Nationwide Discrete Choice Experiment on Chinese Guardians’ Preferences for HPV Vaccination for Mothers and Daughters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Recruitment and Data Collection
2.3. Discrete Choice Experiment Scales
2.4. Quality Control
2.5. Statistical Analysis
3. Results
3.1. The Demographic Characteristics of Study Participants
3.2. Willingness for Guardians’ HPV Vaccination
3.3. Willingness for Daughters’ HPV Vaccination
3.4. Willingness to Pay Based on the Attributes of HPV Vaccines
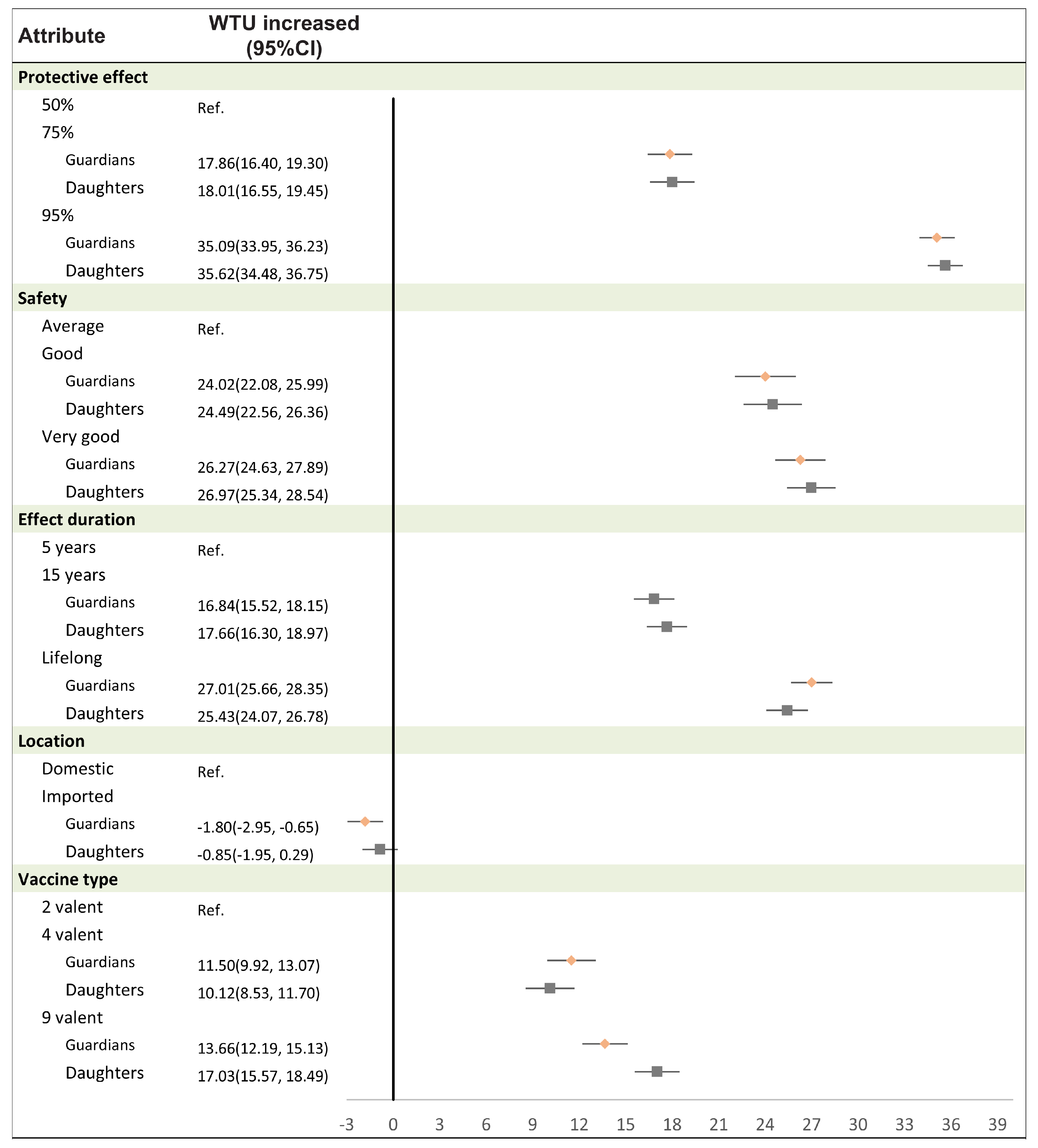
3.5. Willingness to Uptake HPV Vaccines of Different Attributes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2016, 45, D499–D506. [Google Scholar] [CrossRef] [PubMed]
- Braaten, K.P.; Laufer, M.R. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev. Obstet. Gynecol. 2008, 1, 2–10. [Google Scholar] [PubMed] [PubMed Central]
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann. Ig. 2018, 30, 28–32. [Google Scholar] [CrossRef]
- ICO/IARC. Human Papillomavirus and Related Diseases in the World; Bruni, L., Albero, G., Serrano, B., Mena, M., Collado, J.J., Gómez, D., Muñoz, J., Bosch, F.X., de Sanjosé, S., Eds.; IARC: Barcelona, Spain, 2023; Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 24 June 2024).
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2007. Available online: https://publications.iarc.fr/108 (accessed on 17 October 2024).
- Ji, L.; Chen, M.; Yao, L. Strategies to eliminate cervical cancer in China. Front. Oncol. 2023, 13, 1105468. [Google Scholar] [CrossRef]
- Ni-Nan, H.E.; Zhuo-Ru, Z.; Yun-Bo, Z.; Xiao-Qing, H.E.; Su-Qiang, X.; Chang-Xin, L.; Jia-Wei, B.; Ru-Yi, X.; Gui-Hua, Z. Economic burden of human papilloma virus related diseases in China: A systematic review and synthetic analysis. Chin. J. Dis. Control. Prev. 2023, 27, 345–351. [Google Scholar] [CrossRef]
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 24 June 2024).
- Han, L.; Zhang, B. Can prophylactic HPV vaccination reduce the recurrence of cervical lesions after surgery? Review and prospect. Infect. Agents Cancer 2023, 18, 66. [Google Scholar] [CrossRef]
- Lee, C.H.J.; Overall, N.C.; Sibley, C.G. Maternal and paternal confidence in vaccine safety: Whose attitudes are predictive of children’s vaccination? Vaccine 2020, 38, 7057–7062. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, W.M.; Weber, E.U. Content and Discontent: Indications and Implications of Domain Specificity in Preferential Decision Making. In Psychology of Learning and Motivation; Busemeyer, J., Hastie, R., Medin, D.L., Eds.; Academic Press: Cambridge, MA, USA, 1995; pp. 83–136. [Google Scholar] [CrossRef]
- Yim, V.W.C.; Wang, Q.; Li, Y.; Qin, C.; Tang, W.; Tang, S.; Jit, M.; Smith, J.S.; Larson, H.J.; Tucker, J.D.; et al. Between now and later: A mixed methods study of HPV vaccination delay among Chinese caregivers in urban Chengdu, China. BMC Public Health 2024, 24, 183. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Y.; Zhang, L.; Sun, X.; Jiang, Q.; Li, Z.; Wu, Y.; Fu, C. Stages of HPV Vaccine Hesitancy Among Guardians of Female Secondary School Students in China. J. Adolesc. Health 2023, 72, 73–79. [Google Scholar] [CrossRef]
- Zhao, X.L.; Hu, S.Y.; Hu, J.W.; Wang, H.H.; Wen, T.M.; Feng, Y.S.; Qiao, Y.L.; Zhao, F.H.; Zhang, Y. Tackling barriers to scale up human papillomavirus vaccination in China: Progress and the way forward. Infect. Dis. Poverty 2023, 12, 86. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Luo, J.; Zhang, Y.; Ha, Y.; Xu, X.; Tao, L.; Mu, X.; Gao, S.; Han, Y.; et al. A Case-Control Study on Factors of HPV Vaccination for Mother and Daughter in China. Vaccines 2023, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, X.; Zhang, Y.; Liu, Y.; Yang, C.; Wang, Y.; Wang, Y.; Yu, Y.; Hong, Y.; Zhang, X.; et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: Implications for vaccine roll-out in mainland China. Vaccine 2021, 39, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cervical Cancer Prevention and Treatment Committee of Guangdong Preventive Medicine Association. Preventive Medicine Association, Guangdong Expert Consensus on HPV Vaccine Application to Eliminate Cervical Cancer. J. Chin. Phys. 2021, 23, 303–315. [Google Scholar] [CrossRef]
- Gao, M.; Hu, S.; Zhao, X.; You, T.; Jit, M.; Liu, Y.; Qiao, Y.; Zhao, F.; Wang, C. Health and economic impact of delaying large-scale HPV vaccination and screening implementation on cervical cancer in China: A modelling study. Lancet Reg. Health West. Pac. 2023, 36, 100768. [Google Scholar] [CrossRef]
- Diks, M.E.; Hiligsmann, M.; van der Putten, I.M. Vaccine preferences driving vaccine-decision making of different target groups: A systematic review of choice-based experiments. BMC Infect. Dis. 2021, 21, 879. [Google Scholar] [CrossRef]
- Gong, T.; Chen, G.; Liu, P.; Lai, X.; Rong, H.; Ma, X.; Hou, Z.; Fang, H.; Li, S. Parental Vaccine Preferences for Their Children in China: A Discrete Choice Experiment. Vaccines 2020, 8, 687. [Google Scholar] [CrossRef]
- Stöckli, S.; Spälti, A.K.; Phillips, J.; Stoeckel, F.; Barnfield, M.; Thompson, J.; Lyons, B.; Mérola, V.; Szewach, P.; Reifler, J. Which vaccine attributes foster vaccine uptake? A cross-country conjoint experiment. PLoS ONE 2022, 17, e0266003. [Google Scholar] [CrossRef]
- MacDonald, N.E. SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Hoogink, J.; Verelst, F.; Kessels, R.; Van Hoek, A.J.; Timen, A.; Willem, L.; Beutels, P.; Wallinga, J.; De Wit, G.A. Preferential differences in vaccination decision-making for oneself or one’s child in The Netherlands: A discrete choice experiment. BMC Public Health 2020, 20, 828. [Google Scholar] [CrossRef]
- Goldman, R.D.; Yan, T.D.; Seiler, M.; Cotanda, C.P.; Brown, J.C.; Klein, E.J.; Hoeffe, J.; Gelernter, R.; Hall, J.E.; Davis, A.L.; et al. Caregiver willingness to vaccinate their children against COVID-19: Cross sectional survey. Vaccine 2020, 38, 7668–7673. [Google Scholar] [CrossRef]
- Vasudevan, L.; Ostermann, J.; Wang, Y.; Harrison, S.E.; Yelverton, V.; Fish, L.J.; Williams, C.; Walter, E.B. Association of caregiver attitudes with adolescent HPV vaccination in 13 southern US states. Vaccine X 2022, 11, 100181. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Maitland, E.; Wang, S.; Nicholas, S.; Liu, R.; Wang, J. Individual preferences for COVID-19 vaccination in China. Vaccine 2021, 39, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Prosser, L.A.; Wagner, A.L.; Wittenberg, E.; Zikmund-Fisher, B.J.; Rose, A.M.; Pike, J. A Discrete Choice Analysis Comparing COVID-19 Vaccination Decisions for Children and Adults. JAMA Netw. Open 2023, 6, e2253582. [Google Scholar] [CrossRef]
- Burdier, F.R.; Eklund, C.; Baussano, I.; Mariz, F.C.; Téblick, L.; Mugo, N.; Watson-Jones, D.; Stanley, M.; Baay, M.; Vorsters, A. An update on one-dose HPV vaccine studies, immunobridging and humoral immune responses-A meeting report. Prev. Med. Rep. 2023, 35, 102368. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Sampson, J.N.; Porras, C.; Schiller, J.T.; Kemp, T.; Herrero, R.; Wagner, S.; Boland, J.; Schussler, J.; Lowy, D.R.; et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J. Natl. Cancer Inst. 2020, 112, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Luo, W.; Xie, Y. Sexuality in China: A review and new findings. Chin. J. Sociol. 2022, 8, 293–329. [Google Scholar] [CrossRef]
- Chen, G.; Wu, B.; Dai, X.; Zhang, M.; Liu, Y.; Huang, H.; Mei, K.; Wu, Z. Gender differences in knowledge and attitude towards HPV and HPV vaccine among college students in Wenzhou, China. Vaccines 2021, 10, 10. [Google Scholar] [CrossRef]
- Joshi, S.; Anantharaman, D.; Muwonge, R.; Bhatla, N.; Panicker, G.; Butt, J.; Poli, U.R.R.; Malvi, S.G.; Esmy, P.O.; Lucas, E.; et al. Evaluation of immune response to single dose of quadrivalent HPV vaccine at 10-year post-vaccination. Vaccine 2023, 41, 236–245. [Google Scholar] [CrossRef]
- Wong, L.P.; Wong, P.F.; Megat Hashim, M.M.A.A.; Han, L.; Lin, Y.; Hu, Z.; Zhao, Q.; Zimet, G.D. Multidimensional social and cultural norms influencing HPV vaccine hesitancy in Asia. Hum. Vaccines Immunother. 2020, 16, 1611–1622. [Google Scholar] [CrossRef]
- Hildesheim, A.; Herrero, R. Human papillomavirus vaccine should be given before sexual debut for maximum benefit. J. Infect. Dis. 2007, 196, 1431–1432. [Google Scholar] [CrossRef][Green Version]
- Shen, X.; Cheng, Y.; Ren, F.; Shi, Z. The burden of cervical cancer in China. Front. Oncol. 2022, 12, 979809. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Ma, Y.; Jiao, Y.Z.; Liang, X.F.; Jin, N.; Bao, J.; Jiang, N.; Zhang, X.S. Real-World Data of China: Analysis of HPV Vaccine Coverage and Post-Vaccination Adverse Reaction Monitoring in Western Chinese Provinces from 2018 to 2021. Hum. Vaccines Immunother. 2024, 20, 2315653. [Google Scholar] [CrossRef] [PubMed]
- Hensher, D. Hypothetical bias, choice experiments and willingness to pay. Transp. Res. Part B Methodol. 2010, 44, 735–752. [Google Scholar] [CrossRef]
| Characteristics | N | % | |
|---|---|---|---|
| Relationship with daughter | Mother | 4179 | 84.72 |
| Father | 754 | 15.28 | |
| Age | 25–34 | 527 | 10.68 |
| 35–44 | 3138 | 63.61 | |
| 45–55 | 1268 | 25.70 | |
| Daughter’s age | 9–14 | 2622 | 53.15 |
| 15–18 | 2311 | 46.85 | |
| Occupation | Enterprise staff | 2751 | 55.77 |
| Farmers | 2182 | 44.23 | |
| Education | Junior high school or below | 2910 | 58.99 |
| Senior high school | 734 | 14.88 | |
| Bachelor or above | 1289 | 26.13 | |
| Daughter’s grade | Grade 4 | 551 | 11.17 |
| Grade 5 | 446 | 9.04 | |
| Grade 6 | 543 | 11.01 | |
| Grade 7 | 370 | 7.50 | |
| Grade 8 | 480 | 9.73 | |
| Grade 9 | 624 | 12.65 | |
| Grade 10 | 659 | 13.36 | |
| Grade 11 | 605 | 12.26 | |
| Grade 12 | 655 | 13.28 | |
| Family income | <423 | 2003 | 40.60 |
| 423–704 | 1811 | 36.71 | |
| >704 | 1119 | 22.68 | |
| Residence | Rural | 3468 | 70.30 |
| Urban | 1465 | 29.70 |
| Attribute | Levels | β | 95% CIc | 95% CIa | p | Rural | Urban | ||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CIc | β | 95% CIc | ||||||
| Protective effect | 50% | Ref. | Ref. | Ref. | |||||
| 75% | 0.36 | (0.33, 0.39) | (0.33, 0.39) | <0.001 | 0.35 | (0.32, 0.39) | 0.38 | (0.33, 0.44) | |
| 95% | 0.73 | (0.71, 0.76) | (0.71, 0.76) | <0.0001 | 0.72 | (0.69, 0.75) | 0.77 | (0.72, 0.81) | |
| Safety | Average | Ref. | Ref. | Ref. | |||||
| Good | 0.49 | (0.45, 0.53) | (0.45, 0.53) | <0.001 | 0.50 | (0.45, 0.55) | 0.47 | (0.39, 0.54) | |
| Very good | 0.54 | (0.50, 0.57) | (0.50, 0.57) | <0.001 | 0.55 | (0.51, 0.59) | 0.51 | (0.45, 0.57) | |
| Effect duration | 5 years | Ref. | Ref. | Ref. | |||||
| 15 years | 0.34 | (0.31, 0.37) | (0.31, 0.37) | <0.001 | 0.34 | (0.30, 0.37) | 0.35 | (0.30, 0.40) | |
| Lifelong | 0.55 | (0.53, 0.58) | (0.53, 0.58) | <0.001 | 0.59 | (0.55, 0.62) | 0.48 | (0.43, 0.53) | |
| Location | Domestic | Ref. | Ref. | Ref. | |||||
| Imported | −0.04 | (−0.06, −0.01) | (−0.06, −0.01) | 0.002 | −0.07 | (−0.10, −0.05) | 0.05 | (0.01, 0.09) | |
| Vaccine type | 2-valent | Ref. | Ref. | Ref. | |||||
| 4-valent | 0.23 | (0.20, 0.26) | (0.20, 0.26) | <0.001 | 0.24 | (0.20, 0.28) | 0.21 | (0.15, 0.27) | |
| 9-valent | 0.28 | (0.25, 0.31) | (0.25, 0.31) | <0.001 | 0.27 | (0.24, 0.31) | 0.28 | (0.22, 0.33) | |
| Full payment | −0.09 | (−0.10, −0.08) | (−0.10, −0.08) | <0.001 | −0.10 | (−0.11, −0.09) | −0.06 | (−0.08, −0.05) | |
| Attribute | Levels | β | 95% CIc | 95% CIa | p | Rural | Urban | ||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CIc | β | 95% CIc | ||||||
| Protective effect | 50% | Ref. | Ref. | Ref. | |||||
| 75% | 0.36 | (0.33, 0.39) | (0.33, 0.39) | <0.001 | 0.35 | (0.32, 0.39) | 0.39 | (0.34, 0.45) | |
| 95% | 0.75 | (0.72, 0.77) | (0.72, 0.77) | <0.001 | 0.72 | (0.69, 0.75) | 0.80 | (0.75, 0.85) | |
| Safety | Average | Ref. | Ref. | Ref. | |||||
| Good | 0.50 | (0.46, 0.54) | (0.46, 0.54) | <0.001 | 0.51 | (0.46, 0.56) | 0.49 | (0.41, 0.56) | |
| Very good | 0.55 | (0.52, 0.59) | (0.52, 0.59) | <0.001 | 0.57 | (0.52, 0.61) | 0.52 | (0.46, 0.59) | |
| Effect duration | 5 years | Ref. | Ref. | Ref. | |||||
| 15 years | 0.34 | (0.33, 0.38) | (0.33, 0.38) | <0.001 | 0.35 | (0.31, 0.38) | 0.38 | (0.33, 0.44) | |
| Lifelong | 0.52 | (0.49, 0.55) | (0.49, 0.55) | <0.001 | 0.55 | (0.52, 0.59) | 0.45 | (0.40, 0.50) | |
| Location | Domestic | Ref. | Ref. | Ref. | |||||
| Imported | −0.02 | (−0.04, 0.01) | (−0.04, 0.01) | 0.151 | −0.05 | (−0.08, −0.03) | 0.07 | (0.03, 0.11) | |
| Vaccine type | 2-valent | Ref. | Ref. | Ref. | |||||
| 4-valent | 0.20 | (0.17, 0.24) | (0.17, 0.24) | <0.001 | 0.20 | (0.16, 0.24) | 0.20 | (0.14, 0.26) | |
| 9-valent | 0.34 | (0.31, 0.37) | (0.31, 0.37) | <0.001 | 0.32 | (0.29, 0.36) | 0.40 | (0.34, 0.45) | |
| Full payment | −0.03 | (−0.04, −0.02) | (−0.04, −0.02) | <0.001 | −0.04 | (−0.05, −0.03) | 0.00 | (−0.02, 0.02) | |
| Attribute | All | Education | Family Income | ||||
|---|---|---|---|---|---|---|---|
| Junior High School or Below | Senior High School | Bachelor or Above | <423 | 423–704 | >704 | ||
| Protective effect | |||||||
| 50% | |||||||
| 75% | |||||||
| Guardians | 565 (518, 612) | 462 (409, 515) | 755 (603, 905) | 806 (683, 928) | 369 (316, 419) | 583 (501, 663) | 2165 (1843, 2483) |
| Daughters | 1710 (1568, 1850) | 1131 (1002, 1258) | 4133 (3319, 4937) | 7820 (6634, 8996) | 854 (741, 969) | 1928 (1656, 2197) | 3313 (2810, 3816) |
| 95% | |||||||
| Guardians | 1147 (1106, 1188) | 946 (901, 991) | 1460 (1332, 1589) | 1650 (1544, 1756) | 759 (714, 804) | 1210 (1139, 1282) | 4172 (3893, 4453) |
| Daughters | 3498 (3376, 3620) | 2323 (2213, 2430) | 7934 (7233, 8644) | 16,456 (15,430, 17,477) | 1701 (1605, 1795) | 4182 (3946, 4422) | 6520 (6081, 6962) |
| Safety | |||||||
| Average | Ref. | ||||||
| Good | |||||||
| Guardians | 767 (703, 833) | 668 (597, 740) | 889 (685, 1093) | 1034 (867, 1201) | 486 (415, 557) | 813 (702, 923) | 2939 (2498, 3378) |
| Daughters | 2347 (2155, 2535) | 1607 (1433, 1778) | 4948 (3843, 6042) | 10,753 (9134, 12,362) | 1103 (946, 1260) | 2766 (2398, 3133) | 4653 (3961, 5344) |
| Very good | |||||||
| Guardians | 842 (787, 897) | 743 (683, 804) | 951 (777, 1124) | 1118 (975, 1260) | 540 (480, 600) | 901 (806, 995) | 3127 (2747, 3507) |
| Daughters | 2596 (2432, 2756) | 1844 (1696, 1990) | 5167 (4211, 6119) | 11,244 (9872, 12,628) | 1260 (1127, 1394) | 3063 (2750, 3377) | 4889 (4304, 5467) |
| Effect duration | |||||||
| 5 years | Ref. | ||||||
| 15 years | |||||||
| Guardians | 532 (490, 574) | 433 (386, 481) | 705 (569, 840) | 765 (656, 876) | 333 (286, 380) | 553 (478, 627) | 2144 (1843, 2443) |
| Daughters | 1676 (1545, 1803) | 1065 (950, 1180) | 4078 (3307, 4843) | 8233 (7134, 9339) | 1292 (1183, 1402) | 2938 (2677, 3196) | 3827 (3339, 4314) |
| Lifelong | Ref. | ||||||
| Guardians | 867 (822, 912) | 792 (742, 842) | 998 (855, 1142) | 1038 (920, 1157) | 635 (586, 685) | 903 (824, 980) | 2660 (2348, 2968) |
| Daughters | 2441 (2305, 2577) | 792 (742, 842) | 998 (855, 1142) | 1038 (920, 1157) | 1292 (1183, 1402) | 2938 (2677, 3196) | 3827 (3339, 4314) |
| Location | |||||||
| Domestic | Ref. | ||||||
| Imported | |||||||
| Guardians | −56 (−92, −20) | −111 (−150, −71) | −9 (−123, 104) | 109 (14, 202) | −90 (−130, −52) | −22 (−83, 40) | 63 (−179, 301) |
| Daughters | −80 (−184, 28) | −190 (−285, −93) | 248 (−379, 866) | 1105 (199, 2011) | −99 (−185, −14) | −107 (−310, 101) | 259 (−121, 634) |
| Vaccine type | |||||||
| 2 valent | Ref. | ||||||
| 4 valent | |||||||
| Guardians | 362 (311, 412) | 351 (295, 406) | 342 (180, 505) | 411 (282, 543) | 263 (207, 319) | 377 (289, 464) | 1154 (803, 1503) |
| Daughters | 953 (803, 1103) | 714 (579, 847) | 1550 (674, 2417) | 4004 (2738, 5258) | 494 (372, 619) | 1160 (867, 1451) | 1554 (1006, 2107) |
| 9 valent | Ref. | ||||||
| Guardians | 430 (383, 477) | 380 (327, 433) | 519 (372, 667) | 546 (423, 668) | 267 (216, 319) | 474 (393, 556) | 1613 (1292, 1932) |
| Daughters | 1615 (1474, 1756) | 1041 (914, 1170) | 3548 (2737, 4355) | 8156 (6975, 9338) | 753 (639, 867) | 1963 (1695, 2236) | 3126 (2625, 3632) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhao, T.; Zhang, S.; Huang, N.; Du, J.; Liu, Y.; Lu, Q.; Wang, C.; Cui, F. Nationwide Discrete Choice Experiment on Chinese Guardians’ Preferences for HPV Vaccination for Mothers and Daughters. Vaccines 2024, 12, 1186. https://doi.org/10.3390/vaccines12101186
Zhao J, Zhao T, Zhang S, Huang N, Du J, Liu Y, Lu Q, Wang C, Cui F. Nationwide Discrete Choice Experiment on Chinese Guardians’ Preferences for HPV Vaccination for Mothers and Daughters. Vaccines. 2024; 12(10):1186. https://doi.org/10.3390/vaccines12101186
Chicago/Turabian StyleZhao, Jun, Tianshuo Zhao, Sihui Zhang, Ninghua Huang, Juan Du, Yaqiong Liu, Qingbin Lu, Chao Wang, and Fuqiang Cui. 2024. "Nationwide Discrete Choice Experiment on Chinese Guardians’ Preferences for HPV Vaccination for Mothers and Daughters" Vaccines 12, no. 10: 1186. https://doi.org/10.3390/vaccines12101186
APA StyleZhao, J., Zhao, T., Zhang, S., Huang, N., Du, J., Liu, Y., Lu, Q., Wang, C., & Cui, F. (2024). Nationwide Discrete Choice Experiment on Chinese Guardians’ Preferences for HPV Vaccination for Mothers and Daughters. Vaccines, 12(10), 1186. https://doi.org/10.3390/vaccines12101186






