Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center
Abstract
:1. Introduction
2. Methods
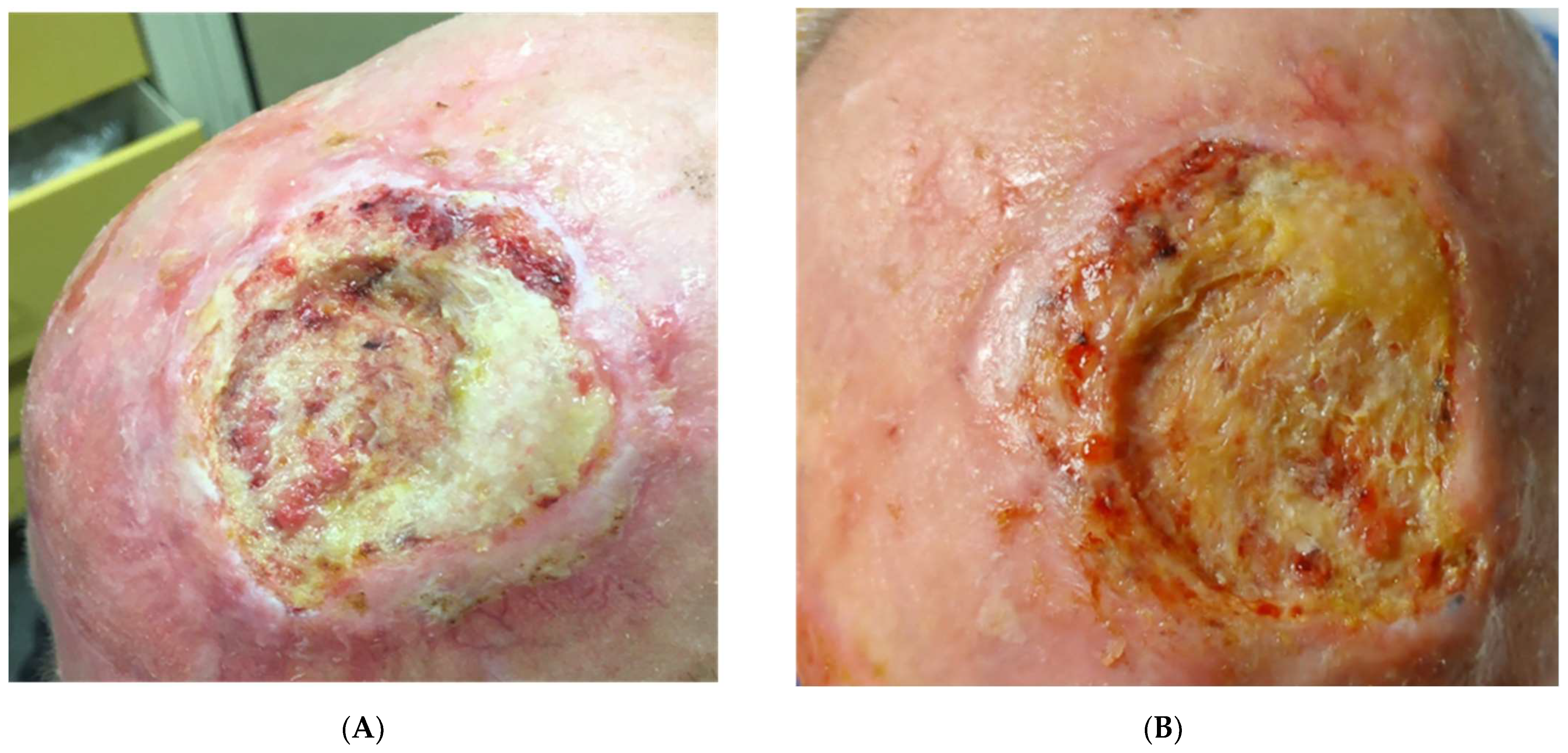
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawlik, L.; Morgenroth, S.; Dummer, R. Recent Progress in the Diagnosis and Treatment of Melanoma and Other Skin Cancers. Cancers 2023, 15, 1824. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.Y.; Goh, M.S.; Porceddu, S.V.; Rischin, D.; Lim, A.M. The Current Treatment Landscape of Cutaneous Squamous Cell Carcinoma. Am. J. Clin. Dermatol. 2023, 24, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Bibee, K.; Swartz, A.; Sridharan, S.; Kurten, C.H.L.; Wessel, C.B.; Skinner, H.; Zandberg, D.P. Cutaneous squamous cell carcinoma in the organ transplant recipient. Oral Oncol. 2020, 103, 104562. [Google Scholar] [CrossRef] [PubMed]
- Work Group; Invited Reviewers; Kim, J.Y.S.; Kozlow, J.H.; Mittal, B.; Moyer, J.; Olenecki, T.; Rodgers, P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 560–578. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Schadendorf, D. Update in the treatment of non-melanoma skin cancers: The use of PD-1 inhibitors in basal cell carcinoma and cutaneous squamous-cell carcinoma. J. Immunother. Cancer 2022, 10, e005082. [Google Scholar] [CrossRef] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Migden, M.R.; Schmults, C.; Khushanlani, N.; Guminski, A.; Chang, A.L.; Lewis, K.; Ansstas, G.; Bowyer, S.E.; Hughes, B.G.M.; Schadendorf, D.; et al. 814P Phase II study of cemiplimab in patients with advanced cutaneous squamous cell carcinoma (CSCC): Final analysis from EMPOWER-CSCC-1 groups 1, 2 and 3. Ann. Oncol. 2022, 33 (Suppl. 7), S918–S919. [Google Scholar] [CrossRef]
- Stuck, A.E.; Siu, A.L.; Wieland, G.D.; Adams, J.; Rubenstein, L.Z. Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet 1993, 342, 1032. [Google Scholar] [CrossRef]
- Gulasingam, P.; Haq, R.; Mascarenhas Johnson, A.; Togo, E.; Moore, J.; Straus, S.E.; Wong, C.L. Using Implementation Science to Promote the Use of the G8 Screening Tool in Geriatric Oncology. J. Am. Geriatr. Soc. 2019, 67, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Rembielak, A.; Yau, T.; Akagunduz, B.; Aspeslagh, S.; Colloca, G.; Conway, A.; Danwata, F.; Del Marmol, V.; O’Shea, C.; Verhaert, M.; et al. Recommendations of the International Society of Geriatric Oncology on skin cancer management in older patients. J. Geriatr. Oncol. 2023, 14, 101502. [Google Scholar] [CrossRef]
- Zavdy, O.; Coreanu, T.; Bar-On, D.Y.; Ritter, A.; Bachar, G.; Shpitzer, T.; Kurman, N.; Mansour, M.; Ad-El, D.; Rozovski, U.; et al. Cutaneous Squamous Cell Carcinoma in Immunocompromised Patients—A Comparison between Different Immunomodulating Conditions. Cancers 2023, 15, 1764. [Google Scholar] [CrossRef]
- Zelin, E.; Maronese, C.A.; Dri, A.; Toffoli, L.; Di Meo, N.; Nazzaro, G.; Zalaudek, I. Identifying Candidates for Immunotherapy among Patients with Non-Melanoma Skin Cancer: A Review of the Potential Predictors of Response. J. Clin. Med. 2022, 11, 3364. [Google Scholar] [CrossRef] [PubMed]
- Rischin, D.; Migden, M.R.; Lim, A.M.; Schmults, C.D.; Khushalani, N.I.; Hughes, B.G.M.; Schadendorf, D.; Dunn, L.A.; Hernandez-Aya, L.; Chang, A.L.S.; et al. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: Primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J. Immunother. Cancer 2020, 8, e000775. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, M.; Leiter, U.; Loquai, C.; Zimmer, L.; Ugurel, S.; Gutzmer, R.; Thoms, K.M.; Enk, A.H.; Hassel, J.C. Programmed cell death protein 1 inhibitors in advanced cutaneous squamous cell carcinoma: Real-world data of a retrospective, multicenter study. Eur. J. Cancer 2020, 138, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Baggi, A.; Quaglino, P.; Rubatto, M.; Depenni, R.; Guida, M.; Ascierto, P.A.; Trojaniello, C.; Queirolo, P.; Saponara, M.; Peris, K.; et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur. J. Cancer 2021, 157, 250–258. [Google Scholar] [CrossRef]
- Strippoli, S.; Fanizzi, A.; Quaresmini, D.; Nardone, A.; Armenio, A.; Figliuolo, F.; Filotico, R.; Fucci, L.; Mele, F.; Traversa, M.; et al. Cemiplimab in an Elderly Frail Population of Patients With Locally Advanced or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Center Real-Life Experience From Italy. Front. Oncol. 2021, 11, 686308. [Google Scholar] [CrossRef]
- Hober, C.; Fredeau, L.; Pham-Ledard, A.; Boubaya, M.; Herms, F.; Celerier, P.; Aubin, F.; Beneton, N.; Dinulescu, M.; Jannic, A.; et al. Cemiplimab for Locally Advanced and Metastatic Cutaneous Squamous-Cell Carcinomas: Real-Life Experience from the French CAREPI Study Group. Cancers 2021, 13, 3547. [Google Scholar] [CrossRef]
- Guillaume, T.; Puzenat, E.; Popescu, D.; Aubin, F.; Nardin, C. Cemiplimab-rwlc in advanced cutaneous squamous cell carcinoma: Real-world experience in a French dermatology department. Br. J. Dermatol. 2021, 185, 1056–1058. [Google Scholar] [CrossRef]
- Valentin, J.; Gérard, E.; Ferte, T.; Prey, S.; Dousset, L.; Dutriaux, C.; Beylot-Barry, M.; Pham-Ledard, A. Real world safety outcomes using cemiplimab for cutaneous squamous cell carcinoma. J. Geriatr. Oncol. 2021, 12, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Caillé, B.; Kottler, D.; Morello, R.; Lecornu, M.; Kao, W.; Meyer, E.; Dompmartin, A.; L’Orphelin, J.M. Real-Life Study of the Benefit of Concomitant Radiotherapy with Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma (cSCC): A Retrospective Cohort Study. Cancers 2023, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Viñuela, E.; Álvarez, P.; Lavernia, J.; Serra-Guillén, C.; Requena, C.; Bernia, E.; Diago, A.; Llombart, B.; Sanmartín, O. Cemiplimab in Advanced Cutaneous Squamous Cell Carcinoma: Real-World Experience in a Monographic Oncology Center. Actas Dermosifiliogr. 2022, 113, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, I.; Salman, S.; Shtamper, N.; Doweck, I.; Popovtzer, A.; Markel, G.; Hendler, D.; Finkel, I.; Moore, A.; Fenig, E.; et al. First-line programmed death-1 inhibitor treatment for locoregionally advanced or metastatic cutaneous squamous cell carcinoma—A real-world experience from Israel. Front. Oncol. 2023, 13, 1117804. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Lang, B.M.; Tsochataridou, A.; Salzmann, M.; Mohr, P.; Schadendorf, D.; Ugurel, S.; Placke, J.-M.; Weichenthal, M.; et al. Response to First-Line Treatment with Immune-Checkpoint Inhibitors in Patients with Advanced Cutaneous Squamous Cell Carcinoma: A Multicenter, Retrospective Analysis from the German ADOReg Registry. Cancers 2022, 14, 5543. [Google Scholar] [CrossRef] [PubMed]
- Merlano, M.C.; Abbona, A.; Denaro, N.; Garrone, O. Knowing the tumour microenvironment to optimise immunotherapy. Acta Otorhinolaryngol. Ital. 2019, 39, 2–8. [Google Scholar] [CrossRef]
- Migden, M.R.; Chandra, S.; Rabinowits, G.; Chen, C.I.; Desai, J.; Seluzhytsky, A.; Sasane, M.; Campanelli, B.; Chen, Z.; Freeman, M.L.; et al. CASE (CemiplimAb-rwlc Survivorship and Epidemiology) study in advanced cutaneous squamous cell carcinoma. Future Oncol. 2020, 16, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Homsi, J.; Park, S.J.; Khushanlani, N.; Panella, T.; Ellison, D.M.; Gentry, R.W.; Venna, S.S.; Strasswimmer, J.; Zuniga, R.M.; et al. CemiplimAb-rwlc Survivorship and Epidemiology (CASE): A prospective study of the safety and efficacy of cemiplimab in patients (pts) with advanced cutaneous squamous cell carcinoma (CSCC) in a real-world setting. Ann. Oncol. 2022, 33 (Suppl. 7), S356–S409. [Google Scholar] [CrossRef]



| G8 | FRAIL |
|---|---|
| Food intake over the past 3 months due to loss of appetite, digestive problems, chewing or swallowing difficulties (range no decrease = +2 severe decrease = 0) | Fatigue A = 1 = most of the time; B = 0 = a little |
| Weight loss during the last 3 months (range >3 kg = 0; no loss = +3) | Resistance: difficulty walking up 10 steps Y = 1 N = 0 |
| Mobility (range bed or chair = 0; goes out = +2) | Ambulation: difficulty walking 300 m or a block y = 1 N = 0 |
| Neuropsychological conditions (severe dementia = 0 no limits = + 2) | Illness * (0–4 = 0; 5–11 = 1) |
| Body mass index (<19 kg/m2 = 0 ≥ 23 kg/m2 = +3) | Loss of weight (y = 1 No = 0) |
| Polypharmacy (≥3) no = +1; yes = 0 | |
| How do you consider the health status (not as good = 0; better = 2) | |
| Age >85 = 0; 80–85 = 1 < 80 = 2 | |
| Range 17 low risk ≥ 14 | Range 5 High risk |
| Patients | Site | Age | Histology | Grading | Comorbidity * | Pharmacologic Therapy ** | G8 Score | FRAIL Score | ECOG PS | Imm- Suppr |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | hnc | 90 | SCC | 2 | 3 | 1 | 12 | 2 | 2 | |
| 2 | hnc | 85 | SCC | 3 | 1 | 1 | 9 | 3 | 1 | |
| 3 | hnc | 91 | SCC | 2 | 1,2,3 | 2 | 15 | 2 | 2 | |
| 4 | back | 86 | SCC | 2 | 1,2,3 | 2 | 14 | 2 | 2 | |
| 5 | hnc | 87 | SCC | 2 | 1 | 2 | 9 | 4 | 1 | |
| 6 | back, hnc, arm | 88 | BCC + SCC | 2 | 1,2,3 | 2 | 12 | 2 | 3 | Y 1 |
| 7 | hnc | 84 | SCC | 3 | 1,2,3 | 3 | 14 | 2 | 2 | Y 2 |
| 8 | hnc | 85 | SCC | 2 | 3 | 2 | 14 | 2 | 1 | |
| 9 | arm | 88 | SCC | 3 | 1 | 1 | 12 | 3 | 2 | |
| 10 | hnc | 80 | SCC | 2 | 1 | 1 | 10 | 4 | 1 | |
| 11 | hnc | 82 | SCC | 2 | 0 | 0 | 14 | 3 | 1 | |
| 12 | hnc | 81 | SCC | 2 | 3 | 2 | 15 | 2 | 1 | |
| 13 | hnc | 80 | SCC | 2 | 3 | 2 | 10 | 3 | 2 | |
| 14 | hnc | 86 | SCC | 3 | 0 | 2 | 14 | 2 | 1 | |
| 15 | arm | 87 | SCC | 2 | 1,2,4 | 1 | 9 | 4 | 2 | |
| 16 | arm | 81 | SCC | 2 | 3 | 2 | 9 | 3 | 3 | |
| 17 | hnc | 86 | BCC + SCC | 2 | 1,3 | 2 | 14 | 2 | 1 | |
| 18 | hnc | 98 | SCC | 2 | 1 | 3 | 9 | 2 | 3 | |
| 19 | hnc | 103 | SCC | 3 | 0 | 0 | 14 | 2 | 1 | |
| 20 | arm | 90 | BCC + SCC | 2 | 1 | 2 | 12 | 2 | 2 |
| Pts | N° of Surgery | RT | RT Doses Gy | Disease Stage | Cycles N° | Tox G1 | Tox G2 | Response |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | N | - | III | 6 | Diarrhea | Pneumonitis | PD |
| 2 | 3 | Y | 50 | IV M0 | 27 | CR | ||
| 3 | 2 | Y | 60 | III | 12 | Myalgia | Diarrhea | PR |
| 4 | 2 | Y | 56 | IV M0 | 7 | Diarrhea | PR | |
| 5 | 3 | Y | 54 | IV M1 | 12 | Fatigue | Transaminitis | SD |
| 6 | 3 | Y | 54 | III | 7 | Diarrhea | Pneumonitis | SD |
| 7 | 3 | Y | 60 | IV M0 | 7 | SD | ||
| 8 | 4 | Y | 66 | III | 8 | Myalgia | PR | |
| 9 | 4 | Y | 54 | IV M1 | 10 | PD | ||
| 10 | 3 | Y | 56 | IV M0 | 30 | PR | ||
| 11 | 3 | N | IV M0 | 6 | Fatigue | SD | ||
| 12 | 3 | Y | 60 | IV M0 | 9 | PR | ||
| 13 | 3 | Y | 60 | IV M0 | 5 | PR | ||
| 14 | 1 | N | IV M0 | 13 | Fatigue | PR | ||
| 15 | 1 | N | IV M0 | 3 | Diarrhea | PR | ||
| 16 | 2 | N | IV M0 | 6 | CR | |||
| 17 | 3 | N | IV M0 | 7 | Fatigue | CR | ||
| 18 | 1 | Y | 66 | IV M0 | 2 | Diarrhea | CR | |
| 19 | 3 | Y | 60 | IV M0 | 6 | Creatinine increase | CR | |
| 20 | 4 | N | IV M1 | 12 | PR |
| Study | Type | Patients (n.) | Median Age, Years (Range) | Response | Toxicity, Any Grade (%) | Special Population |
|---|---|---|---|---|---|---|
| Rischin D 2020 [14] | Phase II | 114 | 71 (38–90) | ORR 51% | 22 G3–4 27 G2 | NR |
| Salzmann MM 2020 [15] | Retrospective observational multicenter | 46 | 76 (39–92) | ORR 58.7% DCR 80.4% | 13 G3–4 8.7 discontinuation | |
| Baggi A 2021 [16] | Retrospective observational multicenter | 131 | 79 (19–95) | ORR 58% DCR 79% | 9.2 G3–4 | 17.7% * |
| Strippoli S 2021 [17] | Retrospective observational monocentric | 30 | 81 (36–95) | ORR 76.7% CR 30% | 10 G3–4 33 G2 | 16% |
| Hober C 2021 [18] | Retrospective observational multicenter | 245 | 77 (64–90) | ORR 48.6% 1 y OS 73% vs. 36%, for pts with PS < 2 vs. ≥ 2 | 9 G3–4 31 G1–2 | 21% |
| Guillaume T 2021 [19] | Retrospective observational monocentric | 18 | 80 (45–96) | ORR 67% CR 33% | 8 G3–4 33 G2 | 16.7% |
| Valentin J 2021 [20] | Retrospective observational monocentric | 22 | 83 (55–93) | ORR 32% DCR 79% | 45 G3–4 32 G1–2 41 discontinuations | 36% |
| Bailly Caillé 2023 [21] | Retrospective observational monocentric | 33 (12 + RT) | 75 (63–88) | ORR 45.5% DCR 70% | 23 G3–4 29 G1–2 | NR |
| Ríos-Viñuela E 2023 [22] | Retrospective observational monocentric | 13 | 81 (56–91) | ORR 62% | 0 G3–4 46 G1–2 | NR |
| Averbuch I 2023 [23] | Retrospective observational | 102 | 78.5 (51–96) | mPFS 29.5 m ORR 80.6% CR 45.2% | 5 G3–4 55 G1–2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denaro, N.; Passoni, E.; Indini, A.; Nazzaro, G.; Beltramini, G.A.; Benzecry, V.; Colombo, G.; Cauchi, C.; Solinas, C.; Scartozzi, M.; et al. Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center. Vaccines 2023, 11, 1500. https://doi.org/10.3390/vaccines11091500
Denaro N, Passoni E, Indini A, Nazzaro G, Beltramini GA, Benzecry V, Colombo G, Cauchi C, Solinas C, Scartozzi M, et al. Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center. Vaccines. 2023; 11(9):1500. https://doi.org/10.3390/vaccines11091500
Chicago/Turabian StyleDenaro, Nerina, Emanuela Passoni, Alice Indini, Gianluca Nazzaro, Giada Anna Beltramini, Valentina Benzecry, Giuseppe Colombo, Carolina Cauchi, Cinzia Solinas, Mario Scartozzi, and et al. 2023. "Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center" Vaccines 11, no. 9: 1500. https://doi.org/10.3390/vaccines11091500
APA StyleDenaro, N., Passoni, E., Indini, A., Nazzaro, G., Beltramini, G. A., Benzecry, V., Colombo, G., Cauchi, C., Solinas, C., Scartozzi, M., Marzano, A. V., & Garrone, O. (2023). Cemiplimab in Ultra-Octogenarian Patients with Cutaneous Squamous Cell Carcinoma: The Real-Life Experience of a Tertiary Referral Center. Vaccines, 11(9), 1500. https://doi.org/10.3390/vaccines11091500








