities in 14 high-burden countries: A Dynamic Measles Immunization Calculation Engine (DynaMICE) modelling study. Lancet Glob. Health 2023, 11, e1194–e1204. [Google Scholar] [CrossRef]
- Portnoy, A.; Jit, M.; Helleringer, S.; Verguet, S. Comparative Distributional Impact of Routine Immunization and Supplementary Immunization Activities in Delivery of Measles Vaccine in Low- and Middle-Income Countries. Value Health 2020, 23, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, A.; Jit, M.; Helleringer, S.; Verguet, S. Impact of measles supplementary immunization activities on reaching children missed by routine programs. Vaccine 2018, 36, 170–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenfeld, K.A.; Frey, K.; McCarthy, K.A. Optimal Timing Regularly Outperforms Higher Coverage in Preventative Measles Supplementary Immunization Campaigns. Vaccines 2024, 12, 820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, L.; Jing, M.; Dapeng, Y. Rubella vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2020, 95, 27. [Google Scholar]
- Ou, A.C.; Zimmerman, L.A.; Alexander, J.P.; Crowcroft, N.S.; O’connor, P.M.; Knapp, J.K. Progress Toward Rubella and Congenital Rubella Syndrome Elimination—Worldwide, 2012–2022. Mmwr-Morb. Mortal. Wkly. Rep. 2024, 73, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Minta, A.A.; Ferrari, M.; Antoni, S.; Portnoy, A.; Sbarra, A.; Lambert, B.; Hatcher, C.; Hsu, C.H.; Ho, L.L.; Steulet, C.; et al. Progress Toward Measles Elimination—Worldwide, 2000–2022. Mmwr-Morbidity Mortal. Wkly. Rep. 2023, 72, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Rachlin, A.; Danovaro-Holliday, M.C.; Murphy, P.; Sodha, S.V.; Wallace, A.S. Routine Vaccination Coverage—Worldwide, 2021. Mmwr-Morbidity Mortal. Wkly. Rep. 2022, 71, 1396–1400. [Google Scholar] [CrossRef]
- Muhoza, P.; Danovaro-Holliday, M.C.; Diallo, M.S.; Murphy, P.; Sodha, S.V.; Requejo, J.H.; Wallace, A.S. Routine Vaccination Coverage—Worldwide, 2020. Mmwr-Morbidity Mortal. Wkly. Rep. 2021, 70, 1495–1500. [Google Scholar] [CrossRef]
- Ho, L.L.; Gurung, S.; Mirza, I.; Nicolas, H.D.; Steulet, C.; Burman, A.L.; Danovaro-Holliday, M.C.; Sodha, S.V.; Kretsinger, K. Impact of the SARS-CoV-2 pandemic on vaccine-preventable disease campaigns. Int. J. Infect. Dis. 2022, 119, 201–209. [Google Scholar] [CrossRef]
- CDC. Global Measles Outbreaks. Global Measles Vaccination. Published 13 May 2024. Available online: https://www.cdc.gov/global-measles-vaccination/data-research/global-measles-outbreaks/index.html (accessed on 15 October 2024).
- e Clinical Medicine Concerning global rise in measles cases. eClinicalMedicine 2024, 68, 102502. [CrossRef]
- Parums, D.V. A Review of the Resurgence of Measles, a Vaccine-Preventable Disease, as Current Concerns Contrast with Past Hopes for Measles Elimination. Med. Sci. Monit. 2024, 30, e944436-1–e944436-10. [Google Scholar] [CrossRef]
- Cutts, F.T.; Danovaro-Holliday, M.C.; Rhoda, D.A. Challenges in measuring supplemental immunization activity coverage among measles zero-dose children. Vaccine 2021, 39, 1359–1363. [Google Scholar] [CrossRef]
- World Health Organization. Vaccination Coverage Cluster Surveys: Reference Manual; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Measles and Measles-Rubella Vaccine Support. Available online: https://www.gavi.org/types-support/vaccine-support/measles-and-measles-rubella (accessed on 15 October 2024).
- Luman, E.T.; Cairns, K.L.; Perry, R.; Dietz, V.; Gittelman, D. Use and abuse of rapid mnitoring to assess coverage during mass vaccination campaigns. Bull. World Health Organ. 2007, 85, 651. [Google Scholar] [CrossRef]
- Kaiser, R.; E Shibeshi, M.; Chakauya, J.M.; Dzeka, E.; Masresha, B.G.; Daniel, F.; Shivute, N. Surveys of measles vaccination coverage in eastern and southern Africa: A review of quality and methods used. Bull. World Health Organ. 2015, 93, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Izurieta, H.S.; Rhoda, D.A. Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys. PLoS Med. 2013, 10, e1001404. [Google Scholar] [CrossRef] [PubMed]
- Utazi, C.E.; Wagai, J.; Pannell, O.; Cutts, F.T.; Rhoda, D.A.; Ferrari, M.J.; Dieng, B.; Oteri, J.; Danovaro-Holliday, M.C.; Adeniran, A.; et al. Geospatial variation in measles vaccine coverage through routine and campaign strategies in Nigeria: Analysis of recent household surveys. Vaccine 2020, 38, 3062–3071. [Google Scholar] [CrossRef] [PubMed]
- Danovaro-Holliday, M.C.; Dansereau, E.; Rhoda, D.A.; Brown, D.W.; Cutts, F.T.; Gacic-Dobo, M. Collecting and using reliable vaccination coverage survey estimates: Summary and recommendations from the “Meeting to share lessons learnt from the roll-out of the updated WHO Vaccination Coverage Cluster Survey Reference Manual and to set an operational research agenda around vaccination coverage surveys”, Geneva, 18–21 April 2017. Vaccine 2018, 36, 5150–5159. [Google Scholar] [CrossRef] [PubMed]
- Biostat Global Consulting. Vaccination Coverage Quality Indicators (VCQI)—Resources [Internet]. 2024. Available online: http://www.biostatglobal.com/VCQI_RESOURCES.HTML (accessed on 23 August 2024).
- Prier, M.L.; Rhoda, D.A. Organ Pipe Plots for Clustered Datasets—Visualize Disparities in Cluster-Level Coverage [Internet]. Stata Conference 2018; Columbus, Ohio. Available online: https://github.com/BiostatGlobalConsulting/organ-pipe-plots/blob/master/opplot_presentation.pptx (accessed on 23 August 2024).
- Cutts, F.T.; Ferrari, M.J.; Krause, L.K.; Tatem, A.J.; Mosser, J.F. Vaccination strategies for measles control and elimination: Time to strengthen local initiatives. BMC Med. 2021, 19, 2. [Google Scholar] [CrossRef]
- World Health Organization. Measles and Rubella Strategic Framework 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Measles Cases in the AFRO Region. South Sudan. Available online: https://www.afro.who.int/sites/default/files/2024-06/Measles%20Outbreak%20and%20Response%20Weekly%20Situation%20Update_Week%2019%2C%202024.pdf (accessed on 23 August 2024).
- Behavioral and Social Drivers of Immunization. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/demand (accessed on 15 October 2024).
- Akpan, G.U.; Mohammed, H.F.; Touray, K.; Kipterer, J.; Bello, I.M.; Ngofa, R.; Stein, A.; Seaman, V.; Mkanda, P.; Cabore, J. Conclusions of the African Regional GIS Summit (2019): Using geographic information systems for public health decision-making. BMC Proc. 2022, 16 (Suppl. 1), 3. [Google Scholar] [CrossRef]
- Utazi, C.E.; Aheto, J.M.; Wigley, A.; Tejedor-Garavito, N.; Bonnie, A.; Nnanatu, C.C.; Wagai, J.; Williams, C.; Setayesh, H.; Tatem, A.J.; et al. Mapping the distribution of zero-dose children to assess the performance of vaccine delivery strategies and their relationships with measles incidence in Nigeria. Vaccine 2022, 41, 170–181. [Google Scholar] [CrossRef]
- Arambepola, R.; Yang, Y.; Hutchinson, K.; Mwansa, F.D.; Doherty, J.A.; Bwalya, F.; Ndubani, P.; Musukwa, G.; Moss, W.J.; Wesolowski, A.; et al. Using geospatial models to map zero-dose children: Factors associated with zero-dose vaccination status before and after a mass measles and rubella vaccination campaign in Southern province, Zambia. BMJ Glob. Health 2021, 6, e007479. [Google Scholar] [CrossRef] [PubMed]
- Gebreslasie, M.T. A review of spatial technologies with applications for malaria transmission modelling and control in Africa. Geospat. Health 2015, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; O’keefe, K.J.; Wei, W.; Arshad, S.; Gruebner, O. Geospatial Analysis of COVID-19: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 2336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seidenberg, A.B.; Moser, R.P.; West, B.T. Preferred Reporting Items for Complex Sample Survey Analysis (PRICSSA). J. Surv. Stat. Methodol. 2023, 11, 743–757. [Google Scholar] [CrossRef]
| Measles Supplementary Immunization Activities (SIAs) | Post-Campaign Coverage Surveys (PCCSs) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basics | Basics | Sampling | |||||||||||||||||||||
| # | Year of PCCS | Country | WHO Region | Target Age Group in Months | Estimated Target Population | Admin Coverage % | SIA Month/Year | Month/Year of Field Work | Delay Between SIA & PCCS (in Months) | Lead Implementer | Ethical Clearance Mentioned | Questionnaire/Data Collection Form Included in Report | # of Clusters Selected/Included | # of Households Interviewed | # of Children Included | Description of Response Rates of Clusters | Description of Response Rates of Households | Primary Sampling Units | Household Enumeration Done | Involvement of NBS/CSO | Data Collection Tools | GPS Coordinates Collected | Software Used for Analysis |
| 1 | 2019 | Democratic People’s Republic of Korea | SEAR | 9 months-15 years old | 5,873,914 | 99.7 | Oct-19 | Winter | NA | Country-led | Yes | Yes | 41 | 1312 (31 HH in each cluster) | 1180 | Yes | Yes | Census enumeration blocks | Yes | Yes | CSPro | No | SPSS |
| 2 | 2019 | Zimbabwe | AFR | 9–59 months | 1,776,546 | 91 | Sep-19 | Nov-19 | 2 months | Not specified | Yes | Yes | 470 | 8110 planned *actual number included missing | 3796 planned *actual number included missing | No | No | Enumeration areas | Yes | Yes | ODK | NR | Stata |
| 3 | 2020 | Burkina Faso | AFR | 9–59 months | 3,078,334 | 106 | Nov-19 | Aug–Sept 2020 | 9–10 months | Independent consultant | Yes | No | 548 | 17,650 | 8457 | Yes | Yes | Enumeration areas | Not mentioned | Sampling frame from NBS | ODK | NA | SPSS and Stata |
| 4 | 2020 | Cameroon | AFR | 9–59 months | 3,339,090 | 92 | Dec-19 | June–July 2020 | 6–7 months | Research Organization | Not mentioned. Informed consent was obtained. | Yes | 396 | 4866 | 4671 | Yes | Yes | Enumeration areas | Yes | Sampling frame from NBS | ODK | Yes | SPSS |
| 5 | 2020 | Ethiopia | AFR | 9–59 months | 14,181,143 | 102.8 | June–July 2020 | Oct 2020–Jan 2021 | 4–6 months | Research Organization | Yes | Yes | 1016 Tigray was excluded | 10,143 | 12,867 | Yes | Yes | Enumeration areas | Yes | Sampling frame from NBS | CSPro | Yes | Stata, VCQI |
| 6 | 2020 | Uganda | AFR | 9 months to less than 15 years old | 18,200,970 | 107 | Oct-19 | Dec 2019–Jan 2020 | 2–3 months | Academic Institution | Yes | No | 363 | 5174 | 12,025 | Yes (written as coverage rate) | Yes | Enumeration areas | Yes | Sampling frame from NBS | ODK | NA | Stata |
| 7 | 2021 | Central African Republic † | AFR | 6–59 months 5–10 years | 1,209,256 | 102 for 6–59 m 85.5 for 5–10 yrs | Phase 1: Mar-2020 Phase 2: Aug-2020 | Dec 2020 Bangui, March–April 2021 rest | 8–11 months | Independent consultant | Yes | Yes | 240 | 3172 | 5674 | Yes | Yes | Enumeration areas | Yes | Sampling frame from NBS | ODK | Yes (difficulties noted) | SPSS |
| 8 | 2021 | Chad | AFR | 9–59 months | 1,792,830 (Phase1) 1,623,518 (Phase2) | 108.8 (Phase1) 107.5 (Phase2) | Jan- 2021 (Phase1) Mar-2021 (Phase2) | Mar–April 2022 | ~1 year | Independent consultant | The survey technical committe considered that the survey was not reasearch and thus it was not needed to present the protocol to an ethical comittee. Informed consent was obtained. | Yes | 695 | 15,793 | 11,601 | Yes | Yes | Enumeration areas, with segmentation | Yes | Yes | ODK | Yes, at least for clusters | SPSS and Stata |
| 9 | 2021 | Democratic Republic of the Congo | AFR | 6–59 months | 18,167,926 | 101.6 | Oct-Dec 2019 | Nov–Dec 2021 | ~2 years | Independent consultant | Yes | Yes | 728 | 81,740 | 21,575 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | SPSS, EpIInfo |
| 10 | 2021 | Kenya ‡ | AFR | 9–59 months | 3,374,464 | 111 | June–July 2021 | Jul-21 | <3 months | NBS | Considered programmatic evaluation. No ethical clearance was sought. Informed consent was obtained. | No | 477 | 4404 | 5409 | Yes, but by Region | Yes | Enumeration areas | Not for the PCCS. Sample was from available up-to-date sampling frame maintained by NBS. | Yes | Survey Solution application with digitization of the questionnaire and integration of maps. | Yes | Not mentioned |
| 11 | 2021 | Nepal | SEAR | 9–59 months | 2,548,336 | 101 | Feb–Mar 2020 (Phase 1) Mar–July 2020 (Phase 2) | Sept–Nov 2021 | >1 year | Family Welfare Division Department of Health Services MOH | Yes | Yes | 342 | 7180 | 3715 | Yes | Yes | List of wards (some were merged or segmented) | Yes | Yes | CSPro | Yes | SPSS |
| 12 | 2021 | Pakistan | EMR | 9 months-15 years old | 90,262,980 | 105 | Nov-21 | Nov 2021–Jan 2022 | <3 months | Contech International Health Consultants | Yes | Yes | 1584 | 15,840 | 31,871 | Yes | Yes | Enumeration areas | Yes | Yes. Technical and sampling frame | Proprietary android-based CAPI application from implementing partner | No | Stata, VCQI |
| 13 | 2021 | Zambia | AFR | 9–59 months | 3,398,230 | 91.3 | Nov-20 | Oct-21 | >1 year | Not specified | No | No | 272 | 5155 | 4590 | No | Yes | Enumeration areas | Yes | Yes | The questionnaire was programmed into a CAPI application using survey solutions, a World Bank software application | Not mentioned? | |
| 14 | 2022 | Burundi | AFR | 9–59 months | 1,683,300 | 93 | Jan-22 | Oct–Nov 2022 | 10–11 months | NBS | Not mentioned | Yes | 540 | 32,177 | 20,618 | Yes | Yes | Ennumeration areas | Yes | Yes | KoboCollect | Yes | SPSS and Stata |
| 15 | 2022 | Madagascar | AFR | 6–59 months | 4,355,433 | 95.11 | May–June 2022 | Aug–Sept 2022 | 3 months | International consultant with Directorate of Demography and Social Statistics (DDSS) of the NBS | Yes | Yes | 123 | 3055 | 1421 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | SPSS and Stata |
| 16 | 2022 | Somalia | EMR | children of 0–59 months for tOPV, 06–59 months for MCV and Vitamin A and 12–59 months for deworming | 2,566,955 | 90 | Nov-22 | Feb-23 | 3 months | Independent consultant/cabinet | Yes | Yes | 450 | 17,539 | 21,740 | No | No | Lists of accessible areas (not mentioned in detail) | Not mentioned | Not mentioned | Survey123 | Yes | SPSS, Stata, ArcGIS |
| 17 | 2022 or 2023 | Syrian Arab Republic | EMR | 6 months-5 years | 2,494,498 | 75.62 | Oct–Nov 2022 | Dates not provided. 2023? | NA | Independent consultant | Yes | No | 99 | 5982 | 3581 | Yes | Yes | Sub-districts | Yes | Yes, technical advise | Paper-based | No | EpiInfo |
| 18 | 2023 | Cameroon | AFR | 9–59 months | 5,564,940 | 94.38 | Jul-23 | Sept–Oct 2023 | <3 months | NBS | No. Informed consent was obtained. | Yes | 395 | 5711 | 3546 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | SPSS, Stata, VCQI |
| 19 | 2023 | Democratic Republic of the Congo | AFR | 6–59 months | 6,454,490 (May) 7,359,339 (August) 5,273,383 (September) | 103.6 (May) 85.7 (August) 101.1 (September) | Three phases: April, June and Aug 2023. | October–December 2023 in Block 1, Phase 2 in Block 2 and part of Block 3 from January to March 2024, then Phase 3 in the rest of the provinces in March–April 2024. | 6 to 10 months | Independent consultant | Yes | Yes | 728 | 10,920 | 9627 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | SPSS, EpIInfo |
| 20 | 2023 | Malawi | AFR | 9–59 months | 3,169,522 | 82.4 | May-23 | Jun-23 | <3 months | International consultant and NBS | Yes | Yes | 205 | 4715 | 8485 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | Stata, VCQI |
| 21 | 2023 | Niger | AFR | 6–59 m or 9–59 m depending on district | 5,098,682 | 105 | Dec 2022–Jan 2023 | Mar–April 2023 | 3 months | Independent consultant | Yes | Yes | 270 | 5334 | 4655 | Yes | Yes | Ennumeration areas | Yes | Yes | ODK | Yes | SPSS |
| 22 | 2023 | Nigeria | AFR | 9–59 months | 4,298,149 (October) 1,090,330 (November) 5,021,611 (December) | 96.67 (October) 103.95 (November) 4,823,266 (December) | Oct 2023–Jan 2024 | Dec 2023–Jan 2024 | <3 months | NBS | No. Informed consent was obtained. | Yes | 740 (560 in 14 states plus 180 in 6 local area governments in Borno) | 7399 | 6987 | Yes | Yes | Enumeration areas | Yes | Yes | CSPro | Yes | Stata, VCQI |
| 23 | 2023 | Yemen ‡ | EMR | 6–59 months | 1,267,083 | 91 | September–October 2023 | Oct-23 | <3 months | Research Organization | Yes | Yes | 1547 | 18,564 | 29,549 | Yes | Yes | Harahs | No | No | ODK | No | Excel |
| 24 | 2024 | Ethiopia | AFR | 98.7 | Dec 2022–April 2023 | Dec 2023–Jan 2024 | >1 year | Research Organization | Yes | Yes | 1272 | 12,702 | 15,763 | Yes | Yes | Enumeration areas | Yes | Sampling frame from NBS | Kobo Toolbox | Yes | Stata, VCQI | ||
| Results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Year of PCCS | Country | WHO Region | MCV1 Coverage in Year of Start of SIA | Admin Coverage % | SIA Cards or Finger Marking Seen | SIA Coverage (95% CI) | % Routine Immunization (RI) Cards Seen (Age Group If Not All) | RI MCV1 Coverage % (95% CI) (Age Group If Not All) | SIA Dose Was the First MCV Dose Received by Child % (95%CI) | SIA Coverage Among Zero-Dose % (95%CI) | Child Remained Measles Zero-Dose After SIA % (95%CI) |
| 1 | 2019 | Democratic People’s Republic of Korea | SEAR | 98 | 99.7 | Almost all were verified by record in Primary Health Facility. Only recall were 7 children who had records in a different health facility | 99.9% (99.09–99.87) | NA | 81% yes, 18.6% unsure, 0.4% no | NR | NA | NA |
| 2 | 2019 | Zimbabwe | AFR | 85 | 91 | NR | 78.7% (77.35– 79.98) | NA | NA | NR | NR | NR |
| 3 | 2020 | Burkina Faso | AFR | 88 | 106 | 38.7% (87.8% received one) | 84.4% (83.6–85.2%) | 93% 12–23 months 81% 24–35 months | 87,8% 12–23 m 84.8% 24–35 m | NR | NR | 4.80% |
| 4 | 2020 | Cameroon | AFR | 61 | 92 | 8.30% | 69.7% (68.34–71.01) | NA | NR | 35.70% | NR | NR |
| 5 | 2020 | Ethiopia | AFR | 59 | 102.8 | 81.10% | 81.5% (80.0–83.0%) | NA | 80.30% | NR | 56.70% | 8.10% |
| 6 | 2020 | Uganda | AFR | 87 | 107 | 48.5 (78.4% received one) | 94.4% (93.0–95.5) | 25.30% | 85.40% | 12.00% | NR | 2.60% |
| 7 | 2021 | Central African Republic † | AFR | 41 | 102 for 6–59 m 85.5 for 5–10 yrs | 25% (83% received one) | 94.6% (92.9–96.0%) | NA | 25.2% 12–23 m 15.3% 24–35 m | NR | NR | 3.40% |
| 8 | 2021 | Chad | AFR | 53 | 108.8 (Phase1) 107.5 (Phase2) | 11% (19% reported not having received a card) | 77.4% (74.8–79.8%) | NA | Available as an analysis added later, not in main report. 41.1% 12–23 m 28.2% 24–35 m | NR | 73.5% (IC95%:70.2–76.5%) | 20% (IC95%: 17.6–22.5%) |
| 9 | 2021 | Democratic Republic of the Congo | AFR | 65 | 101.6 | 6.89%; 4.93% (correctly filled in) | 87.52% (87.50–87.53%) | NR | 80.70% | NR | 61.06%. | 7.61% |
| 10 | 2021 | Kenya ‡ | AFR | 90 | 111 | NR | 84.20% | NA | NA | NR | 80.10% | NR |
| 11 | 2021 | Nepal | SEAR | 87 | 101 | 43% | 84% (82–87) Phase 1: 87% (85–89) phase 2: 81% (77–85) | 51.40% | 95.50% | Small sample size of previously zero-dose | Small sample size of previously zero-dose | NR |
| 12 | 2021 | Pakistan | EMR | 81 | 105 | 80% | 93.6% (92.7–94.4) | 47% | 71.5% (and 10% unkown status) 79% for children under 2 years | NR | NR | NR |
| 13 | 2021 | Zambia | AFR | 96 | 91.3 | NA | 68.90% | 63% had documented evidence | 88.5 | NA | NA | NA |
| 14 | 2022 | Burundi | AFR | 89 | 93 | 15% | 88.60% | 91.8% 12–23 m 86.1% 24–35 m | 85.90% | NR | NR | NR |
| 15 | 2022 | Madagascar | AFR | 44 | 95.11 | 56.30% | 65.3% (56.8–72.9) | NA | 69.50% | NR | NR | 19.20% |
| 16 | 2022 | Somalia | EMR | 46 | 90 | NA | 86.0 (83.7–88.0) | 25% 12–23 | 65% | NR | 33.1% 14.1% 12–23 m 37.2% 24–35 m | NR |
| 17 | 2022 or 2023 | Syrian Arab Republic | EMR | 52 | 75.62 | 54.40% | 80.7% (79.35%–81.94%) | NA | 82.8 12–23 m 93.6 24–59 m | NR | 54.30% | NR |
| 18 | 2023 | Cameroon | AFR | 71 | 94.38 | 31.8% (84.9% received a card) | 69.5 (66.4–72.5) | NA | NR | 22.10% | 62.30% | 13.3% |
| 19 | 2023 | Democratic Republic of the Congo | AFR | 52 | 103.6 (May) 85.7 (August) 101.1 (September) | 12.88% | 94.60% (94.60%–94.61%) | ~3.5% (3.47% vaccinated by card seen) | 78.30% | NR | 75.01% | NA |
| 20 | 2023 | Malawi | AFR | 87 | 82.4 | 38.8% | 75.7% (72.3–79.0) | NA | NR | NR | 27.6% for 12–35 m | NR |
| 21 | 2023 | Niger | AFR | 65 | 105 | 71.8% (86.2% received one) | 92.7% (90.8–94.1) | NA | NA | NR | 91.60% | 4.60% |
| 22 | 2023 | Nigeria | AFR | 60 | 96.67 (October) 103.95 (November) 4,823,266 (December) | 47% card and 9.9% finger marking | 87% (84–90) | NR | NR | 12% | NR | NR |
| 23 | 2023 | Yemen ‡ | EMR | 45 | 91 | NA | 84.2% (95% CI: 83.8–84.6) | NA | NR | NR | 12% | NR |
| 24 | 2024 | Ethiopia | AFR | 55 | 98.7 | 16% | 87.10% | 3940 (~25%) | 73.80% | 14.00% | 72% | NR |
| Number of Measles Vaccine Doses Prior to SIA | Vaccinated During SIA (%) | 95% CI (%) | Vaccinated During SIA (Weighted N) | Weighted N |
|---|---|---|---|---|
| Zero | 37.2 | (31.8, 42.8) | 81 | 217 |
| 1 Dose | 92.8 | (89.5, 95.1) | 540 | 582 |
| 2+ Doses | 90.9 | (86.6, 94.0) | 812 | 894 |
| Somalia 2023 (Total) | 84.7 | (81.6, 87.3) | 1433 | 1692 |
| State | % Vac’d During SIA | Total # Children | Total # of Clusters | # Clusters with 100% Children vac’d’ | # Clusters with >50–99.9% Children vac’d | # Clusters with ≤50% Children vac’d | DEFF | ICC |
|---|---|---|---|---|---|---|---|---|
| Banadir | 89.8 | 3604 | 74 | 13 | 57 | 4 | 11.7 | 0.2216 |
| Galmudug | 86.1 | 3338 | 76 | 10 | 61 | 5 | 10.8 | 0.1634 |
| Hirshabelle | 82.6 | 4372 | 76 | 17 | 47 | 12 | 28.5 | 0.4284 |
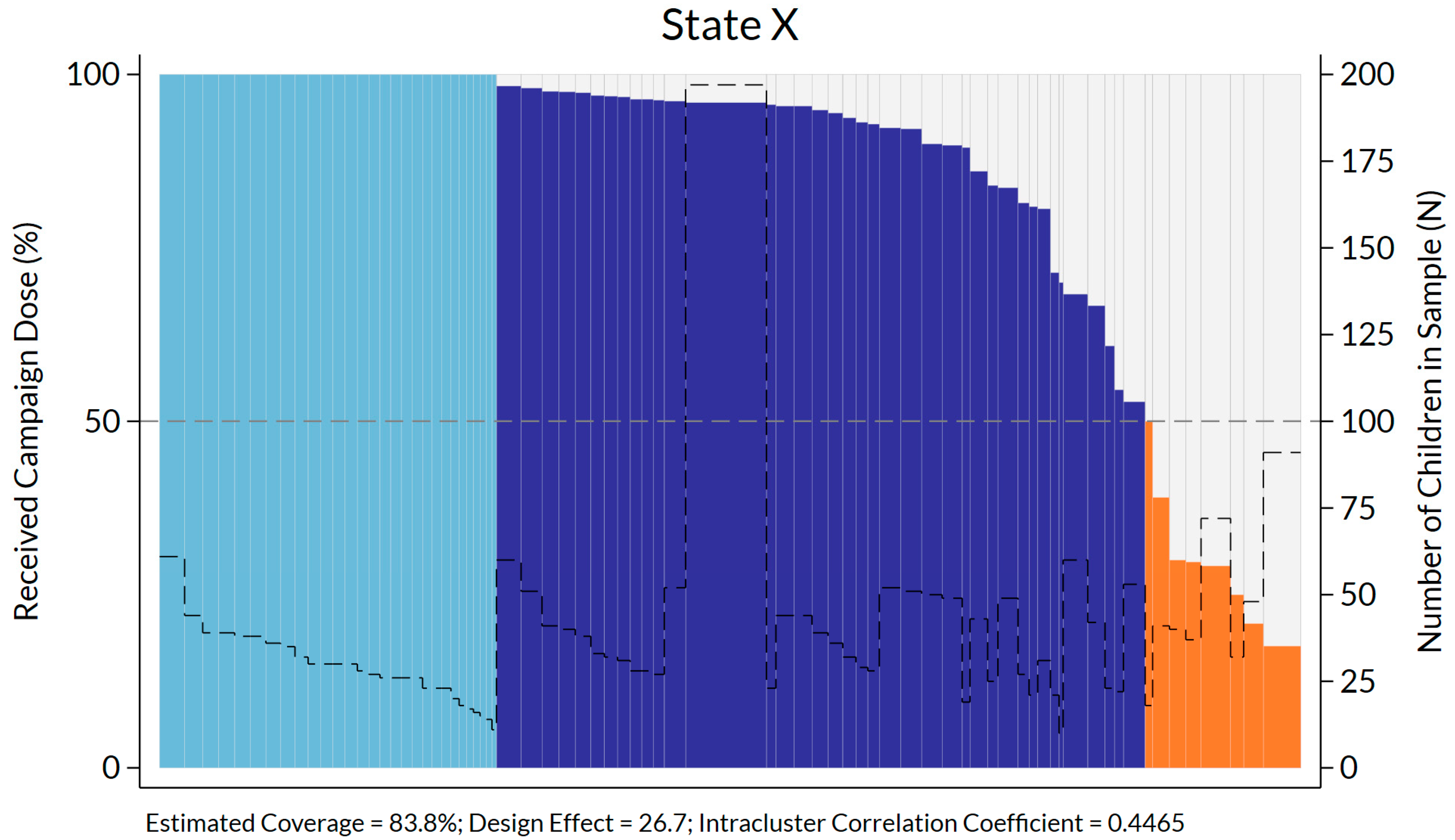
| Jubbaland | 83.8 | 2781 | 76 | 29 | 39 | 8 | 26.7 | 0.4465 |
| Puntland | 88.6 | 3279 | 73 | 18 | 52 | 3 | 14.0 | 0.2361 |
| Southwest | 87 | 3542 | 75 | 20 | 48 | 7 | 17.7 | 0.2791 |
| Total | 87 | 20,916 | 450 | 107 | 304 | 39 | 21.3 | 0.3116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danovaro-Holliday, M.C.; Koh, M.; Steulet, C.; Rhoda, D.A.; Trimner, M.K. Lessons from Recent Measles Post-Campaign Coverage Surveys Worldwide. Vaccines 2024, 12, 1257. https://doi.org/10.3390/vaccines12111257
Danovaro-Holliday MC, Koh M, Steulet C, Rhoda DA, Trimner MK. Lessons from Recent Measles Post-Campaign Coverage Surveys Worldwide. Vaccines. 2024; 12(11):1257. https://doi.org/10.3390/vaccines12111257
Chicago/Turabian StyleDanovaro-Holliday, M. Carolina, Mitsuki Koh, Claudia Steulet, Dale A. Rhoda, and Mary Kay Trimner. 2024. "Lessons from Recent Measles Post-Campaign Coverage Surveys Worldwide" Vaccines 12, no. 11: 1257. https://doi.org/10.3390/vaccines12111257
APA StyleDanovaro-Holliday, M. C., Koh, M., Steulet, C., Rhoda, D. A., & Trimner, M. K. (2024). Lessons from Recent Measles Post-Campaign Coverage Surveys Worldwide. Vaccines, 12(11), 1257. https://doi.org/10.3390/vaccines12111257







