Humoral Response to SARS-CoV-2 Vaccine-Boost in Cancer Patients: A Case Series from a Southern European Cancer Center
Abstract
1. Introduction
2. Materials and Methods
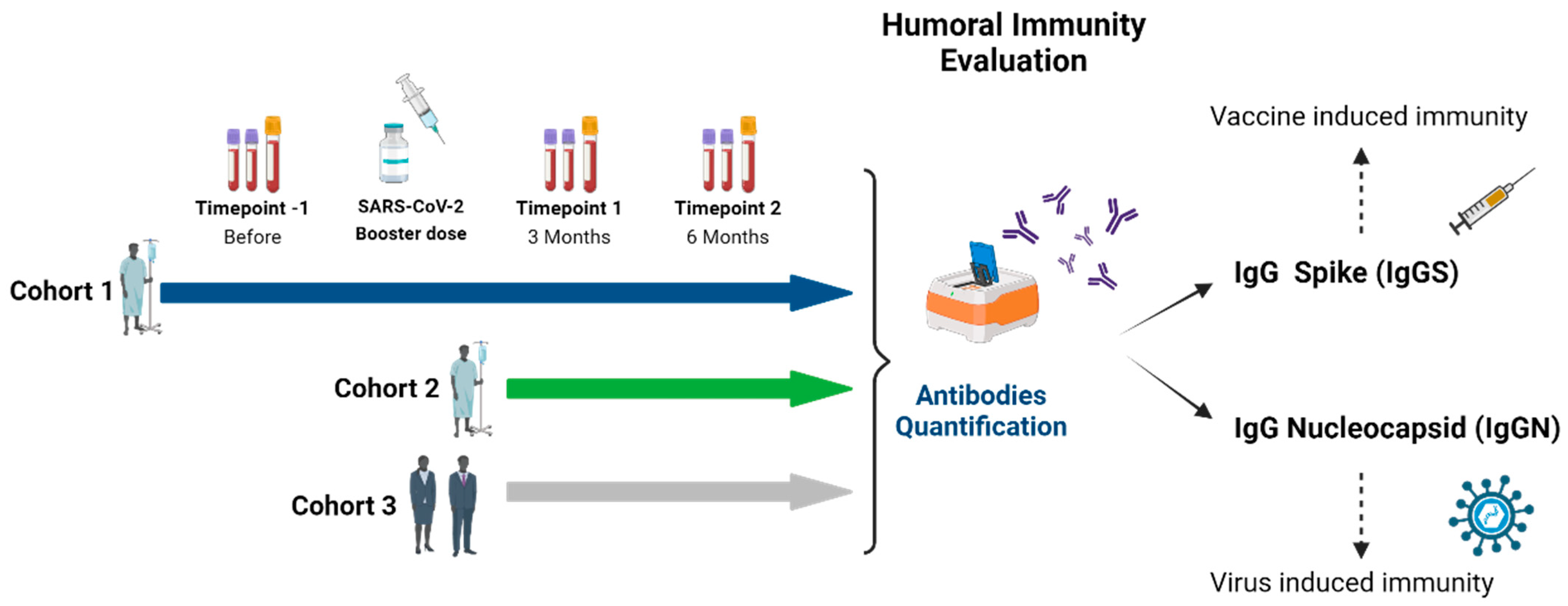
2.1. Study Design
2.2. Study Population
2.3. Humoral Immunity Analysis
2.4. Statistical Analysis
3. Results
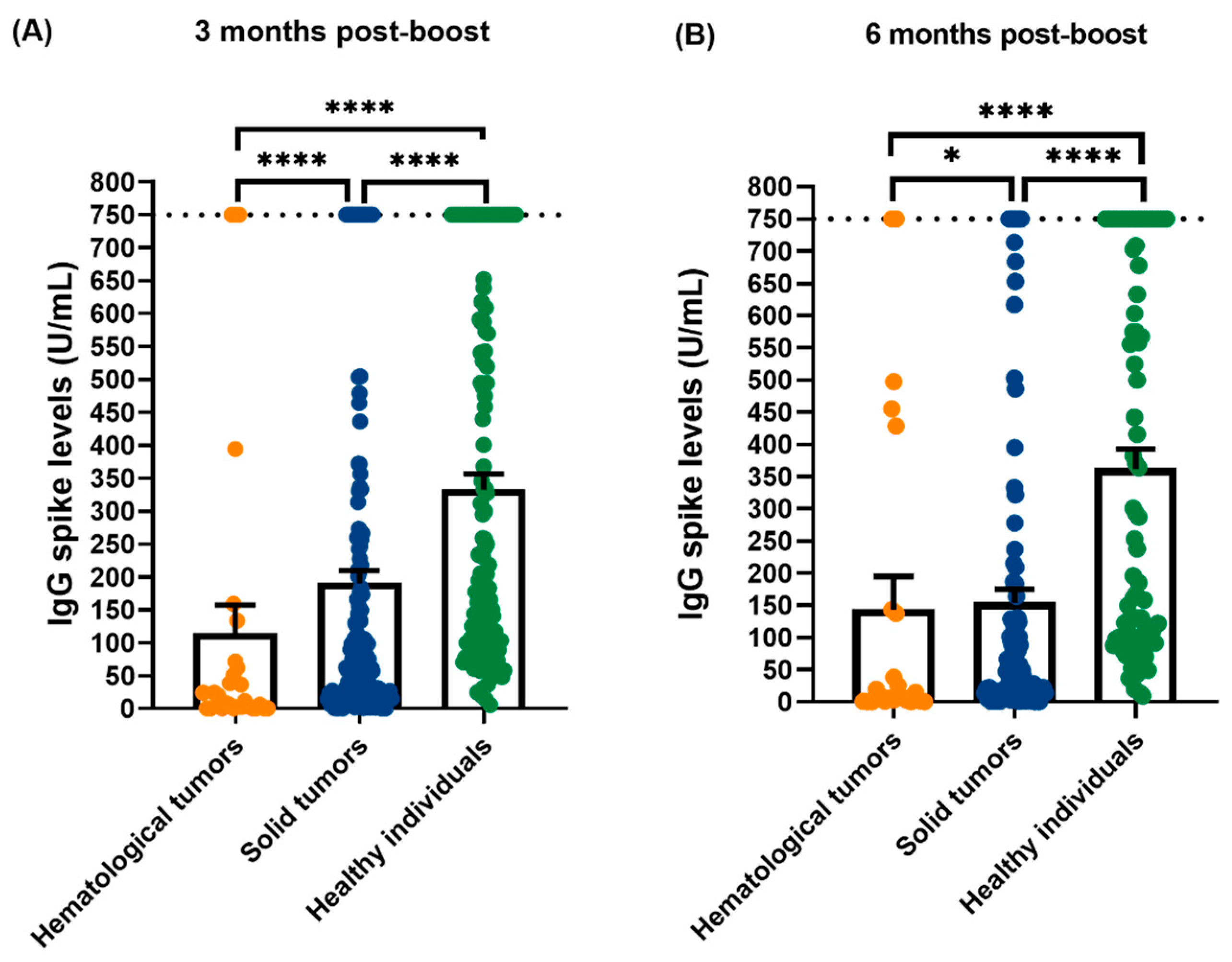
3.1. Quantification of IgG Levels Against SARS-CoV-2 Spike (S) and Nucleocapsid (N)
3.2. Stratified Analyzed of IgG Spike Levels in Hematological Patients
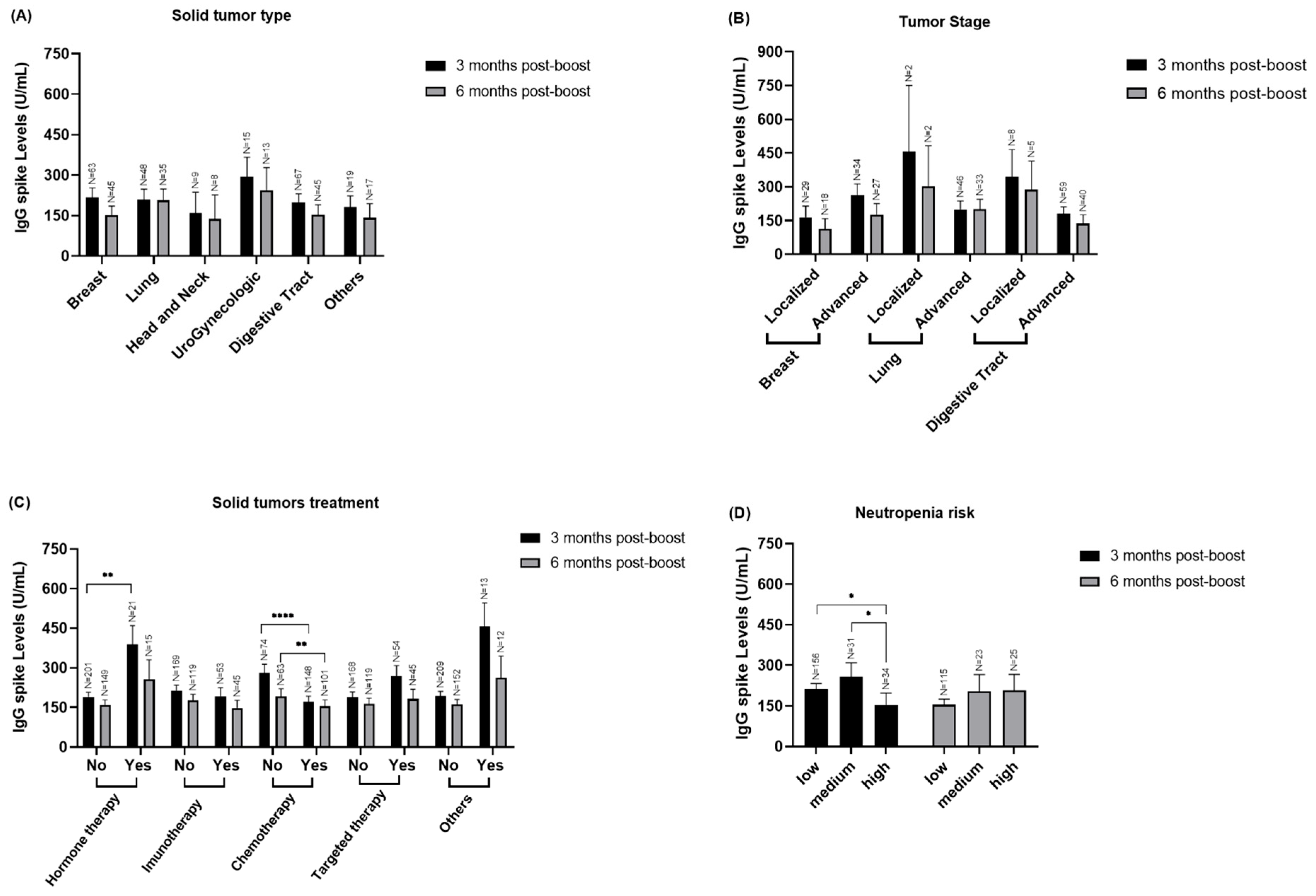
3.3. Stratified Analyzed of IgG Spike Levels in to Solid Cancer Patients
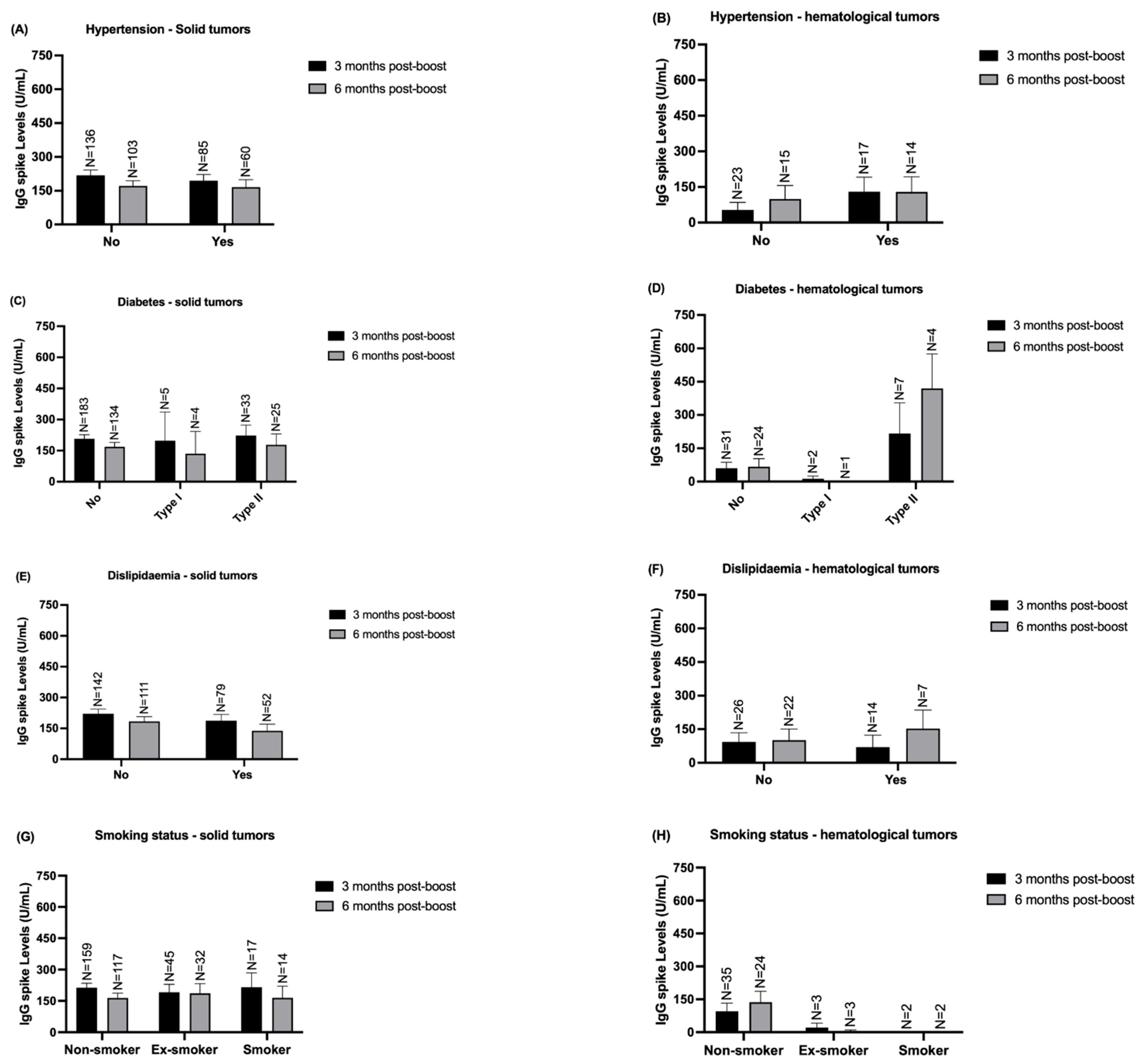
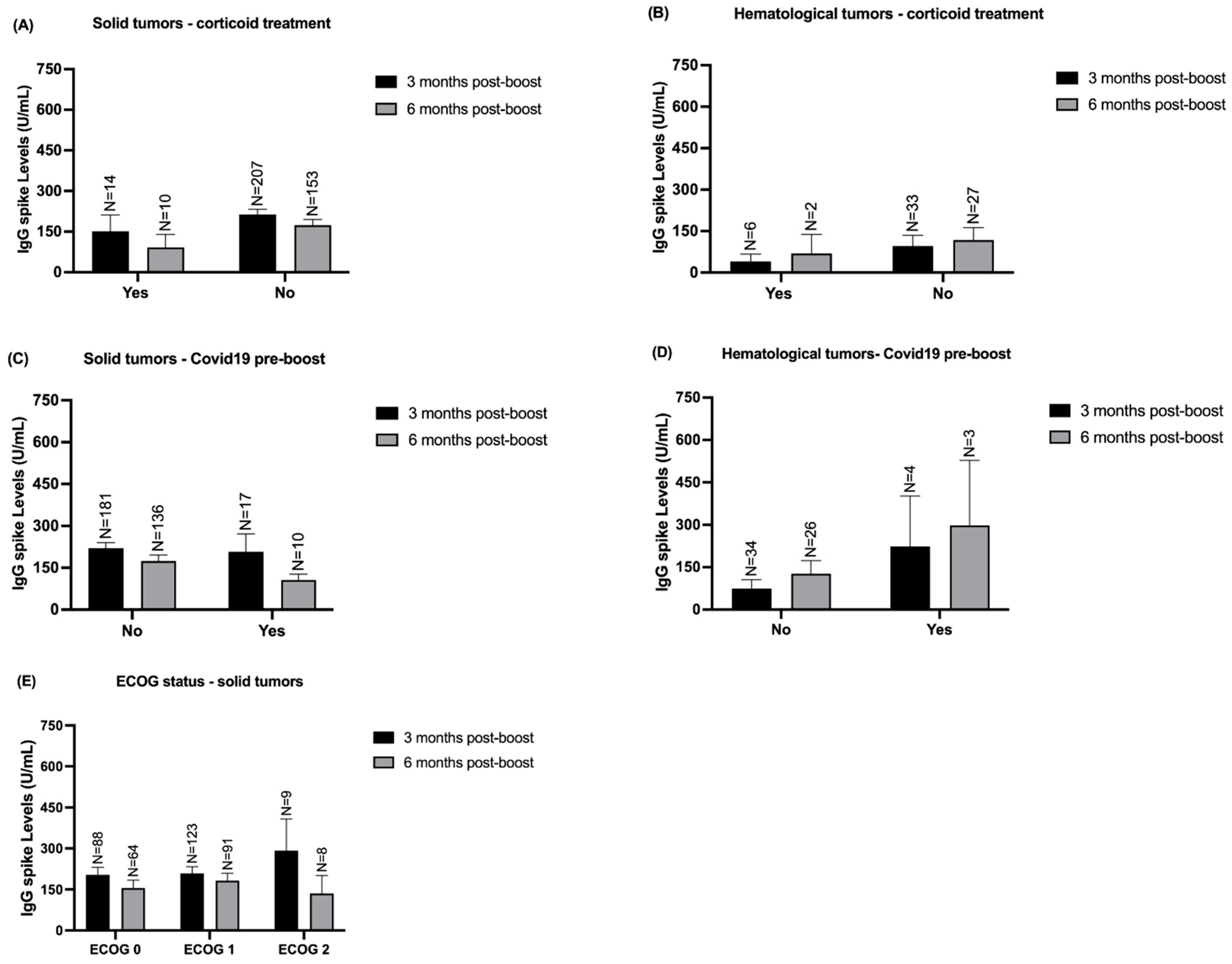
3.4. Stratified Analysis of IgG Spike Levels According to Solid and Hematological Patients’ Comorbidities and Clinical Variables
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, v111–v118. [Google Scholar] [CrossRef] [PubMed]
- Gribben, J.G.; Fowler, N.; Morschhauser, F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2803–2811. [Google Scholar] [CrossRef]
- Voelcker, G. The Mechanism of Action of Cyclophosphamide and Its Consequences for the Development of a New Generation of Oxazaphosphorine Cytostatics. Sci. Pharm. 2020, 88, 42. [Google Scholar] [CrossRef]
- Benito, J.M.; López, M.; Lozano, S.; Ballesteros, C.; González-Lahoz, J.; Soriano, V. Hydroxyurea exerts an anti-proliferative effect on T cells but has no direct impact on cellular activation. Clin. Exp. Immunol. 2007, 149, 171–177. [Google Scholar] [CrossRef]
- Peppas, I.; George, G.; Sollie, S.; Josephs, D.H.; Hammar, N.; Walldius, G.; Karagiannis, S.N.; Van Hemelrijck, M. Association of Serum Immunoglobulin Levels with Solid Cancer: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 527–538. [Google Scholar] [CrossRef]
- Schauenstein, E.; Lahousen, M.; Weblacher, M.; Steinschifter, W.; Estelberger, W.; Schauenstein, K. Selective decrease in serum immunoglobulin G1. A tissue nonspecific tumor marker detecting early stages of gynecologic malignant disease with high efficiency. Cancer 1996, 78, 511–516. [Google Scholar] [CrossRef]
- Kronberger, L.; Steinschifter, W.; Weblacher, M.; Estelberger, W.; Liebmann, P.M.; Rabl, H.; Smola, M.; Lax, S.F.; Mischinger, H.J.; Schauenstein, E.; et al. Selective decrease of serum immunoglobulin G1 as marker for early stages of invasive breast cancer. Breast Cancer Res. Treat. 2000, 64, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Schauenstein, E.; Rabl, H.; Steinschifter, W.; Hirschmann, C.; Estelberger, W.; Schauenstein, K. Selective decrease of serum immunoglobulin G1 as a marker of malignant transformation in colorectal tissue. Cancer 1997, 79, 1482–1486. [Google Scholar] [CrossRef]
- Rieger, C.T.; Liss, B.; Mellinghoff, S.; Buchheidt, D.; Cornely, O.A.; Egerer, G.; Heinz, W.J.; Hentrich, M.; Maschmeyer, G.; Mayer, K.; et al. Anti-infective vaccination strategies in patients with hematologic malignancies or solid tumors—Guideline of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO). Ann. Oncol. 2018, 29, 1354–1365. [Google Scholar] [CrossRef]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef]
- Mackall, C.L.; Fleisher, T.A.; Brown, M.R.; Magrath, I.T.; Shad, A.T.; Horowitz, M.E.; Wexler, L.H.; Adde, M.A.; McClure, L.L.; Gress, R.E. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994, 84, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Kantar, M.; Cetingül, N.; Kansoy, S.; Kütükçüler, N.; Aksu, G. Immune deficiencies following cancer treatment in children. J. Trop. Pediatr. 2003, 49, 286–290. [Google Scholar] [CrossRef][Green Version]
- Pellegrino, R.; Pellino, G.; Selvaggi, L.; Selvaggi, F.; Federico, A.; Romano, M.; Gravina, A.G. BNT162b2 mRNA COVID-19 vaccine is safe in a setting of patients on biologic therapy with inflammatory bowel diseases: A monocentric real-life study. Expert Rev. Clin. Pharmacol. 2022, 15, 1243–1252. [Google Scholar] [CrossRef]
- Kurtenkov, O.; Klaamas, K.; Rittenhouse-Olson, K.; Vahter, L.; Sergejev, B.; Miljukhina, L.; Shljapnikova, L. IgG immune response to tumor-associated carbohydrate antigens (TF, Tn, alphaGal) in patients with breast cancer: Impact of neoadjuvant chemotherapy and relation to the survival. Exp. Oncol. 2005, 27, 136–140. [Google Scholar]
- Hammarlund, E.; Lewis, M.W.; Hansen, S.G.; Strelow, L.I.; Nelson, J.A.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef]
- Yu, X.; Tsibane, T.; McGraw, P.A.; House, F.S.; Keefer, C.J.; Hicar, M.D.; Tumpey, T.M.; Pappas, C.; Perrone, L.A.; Martinez, O.; et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature 2008, 455, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Sedgwick, J.D.; O’Leary, C.; Krska, K.; Leivers, S. Long-lived IgE- and IgG-secreting cells in rodents manifesting persistent antibody responses. Cell. Immunol. 1984, 89, 281–289. [Google Scholar] [CrossRef]
- Ndungu, F.M.; Cadman, E.T.; Coulcher, J.; Nduati, E.; Couper, E.; Macdonald, D.W.; Ng, D.; Langhorne, J. Functional memory B cells and long-lived plasma cells are generated after a single Plasmodium chabaudi infection in mice. PLoS Pathog. 2009, 5, e1000690. [Google Scholar] [CrossRef]
- Whiteside, S.; Markova, M.; Chin, A.; Lam, C.; Dharmani-Khan, P.; Modi, M.; Khan, F.; Storek, J. Influence of Chemotherapy on Allergen-Specific IgE. Int. Arch. Allergy. Immunol. 2018, 177, 145–152. [Google Scholar] [CrossRef]
- Stern, A.; Carrara, E.; Bitterman, R.; Yahav, D.; Leibovici, L.; Paul, M. Early discontinuation of antibiotics for febrile neutropenia versus continuation until neutropenia resolution in people with cancer. Cochrane Database Syst. Rev. 2019, 1, Cd012184. [Google Scholar] [CrossRef]
- De Donno, A.; Lobreglio, G.; Panico, A.; Grassi, T.; Bagordo, F.; Bozzetti, M.P.; Massari, S.; Siculella, L.; Damiano, F.; Guerra, F.; et al. IgM and IgG Profiles Reveal Peculiar Features of Humoral Immunity Response to SARS-CoV-2 Infection. Int. J. Environ. Res. Public Health 2021, 18, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, G.; Mandeville, R.; Poisson, R.; Legault-Poisson, S.; Jolicoeur, R. Biologic markers and breast cancer: A multiparametric study--1. Increased serum protein levels. Cancer 1982, 49, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kuwahara, A.; Kinoshita, T.; Shigemitsu, Y.; Shimoda, K.; Miyahara, M.; Kobayashi, M. Increases in immunoglobulin and complement in patients with esophageal or gastric cancer. Surg. Today 1992, 22, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Miyatani, K.; Kono, Y.; Murakami, Y.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Takano, S.; Osaki, T.; Fujiwara, Y. Decreased Serum Concentration of Total IgG Is Related to Tumor Progression in Gastric Cancer Patients. Yonago Acta Med. 2017, 60, 119–125. [Google Scholar] [CrossRef]
- Dias, T.R.; Dias, F.; Teixeira, A.L.; Sousa, H.; Oliveira, J.; Medeiros, R. MicroRNAs as Potential Tools for Predicting Cancer Patients’ Susceptibility to SARS-CoV-2 Infection and Vaccination Response. Cells 2022, 11, 2279. [Google Scholar] [CrossRef]
- Almeida, B.; Dias, T.R.; Teixeira, A.L.; Dias, F.; Medeiros, R. MicroRNAs Derived from Extracellular Vesicles: Keys to Understanding SARS-CoV-2 Vaccination Response in Cancer Patients? Cancers 2023, 15, 4017. [Google Scholar] [CrossRef]
- Felip, E.; Pradenas, E.; Romeo, M.; Marfil, S.; Trinite, B.; Urrea, V.; Hernandez, A.; Ballana, E.; Cucurull, M.; Mateu, L.; et al. Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors. Mol. Oncol. 2023, 17, 686–694. [Google Scholar] [CrossRef]
- Merli, M.; Costantini, A.; Tafuri, S.; Bavaro, D.F.; Minoia, C.; Meli, E.; Luminari, S.; Gini, G. Management of vaccinations in patients with non-Hodgkin lymphoma. Br. J. Haematol. 2024, 204, 1617–1634. [Google Scholar] [CrossRef]
- Muhsen, I.N.; Heslop, H.E. Time to optimize vaccination strategies in blood cancer patients. Br. J. Haematol. 2024, 205, 406–408. [Google Scholar] [CrossRef]
- Hua, T.; Fan, R.; Fan, Y.; Chen, F. Immune response of COVID-19 vaccines in solid cancer patients: A meta-analysis. Hum. Vaccin. Immunother. 2024, 20, 2357424. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Truong, T.H.; Narendran, A. Evaluation of COVID-19 vaccine response in cancer patients: An interim analysis. Eur. J. Cancer 2021, 159, 259–274. [Google Scholar] [CrossRef] [PubMed]



| Gender | |||
|---|---|---|---|
| Male, n (%) | Female, n (%) | ||
| Patients, n (%) | 284 (100%) | 140 (49.3%) | 144 (50.7%) |
| Patient Age (mean ± sd) | 61.79 ± 11.16 | 63.67 ± 10.50 | 59.96 ± 11.48 |
| SOLID TUMOR CASES, n (%) | 240 (84.5%) | 118 (49.2%) | 122 (50.8%) |
| Tumor type, n (%) | |||
| Breast, n (%) | 68 (23.9%) | 1 (1.5%) | 67 (98.5%) |
| Lung, n (%) | 51 (18.0%) | 41 (80.4%) | 10 (19.6%) |
| Head and Neck, n (%) | 11 (3.9%) | 10 (90.9%) | 1 (9.1%) |
| Urogynecologic, n (%) | 17 (6.0%) | 11 (64.7%) | 6 (35.3%) |
| Digestive Tract, n (%) | 73 (25.7%) | 46 (63.0%) | 27 (37.0%) |
| Other, n (%) | 20 (7.0%) | 9 (45.0%) | 11 (55.0%) |
| Cancer staging (AJCC 8th Edition) | |||
| I–III, n (%) | 76 (31.7%) | 27 (35.5%) | 49 (64.5%) |
| IV, n (%) | 164 (68.3%) | 91 (55.5%) | 73 (44.5%) |
| Cancer Treatment | |||
| ChemoT | 160 (51.6%) | 76 (47.5%) | 84 (52,5%) |
| ImmunoT | 58 (18.7%) | 40 (69.0%) | 18 (31.0%) |
| HormonoT | 21 (6.8%) | 7 (33.3%) | 14 (66.7%) |
| TargetT | 57 (18.4%) | 22 (38.6%) | 35 (61.4%) |
| Others | 14 (4.5%) | 1 (7.1%) | 13 (92.9%) |
| Risk of Febril Neutropenia | |||
| Low (<10%) | 164 (68.3%) | 98 (58.5%) | 68 (41.5%) |
| Medium (10 to 20%) | 35 (14.6%) | 14 (40.0%) | 21 (60.0%) |
| High (>20%) | 39 (17.1%) | 6 (19.5%) | 33 (80.5%) |
| HEMATOLOGICAL MALIGNANCIES, n (%) | 44 (15.5%) | 22 (50.0%) | 22 (50.0%) |
| Tumor type, n (%) | |||
| Lymphoid | 20 (7.0%) | 9 (45.0%) | 11 (55.0%) |
| Leukemia | 2 (0.7%) | 2 (100%) | 0 (0%) |
| Myeloma | 17 (6.0%) | 10 (58.8%) | 7 (41.2%) |
| Others | 5 (1.8%) | 1 (20.0%) | 4 (80.0%) |
| Treatment | |||
| Alkylating antineoplastic agent | 16 (20.8%) | 6 (37.5%) | 10 (62.5%) |
| Anti-CD20 antibodies | 19 (24.7%) | 8 (42.1%) | 11 (57.9%) |
| Anthracyclines | 10 (13%) | 3 (30.0%) | 7 (70.0%) |
| Corticosteroids | 22 (28.5%) | 11 (50.0%) | 11 (50.0%) |
| Immunomodulatory Drugs (IMiDs) | 10 (13%) | 7 (70.0%) | 3 (30.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.; Cruz, P.; Dias, T.R.; Sousa-Pimenta, M.; Almeida, B.; Soares, B.; Sousa, H.; Costa, R.; Ochoa, C.; Dias, F.; et al. Humoral Response to SARS-CoV-2 Vaccine-Boost in Cancer Patients: A Case Series from a Southern European Cancer Center. Vaccines 2024, 12, 1207. https://doi.org/10.3390/vaccines12111207
Oliveira J, Cruz P, Dias TR, Sousa-Pimenta M, Almeida B, Soares B, Sousa H, Costa R, Ochoa C, Dias F, et al. Humoral Response to SARS-CoV-2 Vaccine-Boost in Cancer Patients: A Case Series from a Southern European Cancer Center. Vaccines. 2024; 12(11):1207. https://doi.org/10.3390/vaccines12111207
Chicago/Turabian StyleOliveira, Júlio, Pedro Cruz, Tânia R. Dias, Mário Sousa-Pimenta, Beatriz Almeida, Bruno Soares, Hugo Sousa, Rui Costa, Carlos Ochoa, Francisca Dias, and et al. 2024. "Humoral Response to SARS-CoV-2 Vaccine-Boost in Cancer Patients: A Case Series from a Southern European Cancer Center" Vaccines 12, no. 11: 1207. https://doi.org/10.3390/vaccines12111207
APA StyleOliveira, J., Cruz, P., Dias, T. R., Sousa-Pimenta, M., Almeida, B., Soares, B., Sousa, H., Costa, R., Ochoa, C., Dias, F., & Medeiros, R. (2024). Humoral Response to SARS-CoV-2 Vaccine-Boost in Cancer Patients: A Case Series from a Southern European Cancer Center. Vaccines, 12(11), 1207. https://doi.org/10.3390/vaccines12111207








