Postpartum Interventions to Increase Maternal Vaccination Uptake: Is It Worth It?
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection Process
2.4. Strengths and Limitations
3. Results
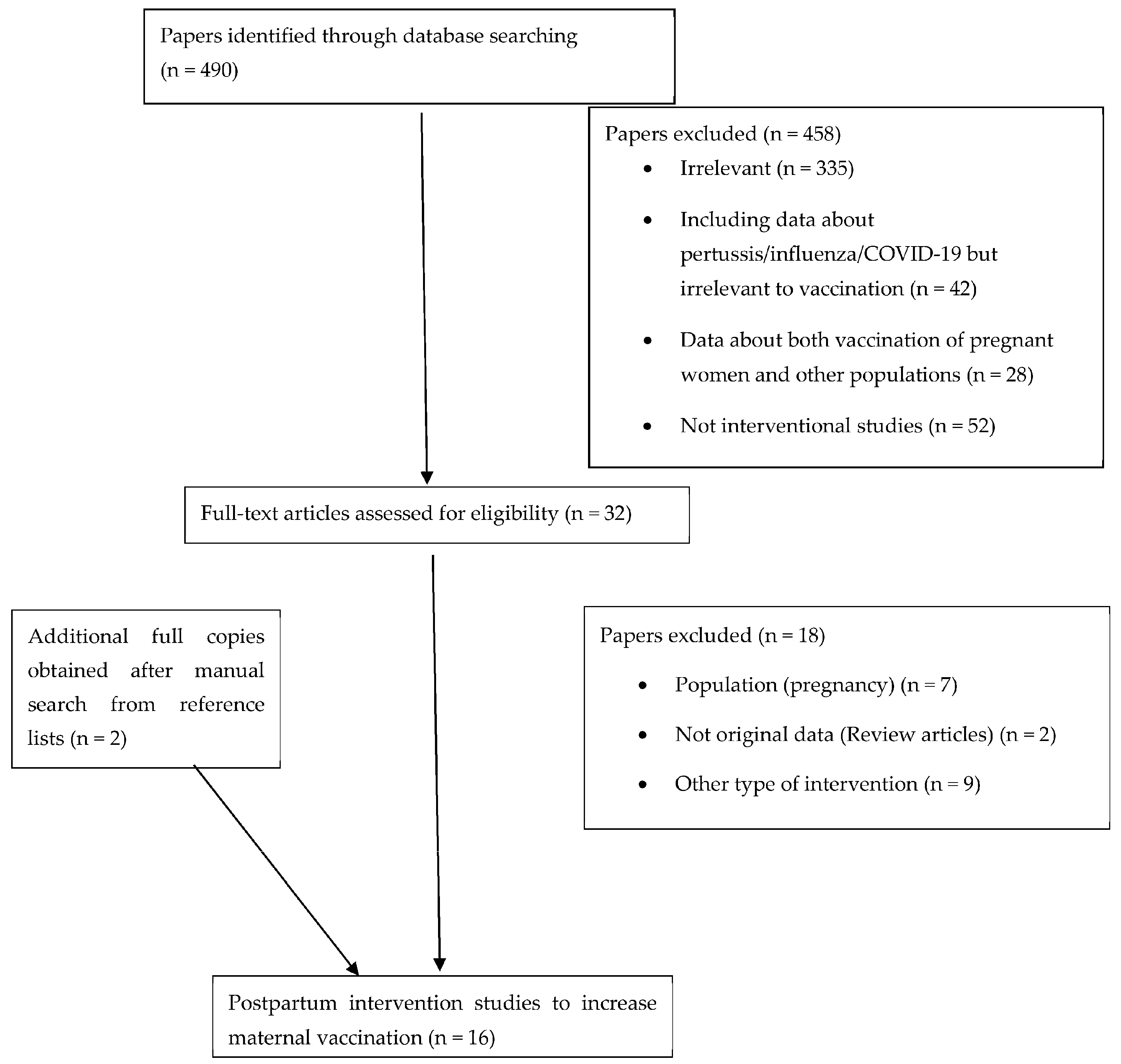
3.1. Study Selection
3.2. Summary of Studies According the Results and Type of Intervention
3.2.1. Primary Outcome According to Study Design
3.2.2. Type of Intervention—The Role of Different Healthcare Professionals Providing Information
3.2.3. Type of Intervention—The Role of Different Methods of Educational Process
3.2.4. Type of Intervention—The Role of Healthcare Provider’s Education as a Method of Intervention
3.2.5. Type of Intervention—The Role of Vaccine Offer
3.2.6. Supplemental Outcomes—Reasons for Vaccine Hesitancy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berti, E.; Venturini, E.; Galli, L.; de Martino, M.; Chiappini, E. Management and prevention of pertussis infection in neonates. Expert. Rev. Anti Infect. Ther. 2014, 12, 1515–1531. [Google Scholar] [CrossRef]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2018, 67, 1–44. [Google Scholar] [CrossRef]
- Decker, M.D.; Edwards, K.M. Pertussis (Whooping Cough). J. Infect. Dis. 2021, 224, S310–S320. [Google Scholar] [CrossRef]
- Straney, L.; Schibler, A.; Ganeshalingham, A.; Alexander, J.; Festa, M.; Slater, A.; MacLaren, G.; Schlapbach, L.J. Burden and Outcomes of Severe Pertussis Infection in Critically Ill Infants. Pediatr. Crit. Care Med. 2016, 17, 735–742. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–2024 Influenza Season. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Sakala, I.G.; Honda-Okubo, Y.; Fung, J.; Petrovsky, N. Influenza immunization during pregnancy: Benefits for mother and infant. Hum. Vaccin. Immunother. 2016, 12, 3065–3071. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F., 3rd; Azziz-Baumgartner, E.; Gilboa, S.M.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Dhir, S.K.; Kumar, J.; Meena, J.; Kumar, P. Clinical Features and Outcome of SARS-CoV-2 Infection in Neonates: A Systematic Review. J. Trop. Pediatr. 2021, 67, fmaa059. [Google Scholar] [CrossRef]
- D’Heilly, C.; Switzer, C.; Macina, D. Safety of Maternal Immunization Against Pertussis: A Systematic Review. Infect. Dis. Ther. 2019, 8, 543–568. [Google Scholar] [CrossRef]
- Winter, K.; Glaser, C.; Watt, J.; Harriman, K.; Centers for Disease Control and Prevention (CDC). Pertussis epidemic—California, 2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1129–1132. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 131–135. [Google Scholar]
- Hellenic Ministry of Health Dophaqol. Hellenic National Vaccination Program 2024. Available online: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/12614-ethniko-programma-emboliasmwn-paidiwn-kai-efhbwn-2024-xronodiagramma-kai-systaseis (accessed on 5 January 2024).
- Manske, J.M. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: A review of the evidence. Matern. Child. Health J. 2014, 18, 1599–1609. [Google Scholar] [CrossRef]
- Lagousi, T.; Gkentzi, D.; Geropeppa, M.; Tsagkli, P.; Spoulou, V. Protecting the Offspring, the Gift of Maternal Immunization: Current Status and Future Perspectives. Vaccines 2022, 10, 1953. [Google Scholar] [CrossRef]
- Eick, A.A.; Uyeki, T.M.; Klimov, A.; Hall, H.; Reid, R.; Santosham, M.; O’Brien, K.L. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch. Pediatr. Adolesc. Med. 2011, 165, 104–111. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Zauche, L.H.; Roper, L.E.; Ellington, S.R.; Olson, C.K.; Sharma, A.J.; Woodworth, K.R.; Tepper, N.; Havers, F.; Oliver, S.E.; et al. Safety and Effectiveness of Maternal COVID-19 Vaccines Among Pregnant People and Infants. Obstet. Gynecol. Clin. N. Am. 2023, 50, 279–297. [Google Scholar] [CrossRef]
- Muyldermans, J.; De Weerdt, L.; De Brabandere, L.; Maertens, K.; Tommelein, E. The Effects of COVID-19 Vaccination on Lactating Women: A Systematic Review of the Literature. Front. Immunol. 2022, 13, 852928. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef]
- ACOG Committee. Opinion No. 732: Influenza Vaccination During Pregnancy. Obstet Gynecol. 2018, 131, e109–e114. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care (accessed on 5 January 2024).
- Razzaghi, H.; Kahn, K.E.; Calhoun, K.; Garacci, E.; Skoff, T.H.; Ellington, S.R.; Jatlaoui, T.C.; Black, C.L. Influenza, Tdap, and COVID-19 Vaccination Coverage and Hesitancy Among Pregnant Women—United States, April 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1065–1071. [Google Scholar] [CrossRef]
- Etti, M.; Calvert, A.; Galiza, E.; Lim, S.; Khalil, A.; Le Doare, K.; Heath, P.T. Maternal vaccination: A review of current evidence and recommendations. Am. J. Obstet. Gynecol. 2022, 226, 459–474. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Siskou, O.; Konstantakopoulou, O.; Katsiroumpa, A.; Kaitelidou, D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 766. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Bottrell, K.; Paterson, P.; Schulz, W.S.; Vandrevala, T.; Larson, H.J.; Jones, C.E. Influenza and pertussis vaccination in pregnancy: Portrayal in online media articles and perceptions of pregnant women and healthcare professionals. Vaccine 2018, 36, 7625–7631. [Google Scholar] [CrossRef]
- Razai, M.S.; Mansour, R.; Ravindran, P.; Freeman, S.; Mason-Apps, C.; Morris, J.; Majeed, A.; Ussher, M.; Hargreaves, S.; Oakeshott, P. Facilitators and barriers to vaccination uptake in pregnancy: A qualitative systematic review. PLoS ONE 2024, 19, e0298407. [Google Scholar] [CrossRef]
- Kiefer, M.K.; Mehl, R.; Costantine, M.M.; Johnson, A.; Cohen, J.; Summerfield, T.L.; Landon, M.B.; Rood, K.M.; Venkatesh, K.K. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: A cross-sectional study. BJOG 2021, 129, 1342–1351. [Google Scholar] [CrossRef]
- Schaal, N.K.; Zollkau, J.; Hepp, P.; Fehm, T.; Hagenbeck, C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch. Gynecol. Obstet. 2022, 306, 365–372. [Google Scholar] [CrossRef]
- Skirrow, H.; Barnett, S.; Bell, S.; Mounier-Jack, S.; Kampmann, B.; Holder, B. Women’s views and experiences of accessing pertussis vaccination in pregnancy and infant vaccinations during the COVID-19 pandemic: A multi-methods study in the UK. Vaccine 2022, 40, 4942–4954. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Hetrick, S.E.; Page, M.J. Updated reporting guidance for systematic reviews: Introducing PRISMA 2020 to readers of the Journal of Affective Disorders. J. Affect. Disord. 2021, 292, 56–57. [Google Scholar] [CrossRef]
- Jordan, E.T.; Bushar, J.A.; Kendrick, J.S.; Johnson, P.; Wang, J. Encouraging Influenza Vaccination Among Text4baby Pregnant Women and Mothers. Am. J. Prev. Med. 2015, 49, 563–572. [Google Scholar] [CrossRef]
- Yeh, S.; Mink, C.; Kim, M.; Naylor, S.; Zangwill, K.M.; Allred, N.J. Effectiveness of hospital-based postpartum procedures on pertussis vaccination among postpartum women. Am. J. Obstet. Gynecol. 2014, 210, 237.e1–237.e6. [Google Scholar] [CrossRef]
- Healy, C.M.; Rench, M.A.; Castagnini, L.A.; Baker, C.J. Pertussis immunization in a high-risk postpartum population. Vaccine 2009, 27, 5599–5602. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.; De Wals, P.; Ovetchkine, P.; Coic, L.; Audibert, F.; Tapiero, B. Evaluation of several approaches to immunize parents of neonates against B. pertussis. Vaccine 2013, 31, 6087–6091. [Google Scholar] [CrossRef] [PubMed]
- Momani, A.; Hamaideh, S.H.; Masadeh, A.B.; Alhalaiqa, F.; Bani Mostafa, F.N.; Weld Ali, H.I.; Masa’Deh, R. The effect of COVID-19 vaccine tele-educational program on vaccine hesitancy and receiving the vaccine among women planning for pregnancy, pregnant or breast-feeding mothers. PLoS ONE 2023, 18, e0282627. [Google Scholar] [CrossRef] [PubMed]
- Kouba, I.; Yaghoubian, Y.; Rochelson, B.; Shan, W.; Combs, A.; Nimaroff, M.; Blitz, M.J. Acceptance of coronavirus disease 2019 vaccination among postpartum women during delivery hospitalization. J. Matern. Fetal. Neonatal. Med. 2022, 35, 10502–10505. [Google Scholar] [CrossRef]
- Cheng, P.J.; Huang, S.Y.; Shaw, S.W.; Kao, C.C.; Chueh, H.Y.; Chang, S.D.; Hsu, T.Y.; Kung, F.T.; Hsieh, T.T. Factors influencing women’s decisions regarding pertussis vaccine: A decision-making study in the Postpartum Pertussis Immunization Program of a teaching hospital in Taiwan. Vaccine 2010, 28, 5641–5647. [Google Scholar] [CrossRef]
- Leboucher, B.; Sentilhes, L.; Abbou, F.; Henry, E.; Grimprel, E.; Descamps, P. Impact of postpartum information about pertussis booster to parents in a university maternity hospital. Vaccine 2012, 30, 5472–5481. [Google Scholar] [CrossRef]
- Hayles, E.H.; Cooper, S.C.; Wood, N.; Sinn, J.; Skinner, S.R. What predicts postpartum pertussis booster vaccination? A controlled intervention trial. Vaccine 2015, 33, 228–236. [Google Scholar] [CrossRef]
- Clarke, C.; Wall, G.C.; Soltis, D.A. An introductory pharmacy practice experience to improve pertussis immunization rates in mothers of newborns. Am. J. Pharm. Educ. 2013, 77, 29. [Google Scholar] [CrossRef]
- Bernstein, H.H.; Monty, M.; Yang, P.; Cohen, A. Increasing Tdap Coverage Among Postpartum Women: A Quality Improvement Intervention. Pediatrics 2017, 139, e20160607. [Google Scholar] [CrossRef]
- Walter, E.B.; Allred, N.; Rowe-West, B.; Chmielewski, K.; Kretsinger, K.; Dolor, R.J. Cocooning infants: Tdap immunization for new parents in the pediatric office. Acad. Pediatr. 2009, 9, 344–347. [Google Scholar] [CrossRef]
- Bucchiotty, M.; El Morabit, S.; Hammou, Y.; Gallouj, R.; Messaadi, N.; Vanderstichele, S.; Roumilhac, M.; Dufour, P.; Subtil, D. Effect of a postpartum prescription for pertussis vaccine: A before-and-after study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102050. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Rench, M.A.; Baker, C.J. Implementation of cocooning against pertussis in a high-risk population. Clin. Infect. Dis. 2011, 52, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hebballi, N.B.; Parker, T.; Garcia, E.I.; Ferguson, D.M.; Lesser, S.; Tsao, K.; Broussard, M.; Wootton, S.H. Pertussis and influenza immunization: Perceived attitude and decision of postpartum patients. BMC Pregnancy Childbirth 2022, 22, 975. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Kalogriopoulou, K.; Pergialiotis, V.; Siahanidou, T.; Skiathitou, A.V.; Katerelos, P.; Goumalatsos, N.; Kostis, E.; Antsaklis, A.; Theodoridou, M. Acceptance of a post-partum influenza vaccination (cocooning) strategy for neonates in Greece. Vaccine 2012, 30, 5871–5874. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country /Year of Publication | Vaccine | Number of Participants and Characteristics | Study Design:Survey/Questionnaire | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Cheng et al. [37] | Taiwan, 2010 | Tdap 1 | 1241 women with uncomplicated delivery (2009) | Decision-making observational study. At first postpartum visit, 25 multiple-choice questions on:
| Information provided to all the participants during pregnancy about Tdap vaccination:
| 53% accepted Tdap vaccination. |
| Leboucher et al. [38] | France, 2012 | Tdap-IPV 2 | 659 postpartum women for Period A (January to March 2008) and 772 women for Period B (January to April 2009). | Prospective single-center observational study. No questionnaire used |
| During Period A, 67.9% of mothers and 63.1% of fathers were vaccinated; During Period B, 68.9% of mothers and 62.4% of fathers were vaccinated. |
| Hayles et al. [39] | Australia 2014 | Tdap | 1404 postpartum women from a maternity hospital of Sydney from November 2010 to July 2012. | Controlled intervention trial. At 0–3 days postpartum, a baseline questionnaire concerning attitudes and beliefs about pertussis and Tdap vaccination was completed. |
| 70% of mothers were vaccinated post-intervention. Rates were similar between ‘gain’, ‘loss’ or ‘control’ groups. Overall pertussis immunization coverage increased from 23% to 77% among women screened. |
| Yeh et al. [32] | USA, 2013 | Tdap | 1252 postpartum women, 648 from the intervention hospital and 605 from the comparison hospital from October 2009 through July 2010. | Prospective controlled trial questionnaire on demographics and prior to receipt of Tdap |
|
|
| Healy et al. [33] | USA, 2009 | Tdap | 1570 postpartum (medically underserved, uninsured) women. January–April 2008 | Single-arm interventional study. No questionnaire |
|
|
| Frere et al. [34] | Canada, 2013 | Tdap | 345 postpartum women
| Multi-arm intervention trial- During Phase I, participants completed a questionnaire regarding:
|
|
|
| Clarke et al. [40] | USA, 2013 | Tdap | A total of 1.263 postpartum women were consulted by the pharmacy students. | Observational study. No questionnaire |
| Following counseling, immunization rates, as a percentage of total births, significantly increased by 18.5%. |
| Bernstein et al. [41] | USA, 2017 | Tdap |
| Quality improvement intervention trial. No questionnaire | 5-step intervention:
| Increase by 33% in the postpartum mothers that received the Tdap vaccine before discharge in the postintervention period. |
| Walter et al. [42] | USA, 2009 | Tdap | 200 parents whose newborns received medical care during the first month of life (5 month intervention in 2007) | Observational study. No questionnaire | Parents were informed about the study and Tdap vaccination (verbal and written). Offer of vaccination to all eligible parents in the pediatric office. | Of the 160 eligible to receive Tdap vaccine, 82 (51.2%) received a dose. |
| Bucchiotty et al. [43] | France, 2021 | Tdap-IPV | Before: 134 postpartum women (September 2011) After: 347 postpartum women (March-April 2015) | Before-and-after comparative study. During pregnancy, the participants each filled out a questionnaire to report their immunization status. | Oral and written information was provided in the “before” and in the “after” period. In the “after” period: before discharge all women who were unimmunized received a prescription for Tdap-IPV. Telephone interview to all the participants at 8–10 weeks after discharge. | Among the women unimmunized at delivery, the percentage vaccinated postpartum climbed from 17 to 42% between 2011 and 2015, while the percentage of their unimmunized partners who were vaccinated remained stable (27 and 29%). |
| Healy et al. [44] | USA, 2011 | Tdap |
| Observational study. A questionnaire was provided asking for:
|
|
|
| Reference | Country/ Year | Vaccine | Number of Participants and Characteristics | Study Design: Survey/Questionnaire | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Hebballi et al. [45] | USA, 2022 | Tdap, Influenza | 200 postpartum women (June–August 2018) | Cross-sectional observational study. The survey included questions on:
|
|
|
| Jordan et al. [31] | USA, 2015 | Influenza | 89,792 pregnant and postpartum women were approached, 28,609 responded to the first contact, 6841 completed the study. (October to November 2012) | Randomized control trial. Text4baby was used (free US national mobile health service)
|
| “Planners”
|
| Maltezou et al. [46] | Greece, 2012 | Influenza |
| Observational study.Demographics, epidemiologic, clinical, pregnancy, and birth data were collected using one standardized form per mother. |
| Of the 224 mothers, 165 (73.7%) received influenza vaccine prior to discharge from the hospital. Of the 224 fathers, 125 received the influenza vaccine (55.8% vaccination rate); 51 (22.7%) of 224 families had all household contacts vaccinated against influenza (complete cocoon). |
| Kouba et al. [36] | USA, 2022 | COVID-19 | 8281 unvaccinated postpartum women during delivery hospitalization at seven hospitals in New York (May 2021–September 2021) | Retrospective cohort study. Sociodemographic characteristics were obtained from medical records |
| 412 of the 8281 unvaccinated postpartum women received the vaccine (5%). |
| Momani et al. [35] | Jordan, 2023 | COVID-19 | 425 women unvaccinated for COVID-19 vaccine (December 2021–April 2022): They were breastfeeding women, pregnant or planning to be pregnant separated into:
| Prospective controlled trial.
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinou, E.; Benou, S.; Hatzidaki, E.; Vervenioti, A.; Dimitriou, G.; Papaevangelou, V.; Jones, C.E.; Gkentzi, D. Postpartum Interventions to Increase Maternal Vaccination Uptake: Is It Worth It? Vaccines 2024, 12, 1130. https://doi.org/10.3390/vaccines12101130
Konstantinou E, Benou S, Hatzidaki E, Vervenioti A, Dimitriou G, Papaevangelou V, Jones CE, Gkentzi D. Postpartum Interventions to Increase Maternal Vaccination Uptake: Is It Worth It? Vaccines. 2024; 12(10):1130. https://doi.org/10.3390/vaccines12101130
Chicago/Turabian StyleKonstantinou, Eleni, Sofia Benou, Eleftheria Hatzidaki, Aggeliki Vervenioti, Gabriel Dimitriou, Vassiliki Papaevangelou, Christine E. Jones, and Despoina Gkentzi. 2024. "Postpartum Interventions to Increase Maternal Vaccination Uptake: Is It Worth It?" Vaccines 12, no. 10: 1130. https://doi.org/10.3390/vaccines12101130
APA StyleKonstantinou, E., Benou, S., Hatzidaki, E., Vervenioti, A., Dimitriou, G., Papaevangelou, V., Jones, C. E., & Gkentzi, D. (2024). Postpartum Interventions to Increase Maternal Vaccination Uptake: Is It Worth It? Vaccines, 12(10), 1130. https://doi.org/10.3390/vaccines12101130






