Hepatitis B Virus-Related Cirrhosis and Hepatocellular Carcinoma Hospital Discharge Rates from 2005 to 2021 in Spain: Impact of Universal Vaccination
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeng, W.J.; Papatheodoridis, G.V.; Lok, A.S.F. Hepatitis B. Lancet 2023, 401, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Tai, D.; Chu, C.M.; Chen, T.J. The development of cirrhosis in patients with chronic type hepatitis B: A prospective study. Hepatology 1998, 8, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.J.; Lok, A.S. Natural history of chronic hepatitis B virus infection: What we knew in 1981 and what we know in 2005. Hepatology 2006, 43, S173–S181. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Goulder, P.; Matthews, P.C. Sexual dimorphism in chronic hepatitis B virus infection: Evidence to inform elimination efforts. Wellcome Open Res. 2022, 7, 32. [Google Scholar] [CrossRef]
- Hamilton, E.; Yang, L.; Mentzer, A.J.; Guo, Y.; Chen, Y.; Lv, J.; Fletcher, R.; Wright, N.; Lin, K.; Walters, R.; et al. Conventional and genetic risk factors for chronic Hepatitis B virus infection in a community-based study of 0.5 million Chinese adults. Sci. Rep. 2022, 12, 12075. [Google Scholar] [CrossRef]
- Ward, J.W.; Wanlapakorn, N.; Poovorawan, Y.; Shouval, D. Hepatitis B vaccines. In Plotkin’s Vaccines, 8th ed.; Orenstein, W.A., Offit, P.A., Edwards, K.M., Plotin, S.A., Eds.; Elsevier: Philadelphia, PA, USA, 2024; pp. 389–432. [Google Scholar]
- Xie, J.; Wang, X.; Wang, X.; Li, J.; Jie, Y.; Hao, Y.; Gu, J. Assessing the impact of comorbid type 2 diabetes mellitus on the disease burden of chronic hepatitis B virus infection and its complications in China from 2006 to 2030: A modeling study. Glob. Health Res. Policy 2024, 9, 5. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Sheena, B.S.; Hiebert, L.; Han, H.; Ippolito, H.; Abbasi-Kangevari, M.; Abbasi-Kangevari, Z.; Abbastabar, H.; Abdoli, A.; Abubaker Ali, H.; Adane, M.M.; et al. Global, regional, and national burden of hepatitis B, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 796–829. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries. 2024. Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 9 April 2024).
- Salleras, L.; Domínguez, A.; Bruguera, M.; Plans, P.; Costa, J.; Cardeñosa, N.; Batalla, J.; Plasència, A. Declining prevalence of hepatitis B virus infection in Catalonia (Spain) 12 years after the introduction of universal vaccination. Vaccine 2007, 25, 8726–8731. [Google Scholar] [CrossRef]
- Limia, A.; Olmedo, C.; Soler, M.; Cantero, E.; Sánchez-Cambronero, L. Committee for Immunization Programme and Registry and changes in the National Immunization Programme in Spain. Rev. Esp. Salud Publica 2020, 94, e202003018. [Google Scholar]
- Arístegui, J.; Díez-Domingo, J.; Marés, J.; Martinón, F. Vaccination against hepatitis B. Impact of vaccination programmes after 20 years of use in Spain. Is it time for a change? Enferm. Infecc. Microbiol. Clin. 2015, 33, 113–118. [Google Scholar]
- Borrás, E.; Urbiztondo, L.; Carmona, G.; Jané, M.; Barrabeig, I.; Sala, M.R.; Balañà, J.P.J.; Company, M.; Parrón, I.; Godoy, P.; et al. Effectiveness and impact of the hepatitis B vaccination program in preadolescents in Catalonia 21 years after its introduction. Vaccine 2019, 37, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad. Vaccination Coverage History. Spain 2000–2013. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/coberturas/docs/Todas_las_tablas2013.pdf (accessed on 20 March 2024).
- Ministerio de Sanidad. Evolution of Primary Vaccination Coverage. Spain 2010–2020. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/coberturas/docs/Todas_las_tablas2020.pdf (accessed on 20 March 2024).
- Hernando, V.; Soler, P.; Garrido, M.; Cano, R.; Llácer, A. Epidemiological surveillance of hepatitis B in Spain, 1997 to 2008. Bol. Epidem. Sem. 2010, 18, 169–180. [Google Scholar]
- Hernando, V.; Ruiz-Algueró, M.; Díaz, A. Analysis of the evolution of acute hepatitis B in Spain, 2008–2018. Bol. Epidem. Sem. 2019, 27, 43–53. [Google Scholar]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224, S343–S351. [Google Scholar] [CrossRef]
- Ruggieri, A.; Gagliardi, M.C.; Anticoli, S. Sex-dependent outcome of hepatitis B and C viruses infections: Synergy of sex hormones and immune responses? Front. Immunol. 2018, 9, 2302. [Google Scholar] [CrossRef]
- Diallo, I.; Ndiaye, B.; Touré, M.; Sow, A.; Mbengue, A.; Diawara, P.S.; Boury Gning, S.; Mbaye, P.S.; Fall, F.; Mbengue, M. Hepatocellular carcinoma in Senegal: Epidemiological, clinical and etiological aspects about 229 cases at Hôpital Principal de Dakar. Pan Afr. Med. J. 2021, 38, 99. [Google Scholar] [CrossRef]
- Khan, M.A.; Haider, M.S.; Nusrat, B.; Abbas Razvi, S.K.; Shah, Z.Z.; Shah, A.M.; Khalid, T.; Haleem, F. Demographics, biochemical characteristics, and phases of chronic hepatitis B virus infection: Retrospective analysis from a secondary care setup. Cureus 2021, 13, e16558. [Google Scholar]
- Liu, M.; Li, L.; Zhao, J.; Ungvari, G.S.; Ng, C.H.; Duan, Z.; Zheng, S.J.; Xiang, Y.T. Gender differences in demographic and clinical characteristics in patients with HBV-related liver diseases in China. PeerJ 2022, 10, e13828. [Google Scholar] [CrossRef]
- Stroffolini, T.; Esvan, R.; Biliotti, E.; Sagnelli, E.; Gaeta, G.B.; Almasio, P.L. Gender differences in chronic HBsAg carriers in Italy: Evidence for the independent role of male sex in severity of liver disease. J. Med. Virol. 2015, 87, 1899–1903. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Fernández Carrillo, C.; Iruzubieta, P.; Hernández-Conde, M.; Rasines, L.; Jorquera, F.; Albillos, A.; Bañares, R.; Mora, P.; Fernández-Vásquez, I.; et al. Massive impact of coronavirus disease 2019 pandemic on gastroenterology and hepatology departments and doctors in Spain. J. Gastroenterol. Hepatol. 2021, 36, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, J.; Nielsen, P.B.; Søgaard, M.; Dalager-Pedersen, M.; Zacho Speiser, L.O.; Yndigegn, T.; Nielsen, H.; Larsen, T.B.; Skjøth, F. Hospital admission and mortality rates for non-covid diseases in Denmark during the Covid-19 pandemic: Nationwide population based cohort study. BMJ 2021, 373, n1135. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Idilman, R.; Dalekos, G.; Buti, M.; Chi, H.; van Boemmel, F.; Calleja, J.L.; Sypsa, V.; Goulis, J.; Manolakopoulos, S.; et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017, 66, 1444–1453. [Google Scholar] [CrossRef]
- Negro, F.; Müllhaupt, B.; Semela, D.; Blach, S.; Bruggmann, P.; De Gottardi, A.; Dufour, J.F.; Fraga, M.; Galante, A.; Razavi, H.; et al. The current and future burden of hepatitis B in Switzerland: A modelling study. Swiss Med. Wkly. 2023, 153, 40086. [Google Scholar] [CrossRef]
- Huang, C.; Wu, Y.; Zhang, C.; Ji, D.; Wang, F.S. The burden of cirrhosis and other chronic liver diseases due to hepatitis B in children and adolescents: Results from global burden of disease study 2019. Front. Public Health 2023, 11, 1315392. [Google Scholar] [CrossRef]
- Sagnelli, E.; Stroffolini, T.; Sagnelli, C.; Morisco, F.; Coppola, N.; Smedile, A.; Pisaturo, M.; Colloredo, G.; Babudieri, S.; Licata, A.; et al. Influence of universal HBV vaccination on chronic HBV infection in Italy: Results of a cross-sectional multicenter study. J. Med. Virol. 2017, 89, 2138–2143. [Google Scholar] [CrossRef]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lai, M.W.; Wu, T.C.; Wu, S.F.; Lee, C.M.; Yang, S.S.; Chu, H.C.; et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 2016, 151, 472–480.E1. [Google Scholar] [CrossRef]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef]
- Chiang, C.J.; Jhuang, J.R.; Yang, Y.W.; Zhuang, B.Z.; You, S.L.; Lee, W.C.; Chen, C.J. Association of nationwide hepatitis B vaccination and antiviral therapy programs with end-stage liver disease burden in Taiwan. JAMA Netw. Open 2022, 5, e2222367. [Google Scholar] [CrossRef]
- Qu, C.; Chen, T.; Fan, C.; Zhan, Q.; Wang, Y.; Lu, J.; Lu, L.L.; Ni, Z.; Huang, F.; Yao, H.; et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: A cluster randomized controlled trial. PLoS Med. 2014, 11, e1001774. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.J.; Bulkow, L.R.; Sigleton, R.J.; Williams, J.; Snowball, M.; Homan, C.; Parkinson, A.J. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011, 54, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, W.Q. The impact of universal hepatitis B vaccine on the trend of liver cancer from the Global Burden of Disease Study 2017. Liver Int. 2021, 41, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, P.A.; Fornari, C.; Conti, S.; Cosimo Antonazzo, I.; Ferrara, P.; Ahmed, A.; Andrei, C.L.; Andrei, T.; Artamonov, A.A.; Banach, M.; et al. Hepatitis B and C in Europe: An update from the Global Burden of Disease Study 2019. Lancet Public Health 2023, 8, e701–e716. [Google Scholar] [CrossRef]
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. 2016. Available online: https://www.who.int/publications/i/item/combating-hepatitis-b-and-c-to-reach-elimination-by-2030 (accessed on 5 April 2024).
- Cao, G.; Liu, J.; Liu, M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: Findings from the Global Burden of Disease Study. Chin. Med. J. 2022, 135, 2049–2055. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Greco, G.; Serapide, F.; Scaglione, V.; Morrone, H.; Marascio, N.; Giancotti, A.; Liberto, M.C.; Matera, G.; Trecarichi, E.M.; et al. Outcome of HBV screening and vaccination in a migrant population in southern Italy. Infez. Med. 2021, 29, 236–241. [Google Scholar]
- Serraino, R.; Mazzitelli, M.; Greco, G.; Serapide, F.; Scaglione, V.; Marascio, N.; Trecarichi, E.M.; Torti, C. Risk factors for hepatitis B and C among healthy population: A community-based survey from four districts of Southern Italy. Infez. Med. 2020, 28, 223–226. [Google Scholar]
- Rodríguez-Tajes, S.; Domínguez, A.; Carrión, J.A.; Buti, M.; Quer, J.C.; Morillas, R.M.; López, C.; Torras, X.; Baliellas, C.; Vergara, M.; et al. Significant decrease in the prevalence of hepatitis C infection after the introduction of direct acting antivirals. J. Gastroenterol. Hepatol. 2020, 35, 1570–1578. [Google Scholar] [CrossRef]
- García, A.; Vigil, K.J.; Taylor, B.; Kshirsagar, O.; Thamer, M.; Jain, M.K. Persistent low prevalence of hepatitis B vaccination among people with HIV: Time for a change? J. Viral Hepat. 2023, 30, 790–792. [Google Scholar] [CrossRef]
- Soriano, V.; Aguilera, A.; Benito, R.; González-Díez, R.; Miró, E.; Liendo, P.; Rodríguez-Dias, J.C.; Cabezas, T.; Richart, A.; Ramos, J.M.; et al. Susceptibility to hepatitis B virus infection in adults living in Spain. Liver Int. 2023, 43, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Al-Busafi, S.A.; Alwassief, A. Global perspectives on the hepatitis B vaccination: Challenges, achievement, and the road to elimination by 2030. Vaccines 2024, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Boeras, D.I.; Marinucci, F.; Easterbrook, P. The future of viral hepatitis testing: Innovations in testing technologies and approaches. BMC Infect. Dis. 2017, 17 (Suppl. 1), 699. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Hepatitis Report. 2017. Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 24 October 2024).
- Liu, Z.; Li, M.; Hutton, D.W.; Wagner, A.L.; Yao, Y.; Zhu, W.; Cao, L.; Tang, S.; Pan, J.; Wang, Y.; et al. Impact of the national hepatitis B immunization program in China: A modeling study. Infect. Dis. Poverty 2022, 11, 106. [Google Scholar] [CrossRef]
- Boccalini, S.; Bonito, B.; Zanella, B.; Liedl, D.; Bonanni, P.; Bechini, A. The first 30 years of the universal hepatitis-B vaccination-program in Italy: A health strategy with a relevant and favorable economic-profile. Int. J. Environ. Res. Public Health 2022, 19, 16365. [Google Scholar] [CrossRef]
- Umemura, T.; Wattanakamolkul, K.; Nayakama, Y.; Takahashi, Y.; Sbarigia, U.; KyungHwa, L.; Villasis-Keever, A.; Furegato, M.; Gautier, L.; Nowacki, G.; et al. Real-world epidemiology, clinical and economic burden of chronic hepatitis B in Japan: A retrospective study using JMDC claims database. Infect. Dis. Ther. 2023, 12, 1337–1349. [Google Scholar] [CrossRef]
- Ginsberg, G.M.; Shouval, D. Cost-benefit analysis of a nationwide neonatal inoculation programme against hepatitis B in an area of intermediate endemicity. J. Epidemiol. Community Health 1992, 46, 587–594. [Google Scholar] [CrossRef][Green Version]
- Howell, J.; Pedrana, A.; Schroeder, S.E.; Scott, N.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Hirnschall, G.; Hoen, E.; Hutchinson, S.J.; et al. A global investment framework for the elimination of hepatitis B. J. Hepatol. 2021, 74, 535–549. [Google Scholar] [CrossRef]
- World Hepatitis Alliance. The Importance of Hepatitis B and C Control and Elimination. Available online: https://www.worldhepatitisalliance.org/wp-content/uploads/2018/06/20170906_wha_hcvhbv_advocacy_final_update_edited_web_version.pdf (accessed on 8 May 2024).
- Flores, J.E.; Thompson, A.J.; Ryan, M.; Howell, J. The global impact of hepatitis B vaccination on hepatocellular carcinoma. Vaccines 2022, 10, 793. [Google Scholar] [CrossRef]
- Waheed, Y. Progress on global hepatitis elimination targets. World J. Gastroenterol. 2021, 27, 8199–8200. [Google Scholar] [CrossRef] [PubMed]
- Viral hepatitis elimination—Time to act. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 529. [CrossRef] [PubMed]
- Lim, S.G. WHO 2030 HBV elimination goals: A goal too far? Lancet Gastroenterol. Hepatol. 2023, 8, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.S.; Flower, B.; Cunningham, E.; Marshall, A.D.; Lazarus, J.V.; Palayew, A.; Jia, J.; Aggarwal, R.; Al-Mahtab, M.; Tanaka, Y.; et al. Progress towards elimination of viral hepatitis: A Lancet Gastroenterology & Hepatology Commission update. Lancet Gastroenterol. Hepatol. 2024, 9, 346–365. [Google Scholar]
- Bruguera, M.; Bañares, R.; Córdoba, J.; Jardí, R.; González Lahoz, J.; Ladero, J.M.; Pajares, J.M.; Segura, A. Documento de consenso de la AAEEH sobre el tratamiento de las infecciones por los virus de las hepatitis B y C. Gastroenterol. Hepatol. 2006, 29 (Suppl. 1), 216–230. [Google Scholar] [CrossRef]
- Khatun, M.S.; Biswas, M.H.A. Optimal control strategies for preventing hepatitis B infection and reducing chronic liver cirrhosis incidence. Infect. Dis. Model. 2019, 27, 91–110. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Informe del SNS 2020–2021. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnualSNS2020_21/ASPECTOS_RELEVANTES_2020-21.pdf (accessed on 10 July 2024).
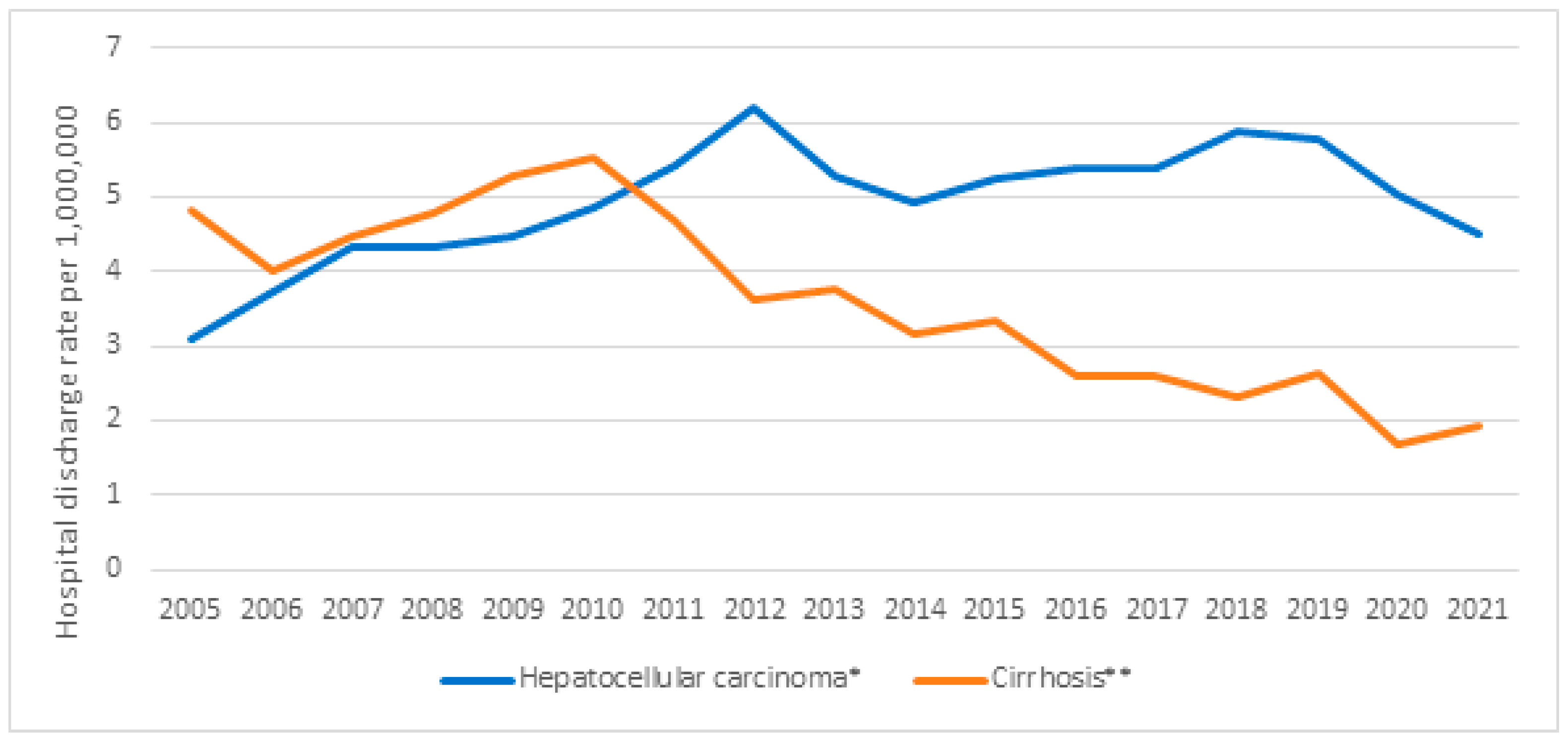

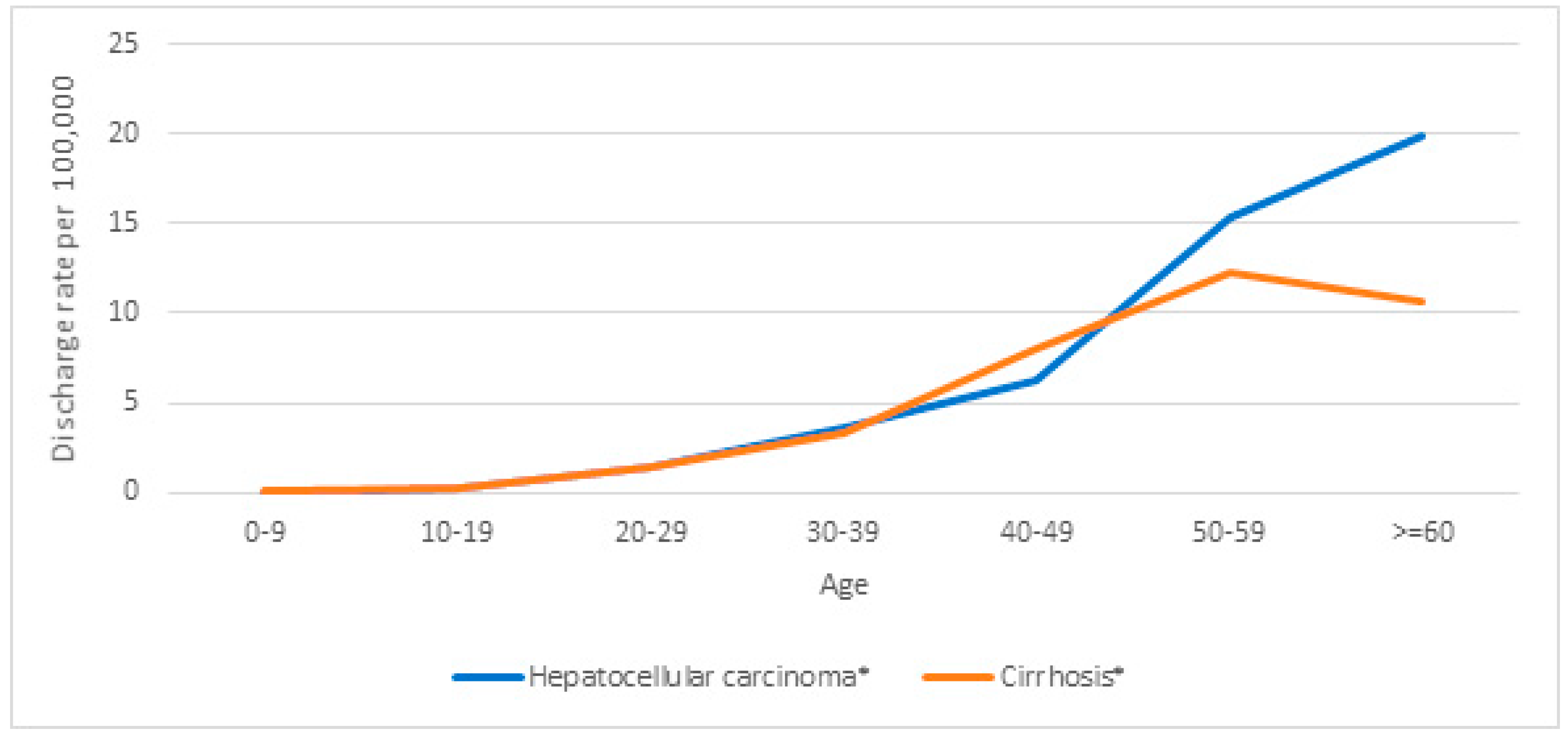
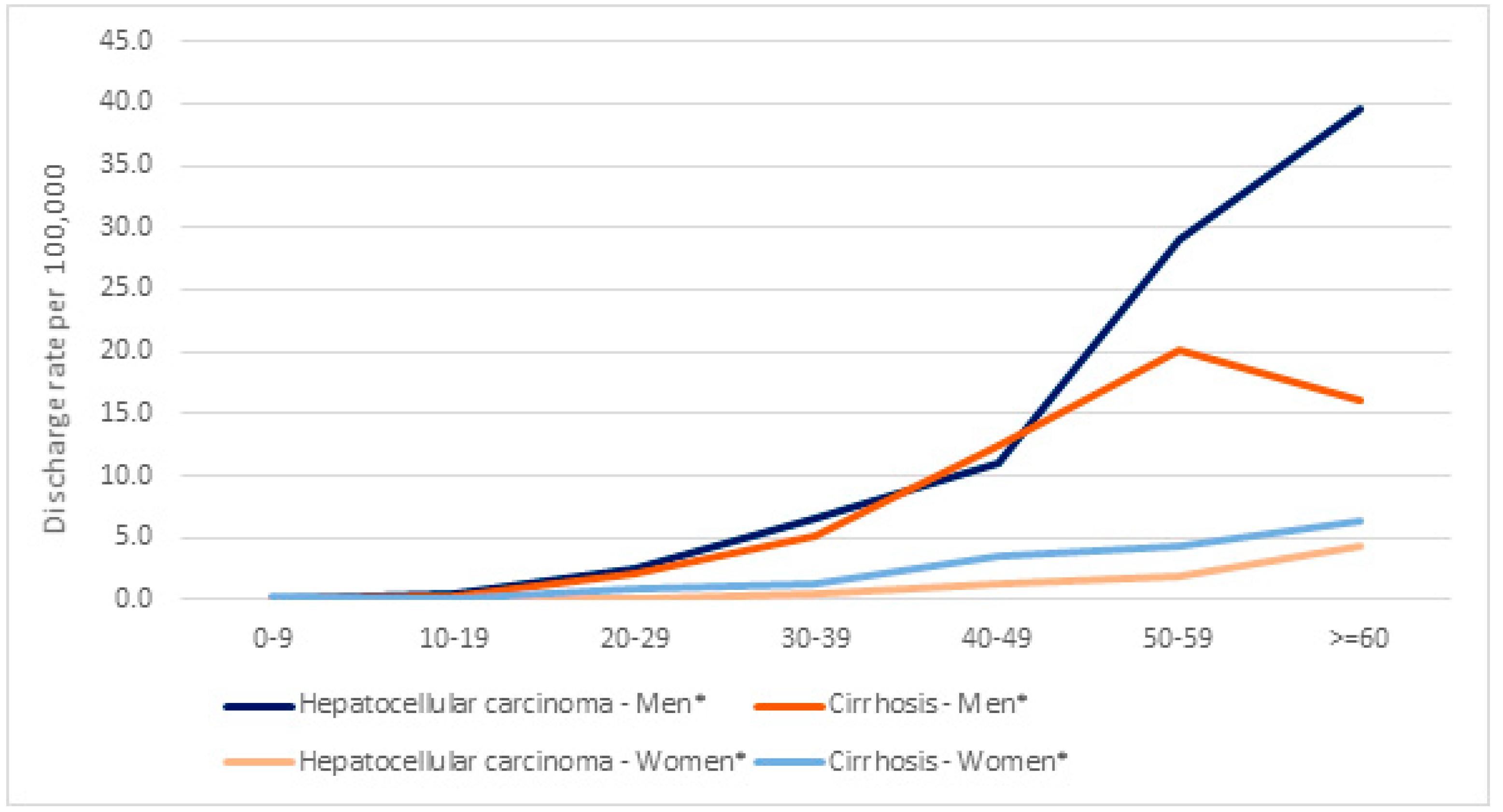
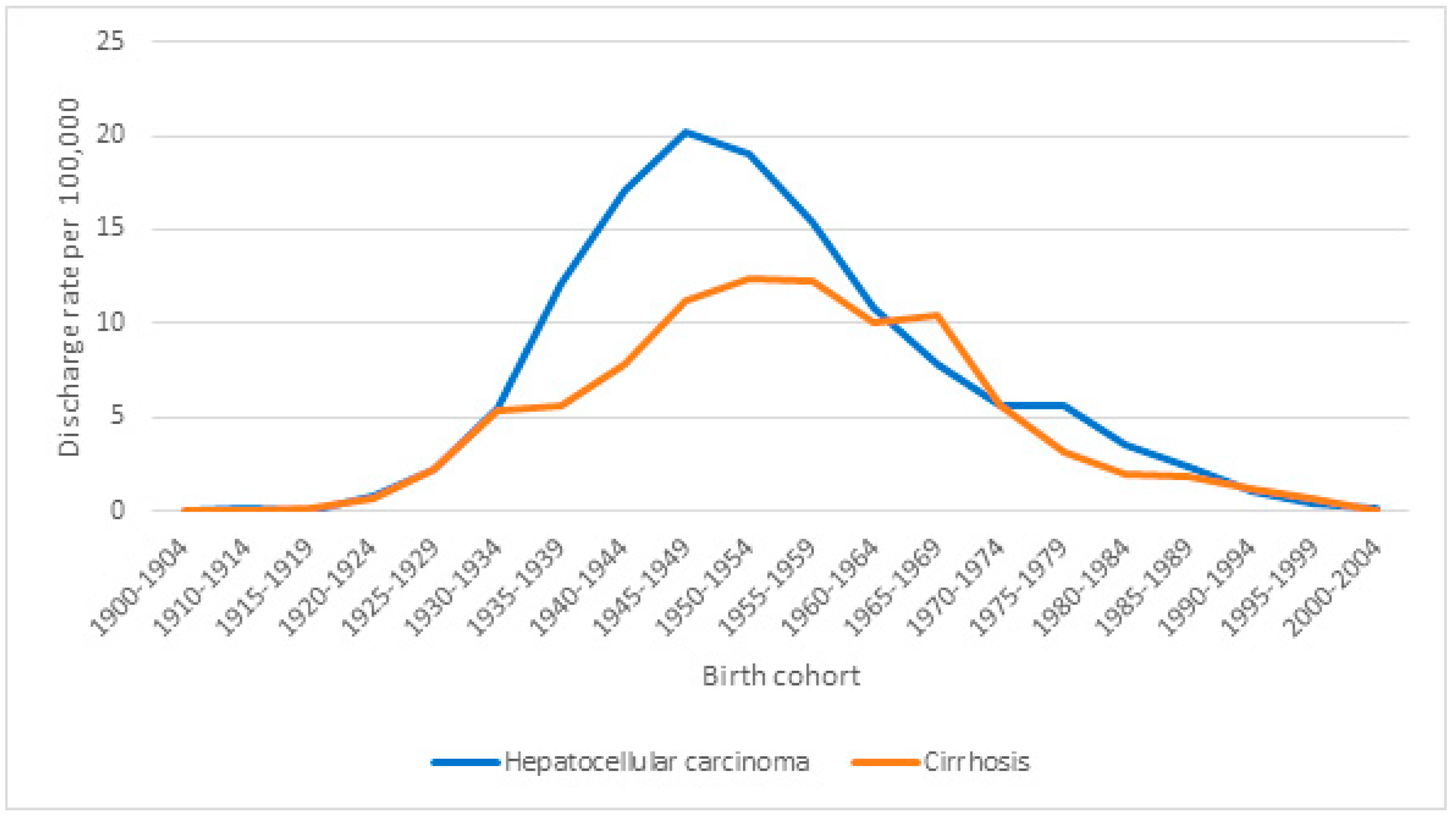
| Hospital Discharges | Hospital Discharges per 1,000,000 | HDRR (CI 95%) | p-Value | |
|---|---|---|---|---|
| All hospitalizations | ||||
| Overall | ||||
| 2005–2013 | 3821 | 9.19 | Ref. | |
| 2014–2021 | 2922 | 7.79 | 0.85 (0.81–0.89) | <0.001 |
| Male | ||||
| 2005–2013 | 3151 | 15.36 | Ref. | |
| 2014–2021 | 2470 | 13.43 | 0.87 (0.83–0.92) | <0.001 |
| Female | ||||
| 2005–2013 | 670 | 3.18 | Ref. | |
| 2014–2021 | 452 | 2.36 | 0.74 (0.66–0.84) | <0.001 |
| Hepatocellular carcinoma | ||||
| Overall | ||||
| 2005–2013 | 1933 | 4.65 | Ref. | |
| 2014–2021 | 1975 | 5.26 | 1.13 (1.06–1.20) | <0.001 |
| Male | ||||
| 2005–2013 | 1737 | 8.45 | Ref. | |
| 2014–2021 | 1776 | 9.65 | 1.14 (1.07–1.22) | <0.001 |
| Female | ||||
| 2005–2013 | 196 | 0.93 | Ref. | |
| 2014–2021 | 199 | 1.04 | 1.12 (0.92–1.36) | 0.27 |
| Cirrhosis | ||||
| Overall | ||||
| 2005–2013 | 1888 | 4.54 | Ref. | |
| 2014–2021 | 947 | 2.52 | 0.56 (0.51–0.60) | <0.001 |
| Male | ||||
| 2005–2013 | 1414 | 6.89 | Ref. | |
| 2014–2021 | 694 | 3.77 | 0.55 (0.50–0.60) | <0.001 |
| Female | ||||
| 2005–2013 | 474 | 2.25 | Ref. | |
| 2014–2021 | 253 | 1.32 | 0.59 (0.50–0.68) | <0.001 |
| Hospital Discharges | Hospital Discharges per 1,000,000 | HDRR (CI 95%) | p-Value | |
|---|---|---|---|---|
| Overall | ||||
| Vaccinated cohorts | 233 | 1.05 | 0.53 (0.45–0.62) | <0.001 |
| Unvaccinated cohorts | 438 | 1.98 | Ref. | |
| Hepatocellular carcinoma | ||||
| Vaccinated cohorts | 138 | 0.62 | 0.66 (0.53–0.82) | <0.001 |
| Unvaccinated cohorts | 209 | 0.94 | Ref. | |
| Cirrhosis | ||||
| Vaccinated cohorts | 95 | 0.43 | 0.41 (0.33–0.53) | <0.001 |
| Unvaccinated cohorts | 229 | 1.03 | Ref. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, A.; Avellón, A.; Hernando, V.; Soldevila, N.; Borràs, E.; Martínez, A.; Izquierdo, C.; Torner, N.; Pericas, C.; Rius, C.; et al. Hepatitis B Virus-Related Cirrhosis and Hepatocellular Carcinoma Hospital Discharge Rates from 2005 to 2021 in Spain: Impact of Universal Vaccination. Vaccines 2024, 12, 1254. https://doi.org/10.3390/vaccines12111254
Domínguez A, Avellón A, Hernando V, Soldevila N, Borràs E, Martínez A, Izquierdo C, Torner N, Pericas C, Rius C, et al. Hepatitis B Virus-Related Cirrhosis and Hepatocellular Carcinoma Hospital Discharge Rates from 2005 to 2021 in Spain: Impact of Universal Vaccination. Vaccines. 2024; 12(11):1254. https://doi.org/10.3390/vaccines12111254
Chicago/Turabian StyleDomínguez, Angela, Ana Avellón, Victoria Hernando, Núria Soldevila, Eva Borràs, Ana Martínez, Conchita Izquierdo, Núria Torner, Carles Pericas, Cristina Rius, and et al. 2024. "Hepatitis B Virus-Related Cirrhosis and Hepatocellular Carcinoma Hospital Discharge Rates from 2005 to 2021 in Spain: Impact of Universal Vaccination" Vaccines 12, no. 11: 1254. https://doi.org/10.3390/vaccines12111254
APA StyleDomínguez, A., Avellón, A., Hernando, V., Soldevila, N., Borràs, E., Martínez, A., Izquierdo, C., Torner, N., Pericas, C., Rius, C., & Godoy, P. (2024). Hepatitis B Virus-Related Cirrhosis and Hepatocellular Carcinoma Hospital Discharge Rates from 2005 to 2021 in Spain: Impact of Universal Vaccination. Vaccines, 12(11), 1254. https://doi.org/10.3390/vaccines12111254







